
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lisette Chavez-Rodríguez | + 1453 word(s) | 1453 | 2021-11-16 07:47:26 | | | |
| 2 | Nora Tang | Meta information modification | 1453 | 2021-11-23 10:25:16 | | |
Video Upload Options
Hepatocellular carcinoma (HCC) accounts for 85% of primary liver cancer, the third most common cause of cancer-related deaths worldwide. Its incidence has been increasing in both men and women. In Western countries, high-calorie diets, mainly rich in carbohydrates such as fructose, represent a significant concern due to their repercussions on the population's health. A high fructose diet is related to the development of Metabolic Associated Fatty Liver Disease (MAFLD), formerly named Non-Alcoholic Fatty Liver Disease (NAFLD), and the progression of HCC since it potentiates the lipogenic pathway and the accumulation of lipids. However, fructose metabolism seems to be different between the stages of the disease, carrying out a metabolic reprogramming to favor the proliferation, inflammation, and metastatic properties of cancer cells in HCC. This review focuses on a better understanding of fructose metabolism in both scenarios: MAFLD and HCC.
1. Introduction
Primary liver cancer is among the most prevalent malignant diseases worldwide. It is the sixth most diagnosed cancer and the third leading cause of death worldwide in 2020 ( Figure 1 ). It is estimated 906,000 new cases and 83,000 deaths due to this malignancy, particularly hepatocellular carcinoma (HCC) [1].
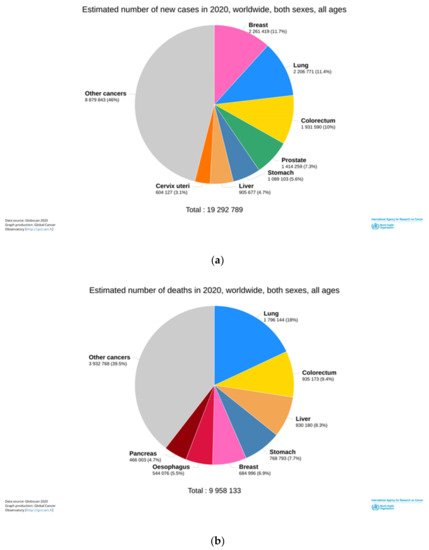
HCC accounts for 75–85% of primary liver cancer. Its incidence has been increasing in both men and women. The high mortality rate is due to inefficient HCC diagnosis in the early stages of the disease, non-optimized treatments, and high aggressivity in these kinds of tumors [2].
One of the most critical risk factors for the development of HCC is the Metabolic-Associated Fatty Liver Disease (MAFLD), formerly named Non-Alcoholic Fatty Liver Disease (NAFLD) [3]. MAFLD represents the hepatic manifestation of a multisystem disorder, and the main difference between MAFLD and NAFLD is the presence of metabolic dysfunction, necessary for MAFLD diagnosis. It does not require excluding patients with alcohol consumption or another chronic liver disease [4]. MAFLD definition is considered more practical for identifying patients with fatty liver disease with a high risk of disease progression. Currently, it has been classified as the most common chronic liver disease in the last years, being one of the leading causes of liver transplantation [5].
The proposed criteria for a positive diagnosis of MAFLD is the evidence of hepatic steatosis in addition to one of the following three criteria: overweight/obesity, Type 2 Diabetes Mellitus (T2DM), or evidence of metabolic dysregulation [3]. The increment of these diseases is closely related to the diet. Indeed, the consumption of high-calorie foods, mainly lipids, and carbohydrates, has increased over the years.
2. Fructose Consumption
Due to the current lifestyle, eating habits have changed, increasing the consumption of fat and sweets related to the increasing incidence of MAFLD, positioning it as the most common chronic liver disease in the last 30 years. The consumption of fructose also has been highly related to the obesity pandemic, T2DM, and risk of cardiometabolic diseases. Mice fed with high fat and fructose diets present insulin resistance, obesity, hypertriglyceridemia, liver steatosis, fibrosis, and HCC development [6][7].
On the initial progression of the disease, the Ketohexokinase C (KHK-C) is more relevant; thus, fructose metabolism is exacerbated in enterocytes and hepatocytes. In the small intestine, fructose-enriched diets induce the rupture of tight junctions between enterocytes allowing the translocation of LPS to the portal blood. Endotoxemia promotes inflammation and lipogenesis in the liver [8].
However, it seems that fructose has a dual role in MAFLD and cancer metabolism (Figure 2 and 3). Some studies have reported that in cancer cells, fructose metabolism is decreased compared to normal cells. Furthermore, some enzymes related to fructose catabolism have lower activity in HCC compared to normal liver tissue [9][10][11]. As a result of this metabolism, reprogramming that favors the Warburg effect has been reported [12]. The canonical Warburg effect is based on reducing mitochondrial oxidation of Pyruvate and the enhancement of glycolysis to obtain higher levels of Lactate and ATP. Lactate is involved in microenvironment reprogramming, angiogenesis, and invasion [13]. However, because the fructose metabolism generates higher ROS levels, it has been proposed that cancer cells under high fructose levels change their metabolism to a reverse Warburg effect [14]. Due to the fructose metabolism, metabolic rewiring enhances proliferation, invasion and metastasis in cancer cells. There is evidence that a change to a lower fructose metabolism favors the invasion and metastatic progression in some types of cancer as breast cancer [15].
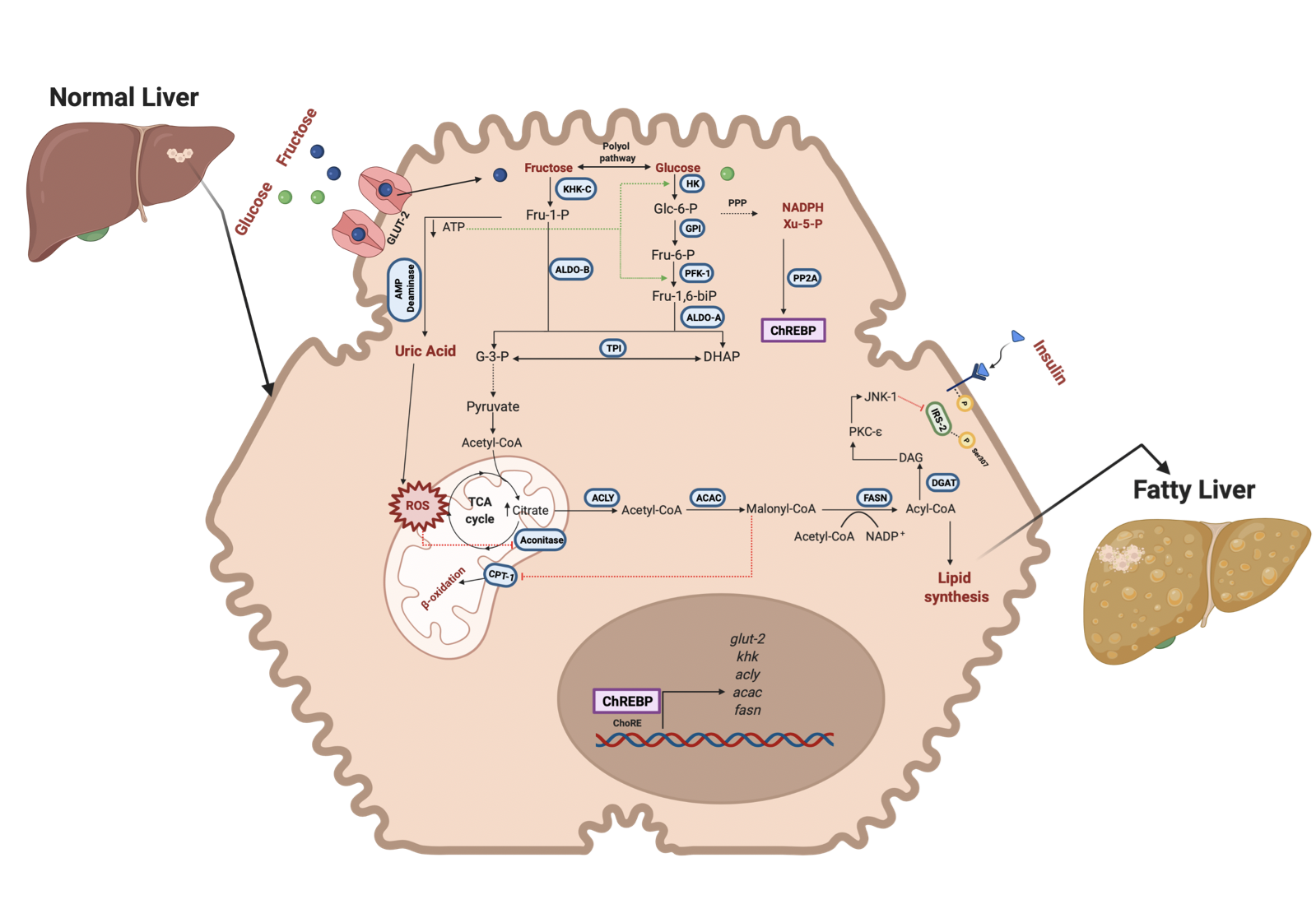
Figure 2. Fructose and glucose metabolism in hepatocytes and steatosis. Fructose and glucose enter the hepatocyte through GLUT-2; the metabolism is different for each molecule. KHK-C first phosphorylates fructose in position 1 to be further cleaved by ALDO-B into intermediary metabolites (DHAP and G-3-P) that render the carbon skeleton to de novo lipid synthesis and PPP. Xy-5-P is an intermediary of PPP that activates ChREBP translocation to the nucleus. ChREBP upreg- ulates the expression of fructose and lipogenesis-related genes. On the other hand, the rapid phosphorylation of fructose by KHK-C induces a decrement of ATP levels and favors the purine deamination pathway to obtain uric acid. Uric acid enhances ROS production in the mitochondrion and affects Aconitase activity, incrementing citrate levels incorporated into the de novo synthesis pathway. Also, higher amounts of Citrate can lead to insulin resistance in the liver through the inhibition of IRS-2. In glucose, fructose metabolism improves the glycolytic pathway by decreasing ATP levels which generates positive pathway feedback. Also, glucose can be converted into fructose through the polyol pathway to exacerbate the fructose effects in the liver. Green lines indicate activation of the enzymes. GLUT-2, Glucose transporter-2; KHK, Ketohexokinase; ALDO-B, Aldolase B; ALDO-A, Aldolase A; TPI, Triosephosphate isomerase; PFK1, Phosphofructokinase; GPI, Glucose 6 phosphate isomerase; HK, Hexokinase; ACLY, ATP citrate lyase; ACAC, Acetyl-CoA carboxylase; FASN, Fatty acid synthase; PP2A, protein phosphatase 2A; ChREBP, carbohydrate response element-binding protein; ChoRE, carbohydrate response elements; IRS-2, insulin receptor substrate 2; DAG, Diacylglycerol; PKC-e, Protein kinase C isoform e; JNK-1, Jun N-terminal kinase 1. Created with BioRender.com
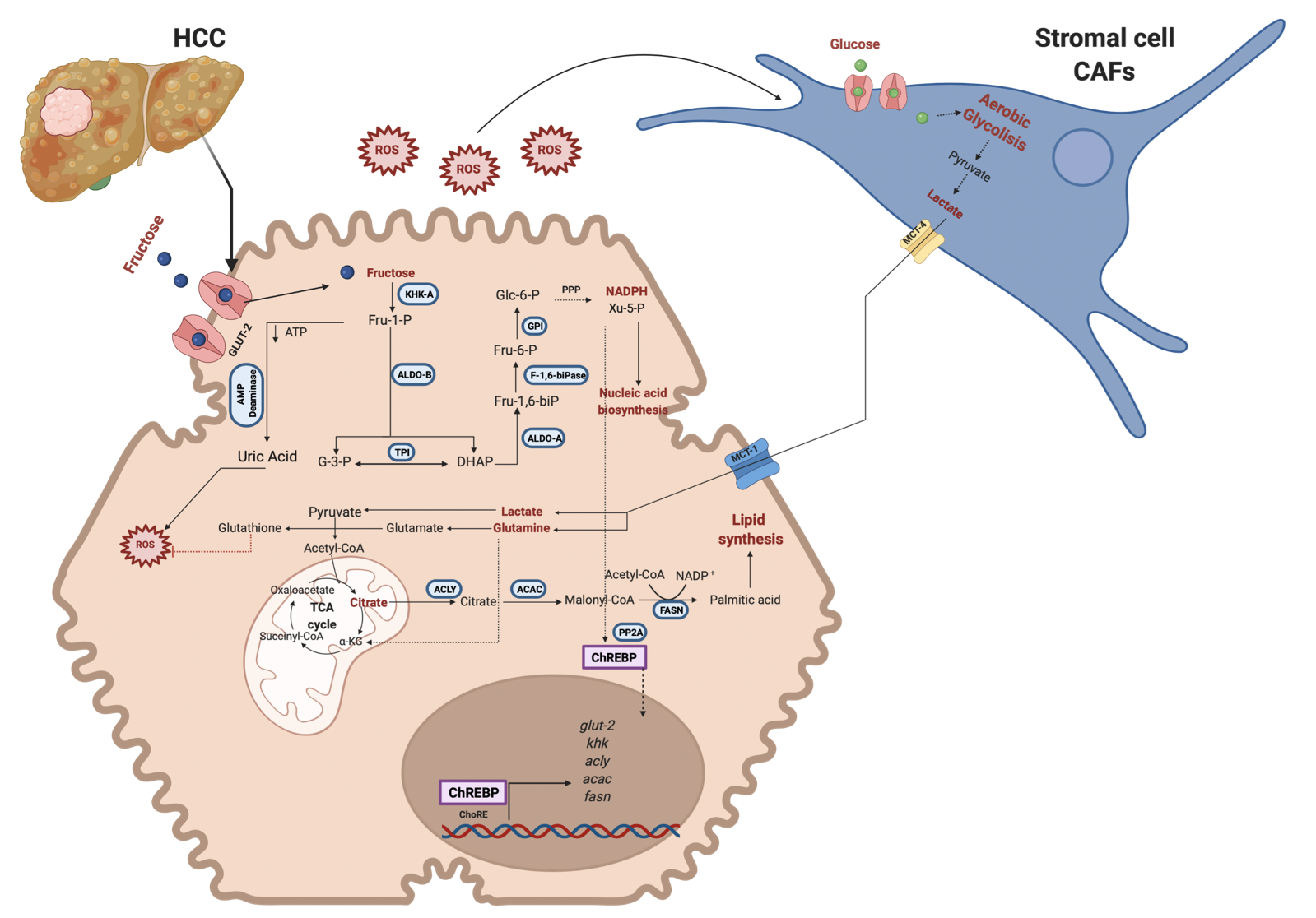
Figure 3. Fructose metabolism reprogramming in cancer cells. Fructose metabolism changes into lower levels of expression and activity of the enzymes implicated in the pathway. First, there is a change in the KHK isoform (KHK-A) to favor the enzyme's lower fructose metabolism and kinase activity. Also, ALDO-B decreases its activity. The intermediary metabolites are diverted into the PPP to obtain reduction power and Xy-5-P, enhancing ChREBP translocation into the nucleus and nucleic acid synthesis. Due to the lower KHK activity, ROS levels diminish compared to the earlier scenario but can activate CAFs. On the other hand, the reverse Warburg effect is representative of this metabolism, and CAFs render metabolic intermediaries to a cancer cell to enhance de novo lipid synthesis and reduction power. Metabolic reprogramming is implicated in improving the survival, proliferation, and metastasis of cancer cells. Red lines indicate inhibition. GLUT-2, Glucose transporter-2; KHK, Ketohexokinase; ALDO-B, Aldolase B; ALDO-A, Aldolase A; TPI, Triosephosphate isomerase; PFK1, Phosphofructokinase; GPI, Glucose 6 phosphate isomerase; HK, Hexokinase; ACLY, ATP citrate lyase; ACAC, Acetyl-CoA carboxylase; FASN, Fatty acid synthase; PP2A, protein phosphatase 2A; ChREBP, carbohydrate response element-binding protein; ChoRE, carbohydrate response elements. Created with BioRender.com
At this point, it is essential to note that fructose metabolism in transformed cells changes from a higher to lower metabolism. Cancer cells need to counteract the elevated ROS levels generated from an exacerbated activity in the cell. That is why it could be observed a metabolic switch in the cell.
3. Conclusions
The present review relates the consumption of a high fructose diet with MAFLD through the lipid accumulation in the liver and its relationship with obesity, T2DM, and metabolic dysfunctions in patients and the hallmarks of cancer. On the other hand, it is essential to note that fructose metabolism exhibits differences related to the state of development of the disease. In the initial steps, there is a high fructose-related enzymes activity that promotes hepatic steatosis. Still, once the cancer is established, a fructose metabolic reprogramming is carried out in transformed cells to favor proliferation, inflammation, and metastatic properties of the cancer cells. In this scenario, the inhibition of KHK seems to be a promising strategy in the initial steps of the disease. Still, once HCC is established, more studies need to be done to determine if the treatment has consistent therapeutic effects. Furthermore, the activities of some enzymes serve as surrogate markers for poor prognoses, such as FASN upregulation, KHK-A isoform change, and ALDO-B downregulation. A better understanding of the role of fructose metabolism in both scenarios is necessary to diagnose and treat patients and find new strategies for possible druggable metabolic points in fructose metabolism.
References
- Hyuna Sung; Jacques Ferlay; Rebecca L. Siegel; Mathieu Laversanne; Isabelle Soerjomataram; Ahmedin Jemal; Freddie Bray; Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians 2021, 71, 209-249, 10.3322/caac.21660.
- Freddie Bray; Jacques Ferlay Me; Isabelle Soerjomataram; Rebecca L. Siegel; Lindsey A. Torre; Ahmedin Jemal; Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018, 68, 394-424, 10.3322/caac.21492.
- Mohammed Eslam; Philip N. Newsome; Shiv K. Sarin; Quentin M. Anstee; Giovanni Targher; Manuel Romero-Gomez; Shira Zelber-Sagi; Vincent Wai-Sun Wong; Jean-François Dufour; Jörn M. Schattenberg; et al.Takumi KawaguchiMarco ArreseLuca ValentiGamal ShihaClaudio TiribelliHannele Yki-JärvinenJian-Gao FanHenning GrønbækYusuf YilmazHelena Cortez-PintoClaudia P. OliveiraPierre BedossaLeon A. AdamsMing-Hua ZhengYasser FouadWah-Kheong ChanNahum Mendez-SanchezSang Hoon AhnLaurent CasteraElisabetta BugianesiVlad RatziuJacob George A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. Journal of Hepatology 2020, 73, 202-209, 10.1016/j.jhep.2020.03.039.
- Su Lin; Jiaofeng Huang; Mingfang Wang; Rahul Kumar; YuXiu Liu; Shiying Liu; Yinlian Wu; Xiaozhong Wang; Yueyong Zhu; Comparison of MAFLD and NAFLD diagnostic criteria in real world. Liver International 2020, 40, 2082-2089, 10.1111/liv.14548.
- Ivana Mikolasevic; Tajana Filipec-Kanizaj; Maja Mijic; Ivan Jakopcic; Sandra Milic; Irena Hrstic; Nikola Sobocan; Davor Stimac; Patrizia Burra; Nonalcoholic fatty liver disease and liver transplantation - Where do we stand?. World Journal of Gastroenterology 2018, 24, 1491-1506, 10.3748/wjg.v24.i14.1491.
- Joanna K. Dowman; Laurence J. Hopkins; Gary M. Reynolds; Nikolaos Nikolaou; Matthew J. Armstrong; Jean C. Shaw; Diarmaid D. Houlihan; Patricia F. Lalor; Jeremy W. Tomlinson; Stefan G. Hübscher; et al.Philip N. Newsome Development of Hepatocellular Carcinoma in a Murine Model of Nonalcoholic Steatohepatitis Induced by Use of a High-Fat/Fructose Diet and Sedentary Lifestyle. The American Journal of Pathology 2014, 184, 1550-1561, 10.1016/j.ajpath.2014.01.034.
- Amon Asgharpour; Sophie C. Cazanave; Tommy Pacana; Mulugeta Seneshaw; Robert Vincent; Bubu A. Banini; Divya P. Kumar; Kalyani Daita; Hae-Ki Min; Faridoddin Mirshahi; et al.Pierre BedossaXiaochen SunYujin HoshidaSrinivas V. KoduruDaniel ContaiferUrszula Osinska WarnckeDayanjan S. WijesingheArun J. Sanyal A diet-induced animal model of non-alcoholic fatty liver disease and hepatocellular cancer. Journal of Hepatology 2016, 65, 579-588, 10.1016/j.jhep.2016.05.005.
- Jelena Todoric; Giuseppe Di Caro; Saskia Reibe; Darren C. Henstridge; Courtney R. Green; Alison Vrbanac; Fatih Ceteci; Claire Conche; Reginald McNulty; Shabnam Shalapour; et al.Koji TaniguchiPeter J. MeikleJeramie D. WatrousRafael MoranchelMahan NajhawanMohit JainXiao LiuTatiana KisselevaMaria T. Diaz-MecoJorge MoscatRob KnightFlorian R. GretenLester F. LauChristian M. MetalloMark A. FebbraioMichael Karin Fructose stimulated de novo lipogenesis is promoted by inflammation. Nature Metabolism 2020, 2, 1034-1045, 10.1038/s42255-020-0261-2.
- Xinjian Li; Xu Qian; Li-Xia Peng; Yuhui Jiang; David H. Hawke; Yanhua Zheng; Yan Xia; Jong-Ho Lee; Gilbert Cote; Hongxia Wang; et al.Liwei WangChao-Nan QianZhimin Lu A splicing switch from ketohexokinase-C to ketohexokinase-A drives hepatocellular carcinoma formation. Nature 2016, 18, 561-571, 10.1038/ncb3338.
- Qi-Fei Tao; Sheng-Xian Yuan; Fu Yang; Sen Yang; Yuan Yang; Ji-Hang Yuan; Zhen-Guang Wang; Qing-Guo Xu; Kong-Ying Lin; Jie Cai; et al.Jian YuWei-Long HuangXiao-Lei TengChuan-Chuan ZhouFang WangShu-Han SunWei-Ping Zhou Aldolase B inhibits metastasis through Ten–Eleven Translocation 1 and serves as a prognostic biomarker in hepatocellular carcinoma. Molecular Cancer 2015, 14, 1-13, 10.1186/s12943-015-0437-7.
- Xuxiao He; Min Li; Hongming Yu; Guijun Liu; Ningning Wang; Chunzhao Yin; Qiaochu Tu; Goutham Narla; Yongzhen Tao; Shuqun Cheng; et al.Huiyong Yin Loss of hepatic aldolase B activates Akt and promotes hepatocellular carcinogenesis by destabilizing the Aldob/Akt/PP2A protein complex. PLOS Biology 2020, 18, e3000803, 10.1371/journal.pbio.3000803.
- Takahiko Nakagawa; Miguel A. Lanaspa; Inigo San Millan; Mehdi Fini; Christopher J. Rivard; Laura G. Sanchez-Lozada; Ana Andres-Hernando; Dean R. Tolan; Richard J. Johnson; Fructose contributes to the Warburg effect for cancer growth. Cancer & Metabolism 2020, 8, 1-12, 10.1186/s40170-020-00222-9.
- Yaojie Fu; Shanshan Liu; Shanghelin Yin; Weihong Niu; Wei Xiong; Ming Tan; GuiYuan Li; Ming Zhou; The reverse Warburg effect is likely to be an Achilles' heel of cancer that can be exploited for cancer therapy. Oncotarget 2017, 8, 57813-57825, 10.18632/oncotarget.18175.
- Brittany Dewdney; Alexandra Roberts; Liang Qiao; Jacob George; Lionel Hebbard; A Sweet Connection? Fructose’s Role in Hepatocellular Carcinoma. Biomolecules 2020, 10, 496, 10.3390/biom10040496.
- Jiyoung Kim; Jengmin Kang; Ye-Lim Kang; Jongmin Woo; Youngsoo Kim; June Huh; Jong-Wan Park; Ketohexokinase-A acts as a nuclear protein kinase that mediates fructose-induced metastasis in breast cancer. Nature Communications 2020, 11, 1-20, 10.1038/s41467-020-19263-1.
- Jiyoung Kim; Jengmin Kang; Ye-Lim Kang; Jongmin Woo; Youngsoo Kim; June Huh; Jong-Wan Park; Ketohexokinase-A acts as a nuclear protein kinase that mediates fructose-induced metastasis in breast cancer. Nature Communications 2020, 11, 1-20, 10.1038/s41467-020-19263-1.




