
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Khaled Machaca | + 859 word(s) | 859 | 2021-05-24 07:50:37 | | | |
| 2 | Rita Xu | Meta information modification | 859 | 2021-05-25 05:13:11 | | |
Video Upload Options
Directional migration of cells is essential for multicellular organisms development and survival. In this review we outline the importance of calcium signaling, and in particular store-operated calcium entry, in regulating cell migration in nomral and metastatic cells. Interestingly, calcium signaling is polarized and differentially regulates focal adhesion and the cytoskeleton at the front and rear ends of the cell.
1. Introduction
Cell migration is essential for the development of multicellular organisms and is critical for many physiological processes, including organ development, morphogenesis, tissue repair and homeostasis, immune response and wound healing [1]. It is also essential for tumor metastasis to colonize remote sites, which is the main cause of death from cancers [2]. For adherent cells to move in a directional fashion multiple coordinated processes need to occur, including extension of lamellipodia at the front of the cell, disassembly of focal adhesions at the rear of the migrating cell and force generation through cytoskeleton attachments to the extracellular matrix (ECM) to pull the cell forward. These events require a complex coordinated machinery that involves environmental cues, signaling and cytoskeleton components, as well as focal adhesion remodeling among other mechanisms. Interestingly, many of these aspects are Ca2+ dependent, highlighting the critical role of Ca2+ signaling in regulating cell movement.
2. Cell Migration
Cell migration is a complex coordinated process that incorporates many cellular components and responds to a plethora of environmental cues. Typically, those environmental signals guide the directional migration of cells [1]. For a cell to move in a directional fashion it needs to polarize, with membrane extensions (lamellipodia) at the front end that are later stabilized by nascent focal adhesions. Lamellipodia are driven by actin polymerization followed by attachment to the extracellular matrix (ECM) to allow for force generation. Attachment is mediated by nascent adhesions, which further mature through interaction with the actin cytoskeleton and myosin mediated force generation (Figure 1). At the rear of the cell, mature focal adhesions disassemble to allow the cell body to be pulled forward thus mediating directional cell movement [3]. Focal adhesions are large dynamic plasma membrane-associated macromolecular assemblies that are rich in integrins, and connect the actin cytoskeleton to the extracellular matrix [4]. The dynamic regulation of focal adhesions is essential for successful cell migration and is mediated by Ca2+ dependent assembly and disassembly cycles [5][6], as will be further discussed below.
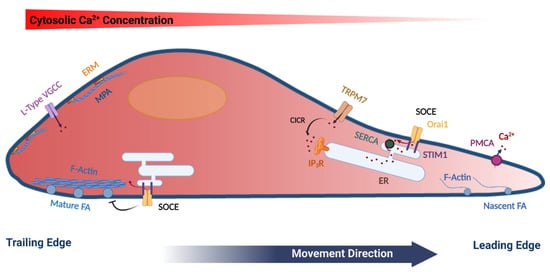
Figure 1. Model summarizing the polarization of various cytoskeletal and Ca2+ signaling components in a migrating cell. Mature focal adhesions (FA), L-type voltage-gated Ca2+ channels (VGCC), ERM (Ezrin, Radixin and Moesin) proteins, as well as cortical actin (Membrane Proximal Actin (MPA) are enriched at the rear end of the cell. TRPM7 and the plasma membrane Ca2+-ATPase (PMCA) are enriched at the leading edge of the migrating cell. Store-operated Ca2+ entry (SOCE), which is mediated by STIM1 and Orai1 have been functionally implicated in disassembly of FA at the rear end, as well as in refilling Ca2+ stores at the leading edge. See text for further details. The figure was created using BioRender.com.
The actin cytoskeleton is vital in the regulation of focal adhesions as well as in generating the forces required for cell migration. In that context, actin dynamics are regulated by small GTPases, including Rac1, Cdc42 and RhoA [7]. Rac regulates protrusive forces in lamellipodia through modulating actin formation via actin nucleation complexes such as SCAR/WAVE and Arp2/3 [7][8]. Rho, Rho-kinase and Ca2+/calmodulin-activated myosin-light chain kinase regulate actomyosin fibers contraction that cause the retraction of the trailing end of the cell and its net forward movement [7][9]. Cdc42 regulates cell polarity in migrating cells through interactions with the PAR complex and the actin cytoskeleton [10][11].
Therefore, the actin cytoskeleton through spatially controlled cycles of polymerization and depolymerization supports membrane deformation and force generation that are required for cell movement. In addition to the actin fibers that crisscross the cell, cells form a shell of cortical actin bundles around their periphery. Cortical actin directly interacts with the PM through the ERM proteins (ezrin, radixin and moesin), that associate with PtdIns (4,5) P2 at the cell membrane through their FERM domain at one end and with actin at the other end, thus linking the PM to the actin cytoskeleton [12]. In addition, cortical actin counters the internal cellular pressure to regulate membrane tension, thus preventing non-specific membrane deformation (blebbing), which impinges on cell migration [13]. The distribution of ERM proteins in migrating cells is polarized with enrichment in the rear of the cell [14] (Figure 1). In contrast, at the front of migrating cells actin is dynamically remodeled through actin severing, nucleation and contraction [15]. Recently, Bisaria et al. developed novel reporters to visualize cortical actin dynamics and more specifically the outer most layer of cortical actin that links directly to the PM, to which they refer as membrane proximal actin (MPA). They found a gradient of MPA density across the migrating cell with high MPA at the trailing edge and membrane protrusions generated from areas with low MPA density [16] (Figure 1). This low density of MPA is maintained by high cofilin activity in the front of the cell, which provides free G-actin monomers to initiate protrusions and decrease MPA density to allow for lamellipodia extension [16][17].
References
- Shellard, A.; Mayor, R. All Roads Lead to Directional Cell Migration. Trends Cell Biol. 2020, 30, 852–868.
- Kraljevic Pavelic, S.; Sedic, M.; Bosnjak, H.; Spaventi, S.; Pavelic, K. Metastasis: New perspectives on an old problem. Mol. Cancer 2011, 10, 22.
- Mo, P.; Yang, S. The store-operated calcium channels in cancer metastasis: From cell migration, invasion to metastatic colonization. Front. Biosci. (Landmark Ed.) 2018, 23, 1241–1256.
- Huang, H.K.; Lin, Y.H.; Chang, H.A.; Lai, Y.S.; Chen, Y.C.; Huang, S.C.; Chou, C.Y.; Chiu, W.T. Chemoresistant ovarian cancer enhances its migration abilities by increasing store-operated Ca2+ entry-mediated turnover of focal adhesions. J. Biomed. Sci. 2020, 27, 36.
- Chang, S.J.; Chen, Y.C.; Yang, C.H.; Huang, S.C.; Huang, H.K.; Li, C.C.; Harn, H.I.; Chiu, W.T. Revealing the three dimensional architecture of focal adhesion components to explain Ca2+-mediated turnover of focal adhesions. Biochim. Biophys. Acta Gen Subj. 2017, 1861, 624–635.
- Derouiche, S.; Warnier, M.; Mariot, P.; Gosset, P.; Mauroy, B.; Bonnal, J.L.; Slomianny, C.; Delcourt, P.; Prevarskaya, N.; Roudbaraki, M. Bisphenol A stimulates human prostate cancer cell migration via remodelling of calcium signalling. Springerplus 2013, 2, 54.
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709.
- Ridley, A.J. Life at the leading edge. Cell 2011, 145, 1012–1022.
- Yang, S.; Huang, X.Y. Ca2+ influx through L-type Ca2+ channels controls the trailing tail contraction in growth factor-induced fibroblast cell migration. J. Biol. Chem. 2005, 280, 27130–27137.
- Mayor, R.; Etienne-Manneville, S. The front and rear of collective cell migration. Nat. Rev. Mol. Cell Biol. 2016, 17, 97–109.
- Petrie, R.J.; Doyle, A.D.; Yamada, K.M. Random versus directionally persistent cell migration. Nat. Rev. Mol. Cell Biol. 2009, 10, 538–549.
- Fehon, R.G.; McClatchey, A.I.; Bretscher, A. Organizing the cell cortex: The role of ERM proteins. Nat. Rev. Mol. Cell Biol. 2010, 11, 276–287.
- Diz-Muñoz, A.; Krieg, M.; Bergert, M.; Ibarlucea-Benitez, I.; Muller, D.J.; Paluch, E.; Heisenberg, C.P. Control of directed cell migration in vivo by membrane-to-cortex attachment. PLoS Biol. 2010, 8, e1000544.
- Liu, Y.J.; Le Berre, M.; Lautenschlaeger, F.; Maiuri, P.; Callan-Jones, A.; Heuzé, M.; Takaki, T.; Voituriez, R.; Piel, M. Confinement and low adhesion induce fast amoeboid migration of slow mesenchymal cells. Cell 2015, 160, 659–672.
- Bravo-Cordero, J.J.; Magalhaes, M.A.; Eddy, R.J.; Hodgson, L.; Condeelis, J. Functions of cofilin in cell locomotion and invasion. Nat. Rev. Mol. Cell Biol. 2013, 14, 405–415.
- Bisaria, A.; Hayer, A.; Garbett, D.; Cohen, D.; Meyer, T. Membrane-proximal F-actin restricts local membrane protrusions and directs cell migration. Science 2020, 368, 1205–1210.
- Oser, M.; Condeelis, J. The cofilin activity cycle in lamellipodia and invadopodia. J. Cell Biochem. 2009, 108, 1252–1262.




