The industrial use of enzymes generally necessitates their immobilization onto solid supports. The well-known high affinity of enzymes for metal-organic framework (MOF) materials, together with the great versatility of MOFs in terms of structure, composition, functionalization and synthetic approaches, has led the scientific community to develop very different strategies for the immobilization of enzymes in/on MOFs. This review focuses on one of these strategies, namely, the one-pot enzyme immobilization within sustainable MOFs, which is particularly enticing as the resultant biocomposite Enzyme@MOFs have the potential to be: (i) prepared in situ, that is, in just one step; (ii) may be synthesized under sustainable conditions: with water as the sole solvent at room temperature with moderate pHs, etc.; (iii) are able to retain high enzyme loading; (iv) have negligible
protein leaching; and (v) give enzymatic activities approaching that given by the corresponding free enzymes. Moreover, this methodology seems to be near-universal, as success has been achieved with different MOFs, with different enzymes and for different applications. So far, the metal ions forming the MOF materials have been chosen according to their low price, low toxicity and, of course, their possibility for generating MOFs at room temperature in water, in order to close the cycle of economic, environmental and energy sustainability in the synthesis, application and disposal life cycle.
1. Scope of this Review
In the last decade, a huge number of metal-organic framework (MOF) materials, enzymes and strategies have been reported as suitable for generating enzyme@MOF biocomposites. This review covers just one of these approaches, specifically that where the solid biocatalysts are formed by the synthesis of a MOF material, acting as a support, in the presence of the enzyme to be immobilized. In other words, this review addresses the strategies for forming enzyme@MOFs known as ‘one-pot’, one-step, in situ or de novo methods. In recognition of the diversity of this field, these terms will be used interchangeably throughout this review. Readers interested in a complete literature review on enzymes are encouraged to check out recent reviews with wider scopes
[1][2].
2. The Origins and Rising Dominance of the Enzyme-Supporting MOF
Enzyme immobilization is a topic with more than half a century of history
[3], with enzymes showing superb advantages that were previously unattainable in industry due to issues with solubility and lability. Apart from methods to achieve enzyme insolubilization, the immobilization on solid supports has been the most widely studied strategy. Much effort has been made since then and thousands of materials have been studied as supports for the immobilization of enzymes, either covalently
[4] or non-covalently
[5][6]. Porous supports offer an extra incentive for enzyme immobilization, as they may ideally trap enzyme molecules without modifying their structure or their active centers.
Table 1 compares some of the most relevant physicochemical properties and performance of some selected porous enzyme supports with different strategies.
Table 1. Comparison of properties and performance of some selected immobilization strategies and porous supports of enzymes: covalent immobilization on amorphous agarose; non-covalent immobilization by post-synthetic or in situ addition to siliceous mesoporous ordered materials (MMO), and in situ immobilization onto MOFs.
| - |
Covalent Post-Synthetic (Agarose) [4][7] |
Non-Covalent Post-Synthetic (MMO) [8] |
Non-Covalent In Situ (MMO) [9][10] |
Non-Covalent In Situ (MOFs) [11][12] |
| Surface area |
Low: ≈200 m2/g |
Moderate/high: ≈700 m2/g |
Moderate/high ≈700 m2/g |
Very high: >1000 m2/g |
| Pore width |
>20 nm |
≈7–10 nm |
≈4–12 nm |
Micropores < 2 nm |
| Pore connectivity |
Amorphous: low |
Excellent |
Excellent |
Excellent |
| Chemical affinity |
Essential |
Necessary |
Unnecessary |
Beneficial |
| Activity preserved |
Low/moderate |
High/moderate |
High/moderate |
High/moderate |
| Enzyme loading |
Moderate/high |
Moderate/high |
Moderate/high |
Moderate/high |
| Enzyme leaching |
None |
Low |
Very low |
Negligible |
| Enzyme stabilization |
High |
Moderate/high |
Moderate |
Moderate/high |
Simply anchoring an enzyme to a support is relatively easy and, in many cases, just enough to catalyze a reaction successfully, but optimizing the biocatalyst and understanding what happens to the immobilized enzyme may be difficult. Covalent immobilization involves the chemical modification of the enzyme, which often leads to decreased activity. However, the formation of several irreversible linkages introduces a noticeable rigidity to the protein molecule: unfolding of the enzyme is prevented and its stability rises
[4][13]. The supports for this kind of enzyme immobilization must display pore diameters wider by several times than the size of the protein dimensions in order to enable good diffusion of the protein along the pore to achieve acceptable enzyme loadings, as well as high surface area.
When pore shape and size are tunable, the possibilities of studying these systems increase significantly. This is the case with siliceous ordered mesoporous materials
[6][14]. These materials display uniform and regular pore systems with high interconnectivity, which facilitates not only a good enzyme diffusion to attain high enzyme loading, but also good substrate and product diffusion to decrease diffusional restrictions. Uniform pores only slightly wider than the enzyme permit high loadings of non-covalently immobilized enzyme, while a covalently-attached enzyme at the mouth of the pore would act as a plug, preventing the access of new ones, reducing the enzyme loading. Non-covalent enzyme immobilization does not require chemical modification of the protein, so the catalytic activity should not be damaged for this reason and may be better preserved. However, the favorable effect of enzyme diffusion may also lead to the unrestricted release of the enzyme, which is not possible with covalent immobilization. But when the surface of the support is coated with functional groups to provide chemical affinity with the enzyme, the situation radically changes: this affinity increases the enzyme load, and also retains the enzyme within the pore so the leaching of the reversibly linked enzyme is prevented
[8]. Therefore, supports with high surface area and uniform pores with a size matching the enzyme dimensions and bearing functional groups to promote attraction, give rise to biocatalysts with high enzyme loading, retained catalytic activity and absence of enzyme leaching. These are the characteristics desired in an immobilized enzyme system.
The use of Pluronics
[6] as a template for siliceous OMM formation allows for uniform pores with window/cage structures, where the large cavities or cages with wide dimensions can widely accommodate a molecule of enzyme, but the windows connecting the cages are often narrower than the enzyme dimensions. The result is a high difficulty (near impossibility) of the enzyme to diffuse through windows and a very low enzyme loading. The harsh synthetic conditions for these siliceous ordered mesoporous materials are not compatible with enzyme activity (i.e., temperatures over 100 °C and pH below 1). It was not until milder conditions to produce these OMMs were studied and developed that in situ synthesis of the biocatalysts could be performed
[9][10]. This is the fundamental idea behind the in-situ immobilization in MOFs: to build the support in the presence of the enzyme, so that a high amount of enzyme is entrapped inside the wide cages (or intercrystalline voids in aggregated nanocrystalline MOFs), and the entrapment is permanent given the narrowness of the surrounding pores, insufficient for enzyme diffusion outwards. Alternatively, in certain biomimetic strategies, the enzymes end up inside of the MOF crystals, which also avoids any leaching.
With the explosion of MOF research beginning in the late 1990s, a new horizon opened up in the field of enzyme immobilization, although this application had not started being studied until a decade later
[15]. Taking advantage of the structural versatility of MOFs and the previous success of enzyme immobilization onto mesoporous materials, the first attempts to prepare biocomposite enzyme@MOF materials which were designed could only encapsulate some of the smallest proteins within crystallographic channels and/or cavities of the most porous MOFs (Ma et al.
[15][16][17][18][19]). Thus, great efforts were made to attain MOFs containing relatively narrow mesopores to confine small proteins like cytochrome C (Cty C)
[17], horseradish peroxidase (HRP)
[20], or trypsin
[21][22]. Crucially, these highly porous MOFs could initially only be attained with the use of very long linkers, resulting in generally unstable systems. In this context, Yang et al. proposed the idea of subjecting the MOF to ozonolysis to generate mesopores wide enough for catalase immobilization
[23].
Alternatively, enzymes may become anchored onto the external surface of the MOF particles, taking advantage of the presumable chemical affinity between enzymes and MOFs in terms of the nature of functional groups, polarity, charge density distribution, etc,. However, in the absence of confinement, the adsorption of enzymes onto the external surface of MOFs is unable to prevent enzyme leaching. Therefore, some authors have proposed covalent linking, via crosslinking with glutaraldehyde
[24][25][26] or EDC/NHS
[27][28]. Also, the inclusion of new components into the composites has been often proposed, either to impart magnetic properties allowing their facile separation from reaction media
[27][29], or to protect the enzyme by incorporating macromolecules like polyvinyl alcohol hydrogels
[30] or by in situ formed self-assembled hybrid nanoflowers
[31].
Probably, the most promising alternative is the set of strategies known as in situ, or de novo methods. As commented above, these consist of the synthesis of the MOF materials in the presence of the enzyme with the aim to entrap enzyme during the MOF formation process, either within a given MOF crystal or within the intercrystalline spaces of the aggregates formed by the fusion of the MOF nanocrystals with each other. Thus, the microporous surroundings of the MOF would prevent protein being released while allowing the diffusion of non-macromolecular substrates and products through them. However, conditions of the media for MOF synthesis are usually far from being ‘enzymatically friendly’. Only when the MOF can be obtained in aqueous media under mild pH and temperature conditions can this approach be addressed
[32]. Zeolitic imidazole frameworks (ZIFs) formed by the metal ions Zn
2+ or Co
2+ can be synthesized quickly and under biocompatible conditions
[2], and therefore many reports have described one-pot immobilization of different enzymes, like cellulase
[33] or catalase
[34], among others, on ZIF-8. Apart from ZIF-8, not many MOFs can be prepared under such mild conditions, mainly due to the very low solubility of organic linkers in water. One-pot immobilization of enzymes in the MOF NH
2-MIL-53(Al) was patented
[35] and then reported for the first time by Gascón et al.
[36], based on the sustainable preparation of the carboxylate-based MOF by simple deprotonation of linkers by a base in water
[37]. After this pioneering work, other enzymes have been immobilized in this material
[11][38] or some other MOFs such as Fe-BTC
[12][39], or CaBDC
[40] which can also be prepared under mild conditions.
In order to preserve catalytic activity, macromolecules have been added in some of these one-pot systems: mixing polyvinylpyrrolidone (PVP) with Cyt C prior to the immobilization process in ZIF-8
[32] to form a double layer to protect its activity and stability. A lignin derivative (DDVA) has also been used to co-precipitate enzymes with Ca
2+ or Zn
2+ to yield enzyme@MOM composites
[41]. Additionally, Fe
3O
4 has successfully been added to provide particles with magnetic properties such as in the one-pot synthesis involving 2-methylimidazole and zinc acetate with lipase from
Candida rugosa in the MOF CRL/MNP@ZIF-8
[42].
As mentioned above, there is a high affinity between MOFs and enzymes. This can be increased, for example, by making the environment of the enzyme more or less hydrophobic or hydrophilic. Thus, Liang et al.
[43] described enhanced activity of catalase immobilized via one-pot synthesis in a hydrophilic environment when the linker of the MOF was 3-methyl-1,2,4-triazole (FCAT@MAF-7) compared to the hydrophobic FCAT-ZIF-8, where the enzyme undergoes inactivation. Lipase, being an enzyme which displays more activity in hydrophobic interfaces, was found to increase its activity in the hydrophobic environment created in the immobilization of lipase onto ZIF-L (AOL@PDMS-ZIF-L) and improves its stability in ZIF-8 (AOL@PDMS-ZIF-8) by the addition of PDMS (polydimethylsiloxane) to provide a hydrophobic environment .
Thus, it can be seen how throughout the history of enzyme immobilization, each new technique or methodology has learned and taken advantage of previous work up to the newest generations of MOF-based composites. Where previously enzyme immobilization within ordered mesoporous materials has required confined spaces, pore connectivity and chemical affinity, in situ immobilization within MOFs has provided solutions with the close retention of the enzyme in the intra- or intercrystalline spaces, and facile substrate diffusion through the porous network . However, the huge structural versatility of MOFs, with hundreds of new materials discovered every year, which also present significant affinity / compatibility with so many other materials (oxides, hydrocarbons, polymers and, of course, enzymes), greatly opens the range of possibilities not only to immobilize enzymes effectively but also to rationally design an optimal habitat for the enzyme that maximizes its activity, stability and recyclability, and minimizes its leaching and inactivation. The aim of this work is to gather the progress made in the in situ/one-pot synthesis of enzyme@MOF biocatalysts thanks to the advances in the knowledge of the synthesis system and the reaction medium. It seems reasonable to start with the development of synthesis methodologies of MOFs under “enzyme friendly” conditions.
3. Designing MOFs Synthesis Methodologies Compatible with One-Pot Enzyme Immobilization
It is often noted that MOF materials offer a huge versatility in terms of (i) their composition (only limited by the periodic table and the known organic chemistry), (ii) their structures (thousands of different topologies are already known) or (iii) their organic functionalization (incorporated either through pre- or post-synthesis), with the wide range of applications for which these materials have been either reported or postulated . Nevertheless, MOFs also possess other kind of versatility much less both explored and exploited: the variety in their synthesis procedures.
The stability of enzymes is relatively low, particularly their tertiary structure which gives them their biocatalytic performance. Even limited changes in temperature or acidity, the presence of alien chemical species in the media or, of course, the use of a non-aqueous solvent, could lead to the inactivation of the enzymes. Therefore, one-pot immobilization of enzymes implies that the support must be capable of being formed in the presence of enzymes under conditions that do not alter their structure/activity.
The challenge of preparing MOFs under such mild conditions is no small one. Fortunately, from the very beginning of MOF history, their preparation at room temperature has been described . However, the lower quality of the resultant materials compared to their solvothermally-prepared homologues, and the proliferation of other alternative methodologies to the solvothermal one has left room-temperature approaches to be relegated for some time in the academic literature, despite the obvious sustainability benefits. In more recent times, the development of synthetic procedures capable of providing higher quality MOFs,
[37] as well as the temporal proximity of the MOFs to be applied, rekindled certain interest in these more sustainable methods.
Moreover, the materials obtained in this way, although isostructural and iso-compositional to their solvothermal counterparts, possess different physicochemical properties to those of the conventional materials. Thus, it is well-known that MOFs formed by precipitation have more structural defects; indeed, simply being nanocrystalline may make the material more defective. Furthermore, the formation of nanocrystalline MOFs results in higher external surface areas and therefore higher possibilities of creating heterojunction composites . Finally, the tendency of nanocrystalline MOF crystallites to be agglomerated, or rather aggregated, in consistent and robust micron-sized particles, leads them to generate permanent intercrystalline mesoporosity with relatively uniform pore diameters
[37]. Therefore, the so-generated MOF materials are not only much more 3E-sustainable (with 3E standing for economical, energetic, and environmental) but also their resultant properties are more adequate than those of their counterparts for certain applications such as in the direct use as catalysts and the effective immobilization of enzymes in biocomposite Enz@MOFs.
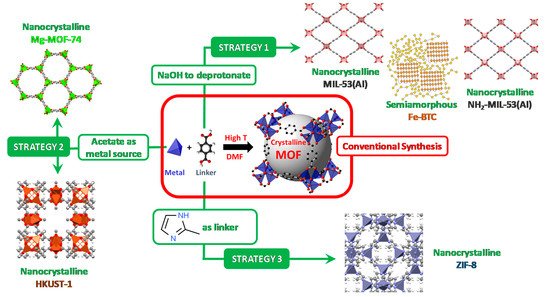
Scheme 1 arranges the general conditions for preparing MOFs via conventional (solvothermal) methodology as well as the sustainable synthetic approaches of the MOFs addressed in this review.
Scheme 1. Schematic representation of the different synthetic strategies (in green) for achieving the MOFs addressed in this work, starting from the conventional formation of a MOF (in red). The gray sphere represents the free volume within the MOF-5 cavity.
In the cases of carboxylate-based MOFs, the acidic form used as the linker source can be deprotonated by a base
[37], which is essential both to favor the dissolution of the linker and also to allow the direct reaction between the metal and the carboxylate groups. Although one could imagine that the use of a base (for instance, NaOH) moves the process away from being sustainable, its role as a deprotonating agent that neutralizes the acid form of the carboxylate-based organic linker, together with its stoichiometry in the synthesis mixture, makes sure that the base cannot be found in the final reaction media. Paradoxically, the use of this base converts this system into being more sustainable, as the corrosive acid by-products generated in the solvothermal crystallization of carboxylate MOFs such as HNO
3, HCl or H
2SO
4 (depending on if the metal sources are nitrates, chlorides or sulfates, respectively) are substituted by the innocuous salts NaNO
3, NaCl or Na
2SO
4 in this sustainable method
[37]. This approach was used for the formation of the biocatalysts Enz@MIL-53(Al) (
Section 4.1), Enz@NH
2-MIL-53(Al) (
Section 4.1) and Enz@Fe-BTC (
Section 4.2).
Alternatively, the use of carboxylates (particularly, acetates) as metal sources allows ion exchange reactions between the carboxylate-containing linkers and the acetates coordinated to the metals, to lead the formation of MOF without any additional energy input and without the addition of any chemical species as deprotonating agents or modulators . This approach was used for the formation of the biocatalysts Enz@HKUST-1 (
Section 4.3), Enz@Zn-MOF-74 (
Section 4.4).
Similarly, the imidazolate-based ZIF-8 does not need any of these stimuli as the simple contact of metal and linker readily leads to the formation of the MOF material . The ease of formation of ZIF-8 is promoted by the high solubility of the 2-methylimidazole linker in water, allowing for spontaneous formation of ZIF-8 at room temperature. This approach has been used for the formation of a large number of Enz@ZIF-8 biocatalysts (
Section 4.5).
It must be noted that, unlike the syntheses outlined in
Scheme 1, the synthesis of the in-situ biocatalysts Enz@MOF implies that the enzyme itself is present in the synthesis media of the MOF support. Therefore, it could potentially alter the chemistry of the synthesis media as well as the formation of the MOF, especially in situations whereby (i) there is significant interactions between the enzymes and the MOF and (ii) the enzyme molecules contain carboxylate groups similar to those of some the above-mentioned linkers that form MOFs by bonding with metallic clusters. As a consequence, the presence of enzymes could change the formation kinetics, the appearance of impurities, the defects, the crystal size, the intercrystalline mesoporosity, etc., of the resultant MOF-based material.
4. One-Pot MOF-Based Biocatalysts
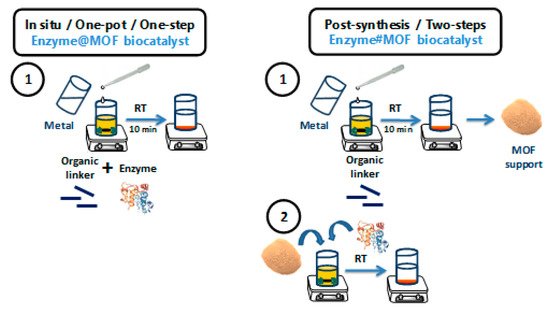
Scheme 2 shows a comparison between the one-pot and the post-synthesis procedure for enzyme immobilizations onto MOF-based supports, as well as the advantages and drawbacks of both methodologies. Although each enzyme@MOF biocatalyst should be individually studied in detail, in general terms, the one-step methods offer greater advantages.
Scheme 2. Schemes of the one-step in situ methodology (left), and the two-step or post-synthesis methodology (right) for the immobilization of enzymes onto MOF-based supports prepared under mild conditions. This figure has been inspired by ref. . Of course, the first step on the post-synthesis methodology, that is, the preparation of the enzyme-free MOF, could be carried out under conventional conditions, making this process potentially more laborious and damaging to the environment.
One-pot enzyme immobilization has previously been described for a variety of MOFs, as indicated in
Scheme 1. However, any given strategy (for instance, the deprotonation approach in
Scheme 1) is dependent on the specific features of the MOF supports, such as the nature of the linker (carboxylates, imidazolates, etc.) and functionalization, their intrinsic intercrystalline mesoporosity, their crystallite size, the pH of their synthetic media, the nature of the metal, etc. These factors strongly influence the compatibility of the specific biocomposite, with subsequent effects on the enzyme catalytic activity, the affinity for enzymes, or the immobilization efficiency. For that reason, this section is divided into different Sections according to the type of MOF material used as support, starting from the carboxylate-based MOF and finishing with the imidazolate-based ZIFs, with ZIF-8 being the most widely used sustainable MOF support for enzymes.
Table 2 summarizes the strategies, the MOF supports and the enzymes forming the biocomposite Enzyme@MOFs discussed in this review.
Table 2. Summary of the strategies, MOF supports, enzymes and biocomposite Enzyme@MOFs covered in this work. The number used for denoting the different strategies is in accordance with those used in
Scheme 1.
| Strategy |
MOF |
Enzyme |
References |
| 1 |
Fe-BTC |
Laccase |
[39] |
| |
|
Lipase |
[12][39] |
| |
|
Alcohol dehydrogenase (ADH)
Glucose oxidase (GOx) |
[12]
[12] |
| |
|
Peroxidase (POx) |
|
| 1 |
NH2-MIL-53(Al) |
β-Glucosidase (β-Glu) |
[36] |
| |
|
Laccase |
[38] |
| |
|
Lipase |
[11] |
| 2 |
HKUST-1 |
Glucose oxidase (Gox) |
|
| |
|
Horseradish peroxidase (HRP)
Laccase |
|
| |
|
Urease |
|
| 2 |
Mg-MOF-74 |
β-Glucosidase (β-Glu) |
[36] |
| 3 |
ZIF-8 |
Alcohol oxidase (AOx) |
|
| |
|
Carbonic anhydrase (CA) |
|
| |
|
Catalase |
[34][43] |
| |
|
Cytochrome C (Cty C) |
[32] |
| |
|
Glucose oxidase (GOx) |
|
| |
|
Horseradish peroxidase (HRP) |
|
| |
|
Laccase |
|
| |
|
Lipase |
|
| |
|
Lysozyme
Pyrroloquinoline quinone
Glucose dehydrogenase (PQQ-GDH) |
|
| |
|
Ribonuclease A |
|
| |
|
Trypsin |
|
| |
|
Urease |
|
| |
|
β-Galactosidase |
|
| 3 |
ZIF-90 |
Catalase |
[43] |
| |
|
Superoxide dismutase |
|
| 3 |
Amorphous-ZIF |
Catalase |
|
| |
|
Glucose oxidase (GOx) |
|
| |
|
Lipase |
|
| 3 |
ZIF-L |
Carbonic anhydrase (CA) |
|
| 3 a |
MAF-7 |
Catalase |
[43] |
5. Conclusions
Sustainability is undoubtedly the main challenge for current advances in chemical processes. Enzymatic catalysis fulfils most requirements of green chemistry regarding reaction conditions, but the necessity of working with immobilized enzymes as a result of the lability and solubility of these proteins threatens to become a new source of environmentally unfriendly processes. Therefore, the development of sustainable methods for the immobilization of enzyme has gained significant attention and some of these methods are reviewed herein. The advantages of the use of MOFs for in situ enzyme immobilization (low cost, leaching prevention of the entrapped enzyme, and sufficient substrate and product diffusion) can be exploited, but the sustainable synthesis of these biocatalysts is not without challenges. Some MOFs, like ZIF-8 and HKUST-1, do not require harsh conditions in their preparation and therefore their synthesis in the presence of enzymes produces one-step biocatalysts in non-polluting conditions. In other cases, like Fe-BTC or NH2-MIL-53(Al), modification of their respective synthetic procedures may be required in order for synthesis to occur in the presence of enzymes. These new conditions are based on the preservation of catalytic activity of the enzymes under sustainable conditions, namely aqueous medium, mild pH, and room temperature. A summary of the field is offered here showing how these systems may offer catalytic activity preservation and/or enzyme stabilization depending also on secondary factors such as the kind of interaction between the enzyme and the organic linker. This revision is meant as a starting point to the further studies of mild-condition synthesis of new Enzyme@MOF catalysts.
With the potential for in situ immobilization in/on MOFs being presented in this manuscript, some issues about future perspectives of these materials and their applications can now be reflected and advised upon. First of all, it is expected that the evolution to an increasingly sustainable world, particularly in chemical processes, will make these methodologies progressively gain ground in the general context of the immobilization of enzymes and MOFs. Secondly, it is worth noting that both the particular MOF support and its synthetic conditions must be optimized according to the nature of the immobilized enzyme, and the intended use of the resultant biocomposite; aspects as relevant as toxicity of metals and linkers, synthetic pH, the nature of deprotonating agents (if any), and the role of the enzyme (biomimetic, intercrystalline mesopore swelling, etc.) could decisively determine the scope of the enzyme@MOF application. Thirdly, the wider scientific community should take advantage of the well-known catalytic potential of MOFs to lead one-pot biocomposite enzyme@MOFs to where MOFs are not mere supports but become active participants that favor chain reactions, provide synergistic effects with the enzymes, or encourage shape selectivity (before, after or during the enzymatic reaction) for further performance. Finally, increasingly powerful computational calculations and characterization techniques should lead to a more exhaustive knowledge of the exact location of the enzymes (inter or intracrystalline) and of the interactions (even at the atomic level) at play in the MOF support, as well as its influence on the catalytic activity of the resulting MOF leading to an acceleration of development in this field.