| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Domenico Caputo | + 5749 word(s) | 5749 | 2020-08-20 05:09:01 | | | |
| 2 | Bruce Ren | -2960 word(s) | 2789 | 2020-08-27 03:29:52 | | |
Video Upload Options
This topic focuses on the use of metal–organic frameworks (MOFs) for adsorbing gas species that are known to weaken the thermal self-regulation capacities of Earth’s atmosphere. A large section is dedicated to the adsorption of carbon dioxide, while another section is dedicated to the adsorption of other different gas typologies, whose emissions, for various reasons, represent a “wound” for Earth’s atmosphere. High emphasis is given to MOFs that have moved enough ahead in their development process to be currently considered as potentially usable in “real-world” (i.e., out-of-lab) adsorption processes. As a result, there is strong evidence of a wide gap between laboratory results and the industrial implementation of MOF-based adsorbents. Indeed, when a MOF that performs well in a specific process is commercially available in large quantities, economic observations still make designers tend toward more traditional adsorbents. Moreover, there are cases in which a specific MOF remarkably outperforms the currently employed adsorbents, but it is not industrially produced, thus strongly limiting its possibilities in large-scale use. To overcome such limitations, it is hoped that the chemical industry will be able to provide more and more mass-produced MOFs at increasingly competitive costs in the future.
1. Introduction
Since its first historical traces, mankind has never had to face global challenges such as those happening in the contemporary age. Among them, climate change is the challenge nowadays considered to be the most threatening for the survival of the whole human race
[1]. Climate change is mainly dependent on the energy transport phenomena occurring between Earth and outer space. In turn, such energy transport phenomena are strongly related to the physicochemical status of Earth’s atmosphere. After the Industrial Revolution took place from the second half of the 18th century to the first half of the 19th century, anthropic activities started to dramatically influence the “health” of the Earth’s atmosphere. Therefore, the energy balance of our planet is being modified in such a way that its average temperature is abnormally increasing (global warming). This temperature increase is already causing anomalous climatic events, which are unprecedented in human history and recall prehistorical periods of planetary crisis such as Late Quaternary extinctions [2]. In order to try reverting global warming, immediate action must be taken to minimize anthropic emissions that modify the chemical composition of Earth’s atmosphere. Among the unit operations of the process industry, adsorption has turned out to be the most efficient for separating gas mixtures in fractions or in pure components [3]. In other words, adsorption represents the cleanest way of capturing pollutants from end-of-process gas streams that are discharged into the atmosphere. When designing an adsorption system, one of the crucial choices concerns the type of adsorbent material that will be used in the packed beds. The materials mostly contemplated for adsorption processes are nanoporous materials [4], in particular, microporous materials (with pore sizes below 2 nm) and mesoporous materials (with pore sizes between 2 and 50 nm). During the last 15 years, microporous metal–organic frameworks (MOFs) have shown great potential for improving the performances of different industrially relevant, adsorption-based applications[5]. This review will focus on the use of MOFs for adsorbing gas species, which are known to weaken the thermal self-regulation capacities of Earth’s atmosphere. Obviously, a large section will be dedicated to the adsorption of carbon dioxide, whose emissions are the main cause of the so-called greenhouse effect, while another section will be dedicated to the adsorption of other different gas typologies (e.g., fluorinated gases, volatile organic compounds, etc.) whose emissions, for various reasons, represent a “wound” for Earth’s atmosphere. Nevertheless, the critical approach to the following literature survey was based on the search for the three main functionalities that an ideal adsorbent should always feature:
i. High adsorption capacity toward the target adsorptive (e., the value of the adsorbed amount of substance observed in saturation conditions);
ii. High selectivity toward the target adsorptive (e., the ability of the adsorbent to preferably adsorb one adsorptive when mixed with others);
iii. High regeneration capacity (e., the possibility of removing the adsorbate by means of simple and inexpensive methods, in order to use the same adsorbent for repeated adsorption cycles).
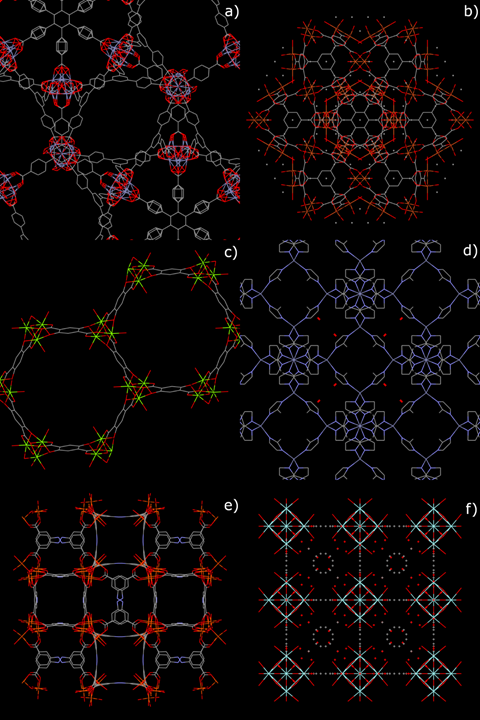
Moreover, high emphasis will be given to studies about MOFs that have moved ahead enough in their development process to be currently considered as potentially usable in “real-world” (i.e., out-of-lab) adsorption processes. The crystal structures of some of these MOFs, whose performances as adsorbents will be detailed in the next sections, are shown in Figure 1.
Figure 1. Crystal structures of some mass-produced metal–organic frameworks (MOFs): (a) MOF-177; (b) Cu-BTC; (c) Mg-MOF-74; (d) ZIF-8; (e) PCN-250 (Fe3); (f) UiO-66.
2. CO2 adsorption on MOFs
Starting from the advent of the Industrial Revolution, anthropic activities caused an increase in carbon dioxide atmospheric concentration of more than 50%, i.e., from about 260 ppm of pre-industrial era [6] the current 400 ppm (and more)[7]. Such anomalous change in the composition of Earth’s atmosphere is significantly reducing the fraction of absorbed solar radiant energy that the planet gives back to outer space. This phenomenon, known as greenhouse effect [8], is the main cause of the abnormal planetary temperature rise registered during the last two centuries. Anthropic CO2 emissions mainly come from three sources:
i. transportation, through combustion of fossil fuels;
ii. electricity production, (again) through combustion of fossil fuels;
iii. industry, through reforming of fossil fuels, gaseous byproducts of industrial steelmaking and cement industry, etc.
As particularly regards the last two scenarios, the implementation of adsorption columns downstream of electricity production and industrial plants can provide a drastic reduction of CO2 emissions [9][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42]. Up to the end of the last century, zeolites were considered the best option among nanoporous adsorbents for CO2 capture and they are still considered the benchmark in terms of benefit-cost ratio [10], also because zeolites can be synthesized starting from several waste materials [11]. In the last two decades, a lot of efforts were focused in studying the CO2 adsorption properties of functionalized mesoporous silicates [12][13][14][15][16]. Eventually, in the last 15 years, there has been an exponential development of studies about the CO2 adsorption properties of MOFs. Readers who are interested only in this specific topic are invited to also read a recent review by Ghanbari et al. [17]. With respect to the latter paper, rather than focusing on the correlation between MOF structures and CO2 adsorption performances, this section of the present review will give emphasis on the actual suitability of MOFs to be used in industrial CO2 adsorption processes. In particular, potential applications in packed bed adsorption columns will be taken into account, whereas membrane-based separation needs specific treatises [18]. An overview of the recent (limited to the last decade) literature about this subject is reported in Table 1.[19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][77]
Table 1. MOFs potentially suitable for CO2 adsorption
|
MOF type |
CO2 adsorption capacity,1 mol/kg (reported working conditions, T and p)2 |
Reference |
|
MOF-177 |
9.02 (298 K, 1400 kPa) |
[19] |
|
1.00 (298 K, 100 kPa) |
[23] |
|
|
0.93 (293 K, 100 kPa) |
[39] |
|
|
Ionic liquid – functionalized MOF-177 |
1.14 (303 K, 100 kPa) |
[24] |
|
Cu-BTC |
7.00 (283 K, 100 kPa) |
[26] |
|
14.00 (303 K, 4000 kPa) |
[28] |
|
|
8.07 (303 K, 1000 kPa) |
[29] |
|
|
11.70 (297 K, 1500 kPa) |
[30] |
|
|
Ionic liquid – functionalized Cu-BTC |
1.70 (303 K, 15 kPa) |
[32] |
|
Li – doped Cu-BTC |
4.85 (298 K, 100 kPa) |
[33] |
|
Mg-MOF-74 |
8.61 (298 K, 100 kPa) |
[37] |
|
9.02 (293 K, 100 kPa) |
[39] |
|
|
15.00 (313 K, 3500 kPa) |
[40] |
|
|
14.80 (303 K, 3000 kPa) |
[41] |
|
|
Mg-MOF-74 – polystyrene composite |
4.98 (298 K, 100 kPa) |
[44] |
|
Tetraethylenepentamine – functionalized Mg-MOF-74 |
6.06 (breakthrough, CO2/N2 mixture, [CO2] = 15 mol%, 333 K) |
[45] |
|
6.11 (298 K, 100 kPa) |
[46] |
|
|
Ethylenediamine – functionalized Mg-MOF-74 |
5.42 (298 K, 100 kPa) |
[47] |
|
ZIF-8 |
8.60 (303 K, 4000 kPa) |
[48] |
|
9.10 (303 K, 4500 kPa) |
[50] |
|
|
Ammonia – functionalized ZIF-8 |
7.50 (298 K, 3000 kPa) |
[51] |
|
Ethylenediamine – functionalized ZIF-8 |
9.85 (298 K, 2500 kPa) |
[52] |
|
Tetraethylenepentamine – functionalized ZIF-8 |
2.18 – 3.19, depending on the amine content (318 K, 500 kPa) |
[53] |
|
3-amino-1,2,4-triazole – functionalized ZIF-8 |
2.51 (308 K, 200 kPa) |
[55] |
|
2-nitrobenzimidazole – functionalized ZIF-8 |
3.39 (273 K, 120 kPa) |
[56] |
|
Thermally annealed ZIF-8 |
3.00 (298 K, 250 kPa) |
[57] |
|
Li – doped polyoxometalate – ZIF-8 composite |
16.00 (298 K, 1000 kPa) |
[58] |
|
Ionic liquid – functionalized ZIF-8 |
0.83 (303 K, 20 kPa) |
[59] |
|
PCN-250 (Fe3) |
5.24 (breakthrough, CO2/N2 mixture, [CO2] = 15 vol%, 303 K) |
[63] |
|
3.02 (298 K, 100 kPa) |
[64] |
|
|
UiO-66 |
7.65 (303 K, 6000 kPa) |
[65] |
|
7.29 (298 K, 3000 kPa) |
[66] |
|
|
1.48 (303 K, 100 kPa) |
[67] |
|
|
4.34 (298 K, 2000 kPa) |
[68] |
|
|
Ti – exchanged UiO-66 |
4.37 (273 K, 120 kPa) |
[72] |
|
Adipic acid – functionalized UiO-66 |
3.76 (273 K, 100 kPa) |
[73] |
|
Ethanolamine – functionalized UiO-66 |
1.70 (298 K, 100 kPa) |
[74] |
|
Polyethylenimine – functionalized UiO-66 |
3.32 (298 K, 100 kPa) |
[75] |
|
Tetraethylenepentamine – functionalized UiO-66 |
3.70 (breakthrough, CO2/He mixture, [CO2] = 10 vol%, 348 K, 100 kPa) |
[76] |
|
Li – doped UiO-66 |
2.80 (298 K, 100 kPa) |
[77] |
A crucial, still overlooked aspect regards the need of shaped adsorbents for packing industrial scale beds, whereas all the examined materials are initially produced in powder form. Actually, commercial MOF providers offer powder shaping as an additional service [78], thus relieving the end user from this laborious task. Anyway, in order to be of any practical use inside adsorption columns, MOF powders need to be processed by means of classical methods such as granulation and pelletization [79]. In both the latter cases, adsorbent shaping comes with a cost, i.e., it negatively affects the structural / textural properties of MOFs. The techniques used for shaping MOFs thus demand further research in order to optimize such manufacturing process [80][81][82]. The most detrimental side effects that must be still minimized are the collapse of the adsorbent crystalline lattice, the reduced access to its pore structure and the diffusion limitations of shaped bodies due to non-optimal void fractions between primary powders [83].
A last, even more crucial issue about the suitability of using MOFs in actual industrial CO2 adsorption processes regards the comparison between the costs and the benefits that such a technological shift would bring along. Unfortunately, current analyses available in literature seem to point out that, despite their declared performance superiority, MOFs cannot compare to much cheaper traditional adsorbents. As an example (more systematic than others already cited ), Danaci et al. assessed 22 different MOFs (some of which are among the ones listed in Table 1) against specific performance constraints and cost in CO2 capture from flue gas using PVSA [84]. At the end of such a significant screening, the MOF that showed the best performance and lowest cost was a still-commercially-unavailable one (i.e., UTSA-16), whose performance was in line with a much cheaper 13X zeolite anyway. It is hoped that studies like the latter one provide specific directions for material scientists to design MOF adsorbents with more focus on actual process needs than on the discovery of new structures per se.
3. MOFs for other adsorption processes significant for the atmospheric environment
As outlined in Section 1, in addition to CO2, there are other greenhouse gases and other gas typologies whose emissions, for various reasons, represent a significant “wound” for Earth’s atmosphere. Also in these cases, adsorption represents the cleanest way for capturing such pollutants from end-of-process gas streams, and MOFs could be considered a valid choice as materials for adsorption column packing.
3.1. Adsorption of sulfur and nitrogen oxides
When emitted into the atmosphere, sulfur and nitrogen oxides (namely, SOx and NOx) contribute to ground-level ozone formation and are responsible for eutrophication, reduction in water quality and, eventually, species richness. They are also associated with adverse effects on human health as high concentrations cause respiratory illnesses. Both NOx and SOx are combustion products that are emitted into the atmosphere within flue gas. Indeed, most anthropogenic SOx and NOx emissions emerge from the combustion of coal and heavy oil. Moreover, other industrial processes are also specifically responsible for significant SO2 emissions [85][86].
During the last 15 years, the scientific community has shown a growing interest in the attempt to capture industrially originating SOx and NOx before their emission into the atmosphere. MOFs, due to their great sorption capacities and their selectivity in capturing a large number of toxic and pollutant gases [87][88][89][90][91], are thus considered promising candidates for packing SOx and NOx adsorption columns. In this regard, the most interesting results are summarized in Table 2.
Table 2. MOFs potentially suitable for SOx and NOx adsorption.[92][93][94]
|
MOF type |
Adsorbate |
Adsorption capacity,1 mol/kg (reported working conditions, T and p)2 |
Reference |
|
Cu-BTC |
SO2 |
0.71 (breakthrough, SO2/O2/He mixture, [SO2] = 50 ppm, 773 K) |
[92] |
|
Ba – doped Cu-BTC |
SO2 |
2.71 (breakthrough, SO2/O2/He mixture, [SO2] = 50 ppm, 773 K) |
|
|
MOF-177 |
SO2 |
25.70 (293 K, 100 kPa) |
[93] |
|
UiO-66 |
NO2 |
1.59 (breakthrough, NO2/N2/air mixture, [NO2] = 1000 ppm, 298 K) |
[94] |
1 Where not explicitly reported in the cited papers, adsorption capacity values were extracted from plots using reverse engineering software. 2 Where not explicitly reported, working conditions imply the collection of single-component adsorption isotherms.
3.2. Adsorption of volatile organic compounds
Volatile organic compounds (VOCs) are a major group of air pollutants, potentially leading to photochemical smog, carcinogenesis, teratogenesis, and mutagenesis, which endanger both ecological environment and human health [95][96]. Anthropogenic VOC emissions emerge from a wide range of industrial processes, including crude oil and natural gas exploration, petroleum refining and basic chemical raw materials manufacturing [97]. Despite the immense amount of research reported regarding MOF adsorbents, literature about the adsorption of VOCs on MOFs is relatively scarce. The results regarding MOFs produced on industrial scale and commercially available are mostly summarized in Table 3.
Table 3. MOFs potentially suitable for adsorption of VOCs[98][99][100][101][102]
|
MOF type |
Adsorbate |
Adsorption capacity,1 mol/kg (reported working conditions, T and p)2 |
Reference |
|
MOF-177 |
acetone |
8.30 (298 K, 10.83 kPa) |
[98] |
|
benzene |
8.82 (298 K, 4.88 kPa) |
||
|
toluene |
3.77 (298 K, 1.44 kPa) |
||
|
ethylbenzene |
2.13 (298 K, 0.39 kPa) |
||
|
m-xylene |
1.92 (298 K, 0.33 kPa) |
||
|
o-xylene |
1.97 (298 K, 0.34 kPa) |
||
|
p-xylene |
1.78 (298 K, 0.32 kPa) |
||
|
ethenylbenzene |
1.61 (298 K, 0.23 kPa) |
||
|
Al-fumarate |
dichloromethane |
3.40 (298 K, 44.70 kPa) |
[101] |
|
trichloromethane |
2.51 (298 K, 21.44 kPa) |
||
|
UiO-66 |
toluene |
1.64 (breakthrough, toluene/O2/argon mixture, [toluene] = 1000 ppm, 298 K) |
[102] |
3.3. Adsorption of fluorinated gases
Historically, another class of gas emissions that are considered extremely harmful for the atmosphere is that including fluorinated compounds. In particular, chlorofluorocarbons (CFCs) constitute a serious threat to the stratospheric ozone layer. During the last decades, the progressive substitution of CFCs with other types of service fluids in refrigeration processes allowed to significantly recover the functionality of the planetary natural UV shield. Anyway, there are other categories of fluorinated gases that should be considered highly dangerous for Earth’s atmosphere. Among them, halogenated general anesthesia gases (HGAGs) represent an emerging threat due to their worldwide growing uncontrolled discharge [103]. Indeed, Cl-containing HGAGs (e.g., enflurane and isoflurane) are also classified as CFCs, while sevoflurane, desflurane and (again) isoflurane are characterized by a global warming potential (GWP) that is three orders of magnitude higher than that of CO2 [104]. Among the convenient technologies for handling emissions of HGAGs, adsorption-based ones are recognized as the most effective [105]. Indeed, non-metabolized anesthetic vapors can be captured in canisters that act as adsorption mini-columns on the vent line of the breathing system connected to the patient. Currently, studies about the potential use of MOFs for packing such canisters are quite rare. To the best of our knowledge, the only paper that deals with this topic and that envisages the use of an already commercially available adsorbent reported the sevoflurane (SF) adsorption properties of MOF-177 [106]. Despite the impressive SF adsorption capacity and affinity showed by this material, its actual implementation as anesthetic scavenger is strongly undermined due to the presence of moisture in the vent line of breathing systems .
In truth, the only MOF that proved to be practically suitable for HGAG capture is the chromium variant of MIL-101, which is not currently available in large quantities as a commercial product. Indeed, MIL-101 showed a significantly higher SF equilibrium adsorption capacity when compared to a reference adsorbent conventionally used for HGAG scavenging [107]. Moreover, when shaped and column-packed, MIL-101 revealed a much higher SF/H2O selectivity with respect to the aforementioned reference adsorbent, which suffered of “roll-up” effects when the test column reached saturation [108]. Under the same dynamic conditions (which obviously include a significant presence of moisture in the feed), the same MOF also showed a much higher performance stability when compared with the reference adsorbent after several adsorption / desorption cycles [109]. Unfortunately, as already outlined, such technologically-relevant results are not destined to improve the performances of the HGAG scavenging systems used in operating rooms (at least for the moment) due to the impossibility of large-scale MIL-101 supplies.
4. Conclusions
Regarding adsorption-based technologies for atmospheric emission control, there is a strong evidence of how MOFs are still far away from passing their “graduation exam”. The wide gap between laboratory results and industrial implementation is due to different factors. First, even when a MOF that performs well in a specific process (e.g., CO2 adsorption) is commercially available in large quantities, economic observations still make designers tend towards more traditional adsorbents (e.g., zeolites, activated carbons). Indeed, the cost per mass unit of commercial MOFs basically is orders of magnitude higher than that of other materials, whose industrial use is already well-established. Moreover, there are cases (like adsorption processes for HGAG scavenging) in which a specific MOF remarkably outperforms currently employed adsorbents, but it is not industrially produced, thus strongly limiting its possibilities of large-scale use. To overcome such limitations, it is to be hoped that chemical industry will be able to provide more and more mass-produced MOFs at increasingly competitive costs in the future.
References
- IPCC, 2014: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. IPCC, Geneva, Switzerland, 151 pp.
- Wan, X.; Zhang, Z. Climate warming and humans played different roles in triggering Late Quaternary extinctions in east and west Eurasia. R. Soc. B 2017, 284, 20162438.
- Mersmann, A.; Fill, B.; Hartmann R.; Maurer S. The Potential of Energy Saving by Gas-Phase Adsorption Processes. Eng. Technol. 2000, 23, 937‑944.
- Broom, D.P.; Thomas, M. Gas adsorption by nanoporous materials: Future applications and experimental challenges. MRS Bull. 2013, 38, 412‑421.
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Soc. Rev. 2009, 38, 1477‑1504.
- Raynaud, D.; Barnol, J.M. An Antarctic ice core reveals atmospheric CO2 variations over the past few centuries. Nature 1985, 315, 309‑311.
- Climate Change: Atmospheric Carbon Dioxide. Available online: https://www.climate.gov/news-features/understanding-climate/climate-change-atmospheric-carbon-dioxide (accessed on 8th January 2020).
- Schneider, S. The Greenhouse Effect: Science and Policy. Science 1989, 243, 771‑781.
- Jiang, L.; Gonzalez-Diaz, A.; Ling-Chin, J.; Roskilly, A.P.; Smallbone, A.J. Post-combustion CO2 capture from a natural gas combined cycle power plant using activated carbon adsorption. Energy 2019, 245, 1‑15.
- Oreggioni, G.D.; Brandani, S.; Luberti, M.; Baykan, Y.; Friedrich, D.; Ahn, H. CO2 capture from syngas by an adsorption process at a biomass gasification CHP plant: its comparison with amine-based CO2 Int. J. Greenhouse Gas Control 2015, 35, 71‑81.
- Gargiulo, N.; Shibata, K.; Peluso, A.; Aprea, P.; Valente, T.; Pezzotti, G.; Shiono, T.; Caputo, D. Reinventing rice husk ash: derived NaX zeolite as a high-performing CO2 Int. J. Environ. Sci. Technol. 2018, 15, 1543‑1550.
- Gargiulo, N.; Pepe, F.; Caputo, D. CO2 adsorption by functionalized nanoporous materials: a review. Nanosci. Nanotechnol. 2014, 14, 1811‑1822.
- Gargiulo, N.; Pepe, F.; Caputo, D. Modeling carbon dioxide adsorption on polyethylenimine-functionalized TUD-1 mesoporous silica. Colloid Interface Sci. 2012, 367, 348‑354.
- Gargiulo, N.; Peluso, A.; Aprea, P.; Pepe, F.; Caputo, D. CO2 Adsorption on polyethylenimine-functionalized SBA-15 mesoporous silica: isotherms and modeling. Chem. Eng. Data 2014, 59, 896‑902.
- Gargiulo, N.; Macario, A.; Iucolano, F.; Giordano, G.; Caputo, D. Modeling the adsorption of CO2/N2 mixtures on siliceous nanoporous materials. Adv. Mater. 2015, 7, 258‑263.
- Gargiulo, N.; Verlotta, A.; Peluso, A.; Aprea, P.; Caputo, D. Modeling the performances of a CO2 adsorbent based on polyethylenimine-functionalized macro-/mesoporous silica monoliths. Microporous Mesoporous Mater. 2015, 215, 1‑7.
- Ghanbari, T.; Abnisa, F.; Wan Daud, W.M.A. A review on production of metal organic frameworks (MOF) for CO2 Sci. Total Environ. 2020, 707, 135090.
- Saqib, S.; Rafiq, S.; Chawla, M.; Saeed, M.; Muhammad, N.; Khurram, S.; Majeed, K.; Khan, A.L.; Ghauri, M.; Jamil, F.; Aslam, M. Facile CO2 separation in composite membranes. Eng. Technol. 2019, 42, 30‑44.
- Saha, D.; Bao, Z.; Jia, F.; Deng, S. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and Zeolite 5A. Sci. Technol. 2010, 44, 1820‑1826.
- Tsivadze, A.Y.; Aksyutin, O.E.; Ishkov, A.G.; Knyazeva, M.K.; Solovtsova, O.V.; Men'shchikov, I.E.; Fomkin, A.A.; Shkolin, A.V.; Khozina, E.V.; Grachev, V.A. Metal-organic framework structures: adsorbents for natural gas storage. Chem. Rev. 2019, 88, 925‑978.
- Sircar, S. Pressure Swing Adsorption. Eng. Chem. Res. 2002, 41, 1389‑1392.
- Chou, C.-T.; Chen, C.-Y. Carbon dioxide recovery by vacuum swing adsorption. Purif. Technol. 2004, 39, 51‑65.
- Ullah, S.; Bustam, M.A.; Assiri, M.A.; Al-Sehemi, A.G.; Sagir, M.; Abdul Kareem, F.A.; Elkhalifah, A.E.I.; Mukhtar, A.; Gonfa, G. Synthesis, and characterization of metal-organic frameworks -177 for static and dynamic adsorption behavior of CO2 and CH4. Microporous Mesoporous Mater. 2019, 288, 109569.
- Mohamedali, M.; Henni, A.; Ibrahim, H. Investigation of CO2 capture using acetate-based ionic liquids incorporated into exceptionally porous metal–organic frameworks. Adsorption 2019, 25, 675‑692.
- Tranchemontagne, D.J.; Hunt, J.R.; Yaghi, O.M. Room temperature synthesis of metal-organic frameworks: MOF-5, MOF-74, MOF-177, MOF-199, and IRMOF-0. Tetrahedron 2008, 64, 8553‑8557.
- Aprea, P.; Caputo, D.; Gargiulo, N.; Iucolano, F.; Pepe, F. Modeling Carbon Dioxide Adsorption on Microporous Substrates: Comparison between Cu-BTC Metal-Organic Framework and 13X Zeolitic Molecular Sieve. Chem. Eng. Data 2010, 55, 3655‑3661.
- Musto, P.; La Manna, P.; Pannico, M.; Mensitieri, G.; Gargiulo, N.; Caputo, D. Molecular interactions of CO2 with the CuBTC metal organic framework: An FTIR study based on two-dimensional correlation spectroscopy. Mol. Struct. 2018, 1166, 326‑333.
- Hamon, L.; Jolimaître, E.; Pirngruber, G.D. CO2 and CH4 Separation by Adsorption Using Cu-BTC Metal-Organic Framework. Eng. Chem. Res. 2010, 49, 7497‑7503.
- Ye, S.; Jiang, X.; Ruan, L.-W.; Liu, B.; Wang, Y.-M.; Zhu, J.-F.; Qiu, L.-G. Post-combustion CO2 capture with the HKUST-1 and MIL-101(Cr) metal–organic frameworks: Adsorption, separation and regeneration investigations. Microporous Mesoporous Mater. 2013, 179, 191‑197.
- Kloutse, F.A.; Hourri, A.; Natarajan, S.; Benard, P.; Chahine, R. Systematic study of the excess and the absolute adsorption of N2/H2 and CO2/H2 mixtures on Cu-BTC. Adsorption 2019, 25, 941‑950.
- Da Silva, F.; Magalhães, G.; Jardim, E.; Silvestre-Albero, J.; Sepúlveda-Escribano, A.; De Azevedo, D.; De Lucena, S. CO2 adsorption on ionic liquid-modified Cu-BTC: Experimental and simulation study. Sci. Technol. 2015, 33, 223‑242.
- Mohamedali, M.; Henni, A.; Ibrahim, H. Markedly improved CO2 uptake using imidazolium-based ionic liquids confined into HKUST-1 frameworks. Microporous Mesoporous Mater. 2019, 284, 98‑110.
- Zhou, L.; Niu, Z.; Jin, X.; Tang, L.; Zhu, L. Effect of Lithium Doping on the Structures and CO2 Adsorption Properties of Metal‐Organic Frameworks HKUST‐1. ChemistrySelect 2018, 3, 12865‑12870.
- Prestipino, C.; Regli, L.; Vitillo, J.G.; Bonino, F.; Damin, A.; Lamberti, C.; Zecchina, A.; Solari, P.L.; Kongshaug, K.O.; Bordiga, S. Local Structure of Framework Cu(II) in HKUST-1 Metallorganic Framework: Spectroscopic Characterization upon Activation and Interaction with Adsorbates. Mater. 2006, 18, 1337‑1346.
- Low, J.J.; Benin, A.I.; Jakubczak, P.; Abrahamian, J.F.; Faheem, S.A.; Willis, R.R. Virtual High Throughput Screening Confirmed Experimentally: Porous Coordination Polymer Hydration. Am. Chem. Soc. 2009, 131, 15834‑15842.
- Al-Janabi, N.; Hill, P.; Torrente-Murciano, L.; Garforth, A.; Gorgojo, P.; Siperstein, F.; Fan, X. Mapping the Cu-BTC metal–organic framework (HKUST-1) stability envelope in the presence of water vapour for CO2 adsorption from flue gases. Eng. J. 2015, 281, 669‑677.
- Bao, Z.; Yu, L.; Ren, Q.; Lu, X.; Deng, S. Adsorption of CO2 and CH4 on a magnesium-based metal organic framework. Colloid Interface Sci. 2011, 353, 549‑556.
- Grajciar, L.; Nachtigall, P.; Bludsky, O.; Rubes, M. Accurate Ab Initio Description of Adsorption on Coordinatively Unsaturated Cu2+ and Fe3+ Sites in MOFs. Chem. Theor. Comput. 2015, 11, 230‑238.
- Mason, J.A.; Sumida, K.; Herm, Z.R.; Krishna, R.; Long, J.R. Evaluating metal–organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption. Energy Environ. Sci. 2011, 4, 3030‑3040.
- Herm, Z.R.; Krishna, R.; Long, J.R. CO2/CH4, CH4/H2 and CO2/CH4/H2 separations at high pressures using Mg2(dobdc). Microporous Mesoporous Mater. 2012, 151, 481‑487.
- Remy, T.; Peter, S.A.; Van Der Perre, S.; Valvekens, P.; De Vos, D.E.; Baron, G.V.; Denayer, J.F.M. Selective Dynamic CO2 Separations on Mg-MOF-74 at Low Pressures: A Detailed Comparison with 13X. Phys. Chem. C 2013, 117, 9301‑9310.
- Qasem, N.A.A.; Ben-Mansour, R. Adsorption breakthrough and cycling stability of carbon dioxide separation from CO2/N2/H2O mixture under ambient conditions using 13X and Mg-MOF-74. Energy 2018, 230, 1093‑1107.
- Mangano, E.; Kahr, J.; Wright, P.A.; Brandani, S. Accelerated degradation of MOFs under flue gas conditions. Faraday Discuss. 2016, 192, 181‑195.
- Moon, H.-S.; Moon, J.-H.; Chun, D.H.; Park, Y.C.; Yun, Y.N.; Sohail, M.; Baek, K.; Kim, H. Synthesis of [Mg2(DOBDC)(DMF)2]@polystyrene composite and its carbon dioxide adsorption. Microporous Mesoporous Mater. 2016, 232, 161‑166.
- Cao, Y.; Song, F.; Zhao, Y.; Zhong, Q. Capture of carbon dioxide from flue gas on TEPA-grafted metal-organic framework Mg2(dobdc). Environ. Sci. 2013, 25, 2081‑2087.
- Su, X.; Bromberg, L.; Martis, V.; Simeon, F.; Huq, A.; Hatton, T.A. Postsynthetic Functionalization of Mg-MOF-74 with Tetraethylenepentamine: Structural Characterization and Enhanced CO2 ACS Appl. Mater. Interfaces 2017, 9, 11299‑11306.
- Bernini, M.C.; García Blanco, A.A.; Villarroel-Rocha, J.; Fairen-Jimenez, D.; Sapag, K.; Ramirez-Pastor, A.J.; Narda, G.E. Tuning the target composition of amine-grafted CPO-27-Mg for capture of CO2 under post-combustion and air filtering conditions: a combined experimental and computational study. Dalton Trans. 2015, 44, 18970‑18982.
- Pérez-Pellitero, J.; Amrouche, H.; Siperstein, F.R.; Pirngruber, G.; Nieto-Draghi, C.; Chaplais, G.; Simon-Masseron, A.; Bazer-Bachi, D.; Peralta, D.; Bats, N. Adsorption of CO2, CH4, and N2 on zeolitic imidazolate frameworks: Experiments and simulations. Eur. J. 2010, 16, 1560‑1571.
- McEwen, J.; Hayman, J.-D.; Ozgur Yazaydin, A. A comparative study of CO2, CH4 and N2 adsorption in ZIF-8, Zeolite-13X and BPL activated carbon. Phys. 2013, 412, 72‑76.
- Danaci, D.; Singh, R.; Xiao, P.; Webley, P.A. Assessment of ZIF materials for CO2 capture from high pressure natural gas streams. Eng. J. 2015, 280, 486‑493.
- Zhang, Z.; Xian, S.; Xi, H.; Wang, H.; Li, Z. Improvement of CO2 adsorption on ZIF-8 crystals modified by enhancing basicity of surface. Eng. Sci. 2011, 66, 4878‑4888.
- Zhang, Z.; Xian, S.; Xia, Q.; Wang, H.; Li, Z.; Li, J. Enhancement of CO2 Adsorption and CO2/N2 Selectivity on ZIF‐8 via Postsynthetic Modification. AIChE J. 2013, 59, 2195‑2206.
- Martínez, F.; Sanz, R.; Orcajo, G.; Briones, D.; Yángüez, V. Amino-impregnated MOF materials for CO2 capture at post-combustion conditions. Eng. Sci. 2016, 142, 55‑61.
- Sayari, A.; Belmabkhout, Y. Stabilization of Amine-Containing CO2 Adsorbents: Dramatic Effect of Water Vapor. Am. Chem. Soc. 2010, 132, 6312‑6314.
- Cho, K.Y.; An, H.; Do, X.H.; Choi, K.; Yoon, H.G.; Jeong, H.-K.; Lee, J.S.; Baek, K.-Y. Synthesis of amine-functionalized ZIF-8 with 3-amino-1,2,4-triazole by postsynthetic modification for efficient CO2-selective adsorbents and beyond. Mater. Chem. A 2018, 6, 18912‑18919.
- Tsai, C.-W.; Niemantsverdriet, J.W.; Langner, E.H.G. Enhanced CO2 adsorption in nano-ZIF-8 modified by solvent assisted ligand exchange. Microporous Mesoporous Mater., 2018, 262, 98‑105.
- Gadipelli, S.; Travis, W.; Zhou, W.; Guo, Z. A thermally derived and optimized structure from ZIF-8 with giant enhancement in CO2 Energy Environ. Sci. 2014, 7, 2232‑2238.
- Ghahramaninezhad, M.; Soleimani, B.; Niknam Shahrak, M. A simple and novel protocol for Li-trapping with a POM/MOF nano-composite as a new adsorbent for CO2 New J. Chem. 2018, 42, 4639‑4645.
- Mohamedali, M.; Ibrahim, H.; Henni, A. Incorporation of acetate-based ionic liquids into a zeolitic imidazolate framework (ZIF-8) as efficient sorbents for carbon dioxide capture. Eng. J. 2018, 334, 817‑828.
- Zeeshan, M.; Keskin, S.; Uzun, A. Enhancing CO2/CH4 and CO2/N2 separation performances of ZIF-8 by postsynthesis modification with [BMIM][SCN]. Polyhedron 2018, 155, 485-492.
- Ferreira, T.J.; Ribeiro, R.P.P.L.; Mota, J.P.B.; Rebelo, L.P.N.; Esperança, J.M.S.S.; Esteves, I.A.A.C. Ionic liquid-impregnated metal-organic frameworks for CO2/CH4 ACS Appl. Nano Mater. 2019, 2, 7933‑7950.
- Idris, I.; Abdullah, A.; Shamsudin, I.K.; Othman, M.R. Comparative analyses of carbon dioxide capture from power plant flue gas surrogate by micro and mesoporous adsorbents. Environ. Chem. Eng. 2019, 7, 103115.
- Wongsakulphasatch, S.; Kiatkittipong, W.; Saupsor, J.; Chaiwiseshphol, J.; Piroonlerkgul, P.; Parasuk, V.; Assabumrungrat, S. Effect of Fe open metal site in metal-organic frameworks on post-combustion CO2 capture performance. Greenhouse Gases Sci. Technol. 2017, 7, 383‑394.
- Chen, Y.; Qiao, Z.; Huang, J.; Wu, H.; Xiao, J.; Xia, Q.; Xi, H.; Hu, J.; Zhou, J.; Li, Z. Unusual moisture-enhanced CO2 capture within microporous PCN-250 frameworks. ACS Appl. Mater. Interfaces 2020, 10, 38638‑38647.
- Yang, Q.; Wiersum, A.D.; Jobic, H.; Guillerm, V.; Serre, C.; Llewellyn, P.L.; Maurin, G. Understanding the thermodynamic and kinetic behavior of the CO2/CH4 gas mixture within the porous zirconium terephthalate UiO-66(Zr): A joint experimental and modeling approach. Phys. Chem. C 2011, 115, 13768‑13774.
- Cavka, J.H.; Grande, C.A.; Mondino, G.; Blom, R. High pressure adsorption of CO2 and CH4 on Zr-MOFs. Eng. Chem. Res. 2014, 53, 15500‑15507.
- Andersen, A.; Divekar, S.; Dasgupta, S.; Cavka, J.H.; Aarti, A.; Nanoti, A.; Spjelkavik, A.; Goswami, A.N.; Garg, M.O.; Blom, R. On the development of Vacuum Swing adsorption (VSA) technology for post-combustion CO2 Energy Procedia 2013, 37, 33‑39.
- Kim, S.-N.; Lee, Y.-R.; Hong, S.-H.; Jang, M.-S.; Ahn, W.-S. Pilot-scale synthesis of a zirconium-benzenedicarboxylate UiO-66 for CO2 adsorption and catalysis. Today 2015, 245, 54‑60.
- Hu, Z.; Sun, Y.; Zeng, K.; Zhao, D. Structural-failure resistance of metal-organic frameworks toward multiple-cycle CO2 Chem. Commun. 2017, 53, 8653‑8656.
- Edubilli, S.; Gumma, S. A systematic evaluation of UiO-66 metal organic framework for CO2/N2 Sep. Purif. Technol. 2019, 224, 85‑94.
- Abdullah, A.; Idris, I.; Shamsudin, I.K.; Othman, M.R. Methane enrichment from high carbon dioxide content natural gas by pressure swing adsorption. Nat. Gas Sci. Eng. 2019, 69, 102929.
- Hon Lau, C.; Babarao, R.; Hill, M.R. A route to drastic increase of CO2 uptake in Zr metal organic framework UiO-66. Commun. 2013, 49, 3634‑3636.
- Hong, D.H.; Suh, M.P. Enhancing CO2 separation ability of a metal-organic framework by post-synthetic ligand exchange with flexible aliphatic carboxylates. Eur. J. 2014, 20, 426‑434.
- Li, L.J.; Liao, P.Q.; He, C.T.; Wei, Y.S.; Zhou, H.L.; Lin, J.M.; Li, X.Y.; Zhang, J.P. Grafting alkylamine in UiO-66 by charge-assisted coordination bonds for carbon dioxide capture from high-humidity flue gas. Mater. Chem. A 2015, 3, 21849‑21855.
- Xian, S.; Wu, Y.; Wu, J.; Wang, X.; Xiao, J. Enhanced dynamic CO2 adsorption capacity and CO2/CH4 selectivity on polyethylenimine-impregnated UiO-66. Eng. Chem. Res. 2015, 54, 11151‑11158.
- Mutyala, S.; Yu, Y.-D.; Jin, W.-G.; Wang, Z.-S.; Zheng, D.-Y.; Ye, C.-R.; Luo, B. CO2 capture using amine incorporated UiO-66 in atmospheric pressure. Porous Mater. 2019, 26, 1831‑1838.
- Niu, Z.; Guan, Q.; Shi, Y.; Chen, Y.; Chen, Q.; Kong, Z.; Ning, P.; Tian, S.; Miao, R. A lithium-modified zirconium-based metal organic framework (UiO-66) for efficient CO2 New J. Chem. 2018, 42, 19764‑19770.
- MOF Manufacturing. Available online: https://www.moftechnologies.com/manufacturing/ (accessed on 5th March 2020).
- Chanut, N.; Wiersum, A.D.; Lee, U-H.; Hwang, Y.K.; Ragon, F.; Chevreau, H.; Bourrelly, S.; Kuchta, B.; Chang, J.-S.; Serre, C.; Llewellyn, P.L. Observing the effects of shaping on gas adsorption in metal-organic frameworks. J. Inorg. Chem. 2016, 2016, 4416‑4423.
- Zheng, J.; Cui, X.; Yang, Q.; Ren, Q.; Yang, Y.; Xing, H. Shaping of ultrahigh-loading MOF pellet with a strongly anti-tearing binder for gas separation and storage. Eng. J. 2018, 354, 1075‑1082.
- Mallick, A.; Mouchaham, G.; Bhatt, P.M.; Liang, W.; Belmabkhout, Y.; Adil, K.; Jamal, A.; Eddaoudi, M. Advances in Shaping of Metal-Organic Frameworks for CO2 Capture: Understanding the Effect of Rubbery and Glassy Polymeric Binders. Eng. Chem. Res. 2018, 57, 16897‑16902.
- Ribeiro, R.P.P.L.; Antunes, C.L.; Garate, A.U.; Portela, A.F.; Plaza, M.G.; Mota, J.P.B.; Esteves, I.A.A.C. Binderless shaped metal-organic framework particles: Impact on carbon dioxide adsorption. Microporous Mesoporous Mater. 2019, 275, 111‑121.
- Valizadeh, B.; Nguyen, T.N.; Stylianou, K.C. Shape engineering of metal–organic frameworks. Polyhedron 2018, 145, 1‑15.
- Danaci, D.; Bui, M.; Mac Dowell, N.; Petit, C. Exploring the limits of adsorption-based CO2 capture using MOFs with PVSA – from molecular design to process economics. Syst. Des. Eng. 2020, 5, 212‑231.
- World Energy Outlook 2016 – Analysis – IEA. Available online: https://www.iea.org/reports/world-energy-outlook-2016 (accessed on 26th March 2020).
- Fioletov, V.E.; McLinden, C.A.; Krotkov, N.; Li, C.; Joiner, J.; Theys, N.; Carn, S.; Moran, M.D. A global catalogue of large SO2 sources and emissions derived from the Ozone Monitoring Instrument. Chem. Phys. 2016, 16, 11497‑11519.
- Britt, D.; Tranchemontagne, D.; Yaghi, O.M. Metal-organic frameworks with high capacity and selectivity for harmful gases. Natl. Acad. Sci. U.S.A. 2008, 105, 11623‑11627.
- Bobbitt, N.S.; Mendonca, M.L.; Howarth, A.J.; Islamoglu, T.; Hupp, J.T.; Farha, O.K.; Snurr, R.Q. Metal-organic frameworks for the removal of toxic industrial chemicals and chemical warfare agents. Soc. Rev. 2017, 46, 3357‑3385.
- Wang, H.; Lustig, W.P.; Li, J. Sensing and capture of toxic and hazardous gases and vapors by metal-organic frameworks. Soc. Rev. 2018, 47, 4729‑4756.
- Han, X.; Yang, S.; Schröder, M. Porous metal-organic frameworks as emerging sorbents for clean air. Rev. Chem. 2019, 3, 108‑118.
- Glomb, S.; Woschko, D.; Makhloufi, G.; Janiak, C. Metal-organic frameworks with internal urea-functionalized dicarboxylate linkers for SO2 and NH3 ACS Appl. Mater. Interfaces 2017, 9, 37419‑37434.
- Dathe, H.; Peringer, E.; Roberts, V.; Jentys, A.; Lercher, J.A. Metal organic frameworks based on Cu2+ and benzene-1,3,5-tricarboxylate as host for SO2 trapping agents. Comptes Rendus Chim. 2005, 8, 753‑763.
- Brandt, P.; Nuhnen, A.; Lange, M.; Möllmer, J.; Weingart, O.; Janiak, C. Metal−organic frameworks with potential application for SO2 separation and flue gas desulfurization. ACS Appl. Mater. Interfaces 2019, 11, 17350‑17358.
- Ebrahim, A.M.; Levasseur, B.; Bandosz T.J., Interactions of NO2 with Zr-based MOF: Effects of the size of organic linkers on NO2 adsorption at ambient conditions. Langmuir 2013, 29, 168‑174.
- Tancrede, M.; Wilson, R.; Zeise, L.; Crouch, E.A.C. The carcinogenic risk of some organic vapors indoors: A theoretical survey. Environ. 1987, 19, 2187‑2205.
- Molhave, L. Indoor climate, air pollution, and human comfort. Expo. Sci. Environ. Epidemiol. 1991, 1, 63‑81.
- Zheng, C.; Shen, J.; Zhang, Y.; Huang, W.; Zhu, X.; Wu, X.; Chen, L.; Gao, X.; Cen, K. Quantitative assessment of industrial VOC emissions in China: Historical trend, spatial distribution, uncertainties, and projection. Environ. 2017, 150, 116‑125.
- Yang, K.; Xue, F.; Sun, Q.; Yue, R.; Lin, D. Adsorption of volatile organic compounds by metal-organic frameworks MOF-177. Environ. Chem. Eng. 2013, 1, 713‑718.
- Saha, D.; Deng, S. Structural Stability of Metal Organic Framework MOF-177. Phys. Chem. Lett. 2010, 1, 73‑78.
- Vellingiri, K.; Szulejko, J.E.; Kumar, P.; Kwon, E.E.; Kim, K-H.; Deep, A.; Boukhvalov, D.W.; Brown, R.J.C. Metal organic frameworks as sorption media for volatile and semi-volatile organic compounds at ambient conditions. Rep. 2016, 6, 27813.
- Zhou, L.; Zhang, X.; Chen, Y. Facile synthesis of Al-fumarate metal–organic framework nano-flakes and their highly selective adsorption of volatile organic compounds. Lett. 2017, 197, 224‑227.
- Zhang, X.; Yang, Y.; Song, L.; Chen, J.; Yang, Y.; Wang Y. Enhanced adsorption performance of gaseous toluene on defective UiO-66 metal organic framework: Equilibrium and kinetic studies. Hazard. Mater. 2019, 365, 597‑605.
- Uzoigwe, C.E.; Sanchez Franco, L.C.; Forrest, M.D. Iatrogenic greenhouse gases: the role of anaesthetic agents. J. Hosp. Med. 2016, 77, 19‑23.
- Ishizawa, Y. General anesthetic gases and the global environment. Analg. 2011, 112 213‑217.
- Anesthetic Gases: Guidelines for Workplace Exposures | Occupational Safety and Health Administration. Available online: https://www.osha.gov/dts/osta/anestheticgases/ (accessed on 1st April 2020).
- Gargiulo, N.; Peluso, A.; Aprea, P.; Eić, M.; Caputo, D. An insight into clustering of halogenated anesthetics molecules in metal-organic frameworks: Evidence of adsorbate self-association in micropores. Colloid Interface Sci. 2019, 554, 463‑467.
- Gargiulo, N.; Peluso, A.; Aprea, P.; Hua, Y.; Filipović, D.; Caputo, D.; Eić, M. A chromium-based metal organic framework as a potential high performance adsorbent for anaesthetic vapours. RSC Adv. 2014, 4, 49478‑49484.
- Hua, Y.; Gargiulo, N.; Peluso, A.; Aprea, P.; Eić, M.; Caputo, D. Adsorption Behavior of Halogenated Anesthetic and Water Vapor on Cr‐Based MOF (MIL‐101) Adsorbent. Part I. Equilibrium and Breakthrough Characterizations. Ing. Tech. 2016, 88, 1730‑1738.
- Hua, Y.; Gargiulo, N.; Peluso, A.; Aprea, P.; Eić, M.; Caputo, D. Adsorption Behavior of Halogenated Anesthetic and Water Vapor on Cr‐Based MOF (MIL‐101) Adsorbent. Part II. Multiple‐Cycle Breakthrough Tests. Ing. Tech. 2016, 88, 1739‑1745.