
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Angela Vincent | + 3418 word(s) | 3418 | 2020-08-11 06:11:05 | | | |
| 2 | Angela Vincent | Meta information modification | 3418 | 2020-09-01 12:02:15 | | | | |
| 3 | Nicole Yin | -27 word(s) | 3391 | 2020-09-02 03:51:09 | | | | |
| 4 | Nicole Yin | Meta information modification | 3391 | 2020-09-02 11:40:44 | | |
Video Upload Options
Recent studies indicate the existence of a maternal-autoantibody-related subtype of autism spectrum disorder (ASD). To date, a large number of studies have focused on describing patterns of brain-reactive serum antibodies in maternal-autoantibody-related (MAR) autism and some have described attempts to define the antigenic targets. This entry describes evidence on MAR autism and the various autoantibodies that have been implicated. Among other possibilities, antibodies to neuronal surface protein Contactin Associated Protein 2 (CASPR2) have been found more frequently in mothers of children with neurodevelopmental disorders or autism, and two independent experimental studies have shown pathogenicity in mice. The N-methyl-D-aspartate receptor (NMDAR) is another possible target for maternal antibodies as demonstrated in mice. Here, we discuss the growing evidence, discuss issues regarding biomarker definition, and summarise the therapeutic approaches that might be used to reduce or prevent the transfer of pathogenic maternal antibodies.
1. Introduction
Early research in this area was closely followed by several critical studies in the description of maternal-autoantibody-related (MAR) autism, a sub-type of ASD in which autoantibodies reactive to foetal brain proteins are present in the maternal serum (Tables 1 and 2).
Table 1. Summary of studies associating maternal antibodies with neurodevelopmental disorder in the child.
|
Author |
Findings |
Antibody Target |
Cohort |
|
Warren et al. (1990)[1] |
6 of 11 mothers with an autistic child had antibodies reactive to lymphocytes of the child. |
Human lymphocytes |
MAU = 11 |
|
Since lymphocyte antigens can be shared with neuronal antigens, the study indicated that maternal antibodies might be associated with the development of autism. |
|||
|
Barnes et al. (1995)[2] |
Four children with AMC born to an initially asymptomatic mother and separate unrelated fathers |
Unclear at this stage |
1 mother with 4 pregnancies affected by AMC and a subsequent diagnosis of MG |
|
It was striking that the diagnosis of AMC was made only after the fourth affected child. |
|||
|
Serum from a mother of children affected by a neuromuscular disorder contained a high titre of antibodies that selectively inhibited the foetal form of acetylcholine receptor. |
Acetylcholine receptors (antibodies inhibiting function of foetal isoform) |
1 healthy mother with 6 consecutive pregnancies affected by AMC |
|
|
First study to implicate clearly maternal antibodies as a cause of developmental disorder in the children of unaffected mothers |
|||
|
Zimmerman et al. (2007)[5] |
Antibodies in the serum of mothers of autistic children recognised different foetal but not adult rat brain proteins compared to controls. |
Adult and foetal rat brain protein preparations |
MAU = 11 |
|
Western blotting procedure as in numerous following studies: |
|||
|
Braunschweig et al. (2008)[6] |
Increased plasma reactivity to 37 kDa band in mothers of autistic children |
Foetal human brain protein preparation |
MAU = 61 |
|
There is inconsistency in the bands identified by the five immunoblotting studies. |
|||
|
Croen et al. (2008)[7] |
Increased plasma reactivity to a 39 kDa band in mothers of autistic children |
Foetal human brain protein preparation |
MAU = 84 |
|
Singer et al. (2008)[8] |
Increased plasma reactivity to 36, 39, 61 and 73 kDa bands in mothers of autistic children |
Human and rat foetal brain protein preparation |
MAU = 100 |
|
Braunschweig et al. (2012)[9] |
Increased plasma reactivity to 37, 39 and 73 kDa bands in mothers of autistic children |
Foetal monkey brain protein preparation |
MAU = 272 |
|
Braunschweig et al. (2013)[10] |
Tandem mass spectrometry and peptide sequencing were used to identify antigen candidates for 37, 39, 73, 70 and 44 kDa proteins. LDH, YBX1, cypin, STIP1, CRMP1 and CRMP2 were confirmed as autoantibody targets in binding inhibition Western blot studies. |
Foetal monkey brain protein preparation |
MAU = 246 |
|
Brimberg et al. (2013)[11] |
Plasmas from mothers of autistic children were four times more likely to contain anti-brain antibodies than control plasmas (10.5% vs. 2.6%). |
Mouse brain sections |
MAU = 2431 |
|
First large cohort study and thus important evidence that mothers of an autistic child have an elevated frequency of anti-brain antibodies |
|||
|
Brimberg et al. (2016)[12] |
Plasma reactivity to CASPR2 was found in 37% of ASD mothers displaying brain-reactive serology compared to 12% of ASD mothers lacking brain-reactive serology. |
Mouse brain in vivo |
MAU with reactive serology = 53 |
|
Coutinho et al. (April 2017)[13] |
CASPR2-Abs found more frequently in mothers of children with MR/DPD (7/171; 4%) than control mothers (1/171; 1%) |
Live human embryonic kidney cells transfected with cDNA encoding CASPR2 or NMDAR |
Gestational week 14–18 mothers of children with mild or unspecified neurodevelopmental disorders (n = 171, cases) and mothers whose child had not received this diagnosis (n = 171, controls) |
|
Jurek et al. (2019)[14] |
Mothers of children with psychiatric disorders had slightly higher titres of NR1-reactive IgG compared to mothers of unaffected children. |
Live HEK cells over-expressing native human NR1 protein |
120 healthy mothers of children with psychiatric disorders; 105 healthy control mothers of unaffected children |
|
No association with any specific psychiatric disorder |
|||
Abbreviations: MAU = mothers of a child with autism spectrum disorder. MTD = mothers with only typically developing children. MDD = mothers of a child with developmental delay. AMC = arthrogryposis multiplex congenita. MG = myasthenia gravis. ASD = autism spectrum disorder. LDH = lactate dehydrogenase, YBX1 = Y-box-binding protein, STIP1 = stress-induced phosphoprotein 1. CRMP1, CRMP2 = collapsin response mediator proteins 1 and 2. MAR = maternal autoantibody related. CASPR2 = contactin associated protein 2. HEK = human embryonic kidney. MR/DPD = mental retardation and/or disorders of psychological development. CASPR2-Abs = CASPR2 antibodies. NMDAR = N-methyl-D-aspartate receptor.
Table 2. Summary of animal studies on maternal antibodies causing neurodevelopmental disorder.
|
Author |
Findings |
Cohort |
|
Jacobson et al. (1999)[15] |
Plasma from four AChR antibody-positive women injected into pregnant mouse dams: foetuses displayed deformities resembling AMC. |
4 AChR antibody-positive women with histories of severe AMC in their babies |
|
First to establish passive transfer animal model for study of maternal antibody-mediated developmental disorder |
||
|
Dalton et al. (2003)[16] |
Plasma from a mother of an autistic child and a child with a severe specific language disorder injected into pregnant mouse dams: offspring displayed a slowed righting reflex, decreased exploration and decreased performance on the multiple static rods test. |
1 mother of 3 children: 1 TD, 1 with autism and 1 with a severe specific language disorder; |
|
First causative study indicating that maternal factor can induce behavioural disorder in offspring |
||
|
Martin et al. (2008)[17] |
Prenatal exposure to purified pooled IgG from mothers of autistic children produced stereotypies and hyperactivity in rhesus macaques. |
MAU = 21 |
|
No changes in sociability were observed. However, the changes observed are particularly compelling in a primate model. |
||
|
Lee et al. (2009)[18] |
NMDAR (NR2) immunisation, with NMDAR antibodies present before and throughout gestation |
|
|
Antibodies in this model cross-reacted with NR2A and NR2B. However, mouse NR2A expression is thought to be strictly postnatal so the observed phenotype was most likely due to antibodies targeting NR2B. In humans, NR2A expression has been detected in the foetal neocortex. |
||
|
Singer et al. (2009)[19] |
Prenatal exposure to purified pooled IgG from mothers of autistic children produced hyperactivity, sociability alterations and anxiety-like behaviour in mice. |
MAU = 63 |
|
Braunschweig et al. (2012)[20] |
Purified IgG from mothers of autistic children with paired 37/73 kDa band reactivity was injected into pregnant mice: Offspring displayed anxiety-like behaviour and delayed physical and sensory-motor development. |
MAU = 3 (selected for paired 37/73 kDa reactivity) |
|
No significant changes in sociability |
||
|
Bauman et al. (2013)[21] |
Purified IgG from mothers of autistic children with paired 37/73 kDa band reactivity was injected into pregnant rhesus macaques: Offspring were treated with heightened protectiveness by their mothers, and on maturing they displayed increased approach of familiar peers (was not reciprocated and didn’t lead to sustained social interactions) and inappropriate approach of unfamiliar peers. Male offspring also had enlarged brains and increased white matter volume most pronounced in the frontal lobes. |
MAU = 4 mothers (selected for paired 37/73 kDa reactivity) |
|
These were very compelling data and the first demonstration of aberrant social development in non-human primates exposed to autism-associated anti-brain antibodies. Increased brain size and white matter volume have been consistently reported in autism[22][23][24][25][26][27][28][29] and have been specifically reported in the MAR autism subtype[30]. |
||
|
Brimberg et al. |
Monoclonal C6 (CASPR2 antibody) derived from mother of autistic child injected into pregnant mice: offspring displayed differences in sociability, repetitive behaviour and flexible learning. |
Monoclonal CASPR2 derived from 1 mother of an autistic child |
|
Martínez-Cerdeño et al. (2016)[31] |
Purified IgG from mothers of autistic children with paired 37/73 kDa band reactivity was injected into pregnant mice: IgG recognised radial glial cells, increased subventricular zone and extra-cortical cell proliferation and increased both the size and weight of adult brains and the size of adult cortical neurons. |
MAU = 4 (selected for paired 37/73 kDa reactivity) |
|
Ariza et al. (2017)[32] |
Purified IgG from mothers of autistic children with paired 37/73 kDa band reactivity was injected into pregnant mice: IgG reduced dendritic spine number and density in the frontal and occipital cortices. |
MAU = 1 (selected for paired high-titre 37/73 kDa reactivity) |
|
Coutinho et al. (October 2017)[33] |
IgG from two patients with CASPR2-antibody encephalitis injected into pregnant mice: Adult offspring showed social interaction deficits, abnormally located glutamatergic neurons in layers V-VI of the somatosensory cortex, microglial overactivation and a reduction in glutamatergic synaptic profile density in the prefrontal and somatosensory cortices. |
2 male patients with CASPR2-antibody encephalitis |
|
Jones et al. (2018)[34] |
Antigen-driven model of MAR autism based on the ASD-specific 37/73 kDa band pattern: offspring of immunised dams showed differences in juvenile sociability and repetitive behaviours. |
|
|
The ELISAs done to confirm antibody generation produced low antibody titres, ranging between 0.3 and 0.5 OD; |
||
|
Jurek et al. (2019)[14] |
Cloned human antibodies were injected into pregnant mice: Offspring had reduced synaptic NMDAR densities, increased mortality, altered physiological functions during early development (elevated blood pH, reduced weight gain) and impaired neurodevelopment. Behavioural changes persisted into adulthood. |
IgG1 cloned from 2 female patients with acute NMDAR encephalitis (obtained during periods of disease exacerbation) |
|
Findings could relate to a spectrum of behavioural disorders, not necessarily autism. |
||
Abbreviations: MAU = mothers of a child with autism spectrum disorder. MTD = mothers with only typically developing children. MDD = mothers of a child with developmental delay. NMDAR = N-methyl-D-aspartate receptor. CASPR2 = contactin associated protein 2. ASD = autism spectrum disorder. ELISA = enzyme-linked immunosorbent assay. OD = optical density
Tables 1 and 2 provide a comprehensive summary of the studies on MAR autism to date. A combination of associative human studies and passive transfer and immunisation studies in animals provides convincing proof of the concept of MAR autism.
2. Early Studies
Table 1 describes 13 studies that have associated patterns of maternal serum reactivity with an outcome of ASD or other neurodevelopmental disorder in the child. The first study to indicate a possible association between maternal antibodies and autism was carried out by Warren et al. (1990)[1], who demonstrated that plasma from six of eleven mothers of an autistic child had antibodies reactive to lymphocytes of the child. Considering the substantial cross-reactivity between lymphocyte and neuronal antigens, this study prompted further investigation into the concept of MAR autism.
In the mid-1990s came two case studies on autoantibody-related neurodevelopmental disorder. Barnes et al. (1995)[2] described a mother whose four pregnancies were affected by foetal arthrogryposis multiplex congenita (AMC), a rare condition characterised by multiple joint contractures, caused by lack of foetal movement in utero, and previously associated with maternal myasthenia gravis (MG). The mother had a history of shoulder and facial muscle weakness, but MG was not diagnosed until after her fourth pregnancy, when she had highly positive serum acetylcholine receptor (AChR) antibodies. Vincent et al. (1995)[3] described a second mother who, despite remaining healthy herself, had six consecutive pregnancies affected by AMC. Serum taken before the sixth pregnancy contained a high titre of AChR antibodies. Foetal movements were initially normal until 15–18 weeks, but repeated plasma exchange from 10 weeks gestation prolonged foetal movements, indicating that onset/progression of AMC could be delayed. This implicated maternal factors over a genetic cause in the pathogenesis. Furthermore, a maternal-autoantibody-mediated cause of AMC was heavily suggested by the recurrence of AMC in all pregnancies (both studies) and the fact that foetal movements were observed until 15–18 weeks, the time at which foetal immunoglobulin G (IgG) levels begin to rise rapidly. This hypothesis was strengthened by demonstration that the maternal serum antibodies selectively inhibited the foetal form of AChR (Riemersma et al., 1997)[4], making this the first study to implicate clearly maternal autoantibodies as a cause of a neurodevelopmental disorder and catalysing further research into this novel field. Witebsky’s postulates for antibody-mediated disorders, as modified by Rose and Bona[35], rely on transfer of disease to an experimental model (as well as transfer of disease from mother to her offspring). To establish an animal model, Jacobson et al. (1999)[15] injected pregnant mouse dams with plasma from four AChR antibody-positive women whose foetuses were affected with severe AMC. Many of the foetuses displayed fixed joint contractures and other deformities resembling AMC. This passive transfer animal model has since been used repeatedly to study the effects of ASD-salient maternal antibodies on foetal development (Table 2).
The first such study looking at autism was done by Dalton et al. (2003)[16] who injected pregnant mouse dams with plasma from a mother who had three surviving children: One typically developing, one with autism and one with a specific language disorder. The mother’s serum contained antibodies that bound to rodent Purkinje cells and to the surface of a neuroblastoma cell line, suggesting a potentially pathogenic neuronal antibody. Compared to controls, the mouse offspring displayed a delayed righting reflex, decreased exploration and decreased ability to balance on the multiple static rods test. While these behavioural measures were far from specific to autism, the reduced exploratory behaviour echoed the characteristic RRBIs seen in autism, and the reduced balancing and changed cerebellar metabolites indicated possible cerebellar dysfunction which has been implicated in the pathophysiology of autism[36]. A strength of this study was its tightly controlled diagnostic criteria for psychiatric disorder; their diagnoses were based on independent clinical assessments by three paediatric specialists. Thus, Dalton et al. were the first to provide causative evidence that maternal factor can induce neurodevelopmental disorder, and with particular direction towards autism.
The story of MAR autism grew significantly from 2007 onwards when a number of studies linked autism with maternal serum reactivity[17][11][5][6]. Western blots of mammalian brain extracts were used as the method of antibody detection in these studies and protein bands were identified by maternal serum IgG in cases but not in controls. Others studied the reactivity of plasma from mothers of an autistic child using mouse brain sections and observed an increased prevalence of anti-brain reactivity in the 10.5% of 2431 cases compared with 2.6% of 653 controls[7]. While the plasma samples used were not mid-gestational, these studies support the existence of MAR autism. However, the method used did not identify specific antigenic targets or identify whether they were intracellular rather than membrane proteins.
Table 2 lists several passive transfer animal studies that have aimed at establishing causative evidence for the association between autism and maternal serum reactivity. Martin et al. (2008)[17] advanced on previous work with the first IgG passive transfer model, in which purified pooled IgG, rather than whole plasma, from mothers of autistic children produced stereotypies and hyperactivity in rhesus macaques. The demonstration of these changes in a primate model makes this particularly compelling evidence. A limitation common to most of the studies listed in Tables 1 and 2, however, was the use of maternal plasma samples collected several years after the children’s birth[1][16][17][11][5][6][7][8][9][20][10][12][19][14], by which time maternal antibody profiles might have changed. Nevertheless, with repetition across studies and in large cohorts the overall significance of this limitation is reduced. Furthermore, a study by Croen et al. was successful in confirming mid-gestational presence of antibodies to an autism-specific pattern of intracellular antigens[5].
The animal studies summarised in Table 2 have helped to strengthen the story of MAR autism. However, it is important to keep in mind the difficulty of comparing animal and human behaviours. It is possible that some or all of the maternal antibodies identified are mediators not specifically of autism but of neurodevelopmental disorder more generally. This issue is further complicated by changes in autism diagnosis over the past 30 years. Baird et al. (2006)[37] described a disparity between the recorded prevalence of childhood autism in South Thames, UK (38.9 per 10,000) and the prevalence measured using a narrower definition which combined clinical consensus with instrument criteria for past and current presentation (24.8 per 10,000). Research subject selection in many of the studies in Table 1, including Brimberg et al. (2013)[11], appear to have been based on the wider definition, meaning there might be inconsistencies depending on where and when the cohorts were recruited and when studied. This could be an explanation for some conflicting results discussed later in this article concerning CASPR2 antibodies (CASPR2-Abs). A simple schema of the placental transfer, the antigenic targets, and possible treatment approaches is shown in Figure 1.
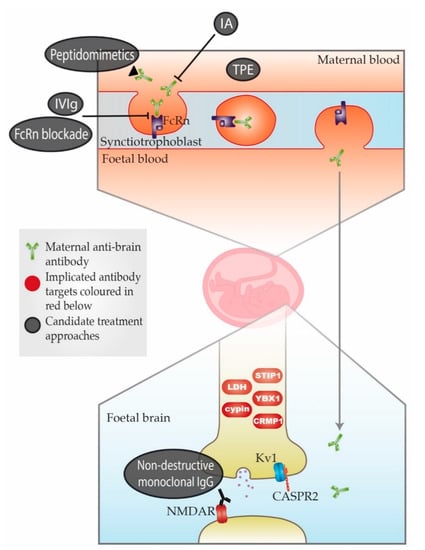
Figure 1. Schematic representation of maternal-autoantibody-related (MAR) autism pathogenesis and potential therapeutic approaches. Placental antibody transfer is mediated by the neonatal Fc receptor (FcRn). Thus, pathogenic maternal antibodies cross into foetal circulation and reach the foetal brain, where they react with foetal brain antigens. Implicated target antigens include Contactin Associated Protein 2 (CASPR2), the N-methyl-D-aspartate receptor (NMDAR) and various intracellular proteins. Potential therapeutic approaches include (1) therapeutic plasma exchange (TPE), (2) immunoadsorption (IA), (3) intravenous immunoglobulin therapy (IVIg), (4) blockade of the neonatal Fc receptor (FcRn), (5) peptidomimetics and (6) non-destructive monoclonal immunoglobulin G (IgG) with the same specificity as the pathogenic antibody.
References
- Reed P. Warren; Phyllis Cole; J. Dennis Odell; Carmen B. Pingree; W. Louise Warren; Ellen White; Jeanne Yonk; Vijendra K. Singh; Detection of Maternal Antibodies in Infantile Autism. Journal of the American Academy of Child & Adolescent Psychiatry 1990, 29, 873-877, 10.1097/00004583-199011000-00005.
- Phillip R.J. Barnes; Dipak J. Kanabar; Louise Brueton; John Newsomdavis; Susan M. Huson; Nicholas P. Mann; David Hilton-Jones; Recurrent congenital arthrogryposis leading to a diagnosis of myasthenia gravis in an initially asymptomatic mother. Neuromuscular Disorders 1995, 5, 59-65, 10.1016/0960-8966(94)e0029-8.
- A. Vincent; C. Newland; L. Brueton; D. Beeson; S. Riemersma; S.M. Huson; J. Newsom-Davis; Arthrogryposis multiplex congenita with maternal autoantibodies specific for a fetal antigen. The Lancet 1995, 346, 24-25, 10.1016/s0140-6736(95)92652-6.
- S Riemersma; A Vincent; D Beeson; C Newland; S Hawke; B Vernet-Der Garabedian; B Eymard; J Newsom-Davis; Association of arthrogryposis multiplex congenita with maternal antibodies inhibiting fetal acetylcholine receptor function.. Journal of Clinical Investigation 1996, 98, 2358-2363, 10.1172/jci119048.
- Andrew W. Zimmerman; Susan L. Connors; Karla J. Matteson; Li-Ching Lee; Harvey S. Singer; Julian A. Castaneda; David A. Pearce; Maternal antibrain antibodies in autism. Brain, Behavior, and Immunity 2007, 21, 351-357, 10.1016/j.bbi.2006.08.005.
- Daniel Braunschweig; Paul Ashwood; Paula Krakowiak; Irva Hertz-Picciotto; Robin Hansen; Lisa A. Croen; Isaac N. Pessah; Judy Van De Water; Autism: Maternally derived antibodies specific for fetal brain proteins. NeuroToxicology 2007, 29, 226-231, 10.1016/j.neuro.2007.10.010.
- Lisa A. Croen; Daniel Braunschweig; Lori Haapanen; Cathleen K. Yoshida; Bruce Fireman; Judith K. Grether; Martin Kharrazi; Robin L. Hansen; Paul Ashwood; Judy Van De Water; et al. Maternal Mid-Pregnancy Autoantibodies to Fetal Brain Protein: The Early Markers for Autism Study. Biological Psychiatry 2008, 64, 583-588, 10.1016/j.biopsych.2008.05.006.
- Harvey S. Singer; Christina M. Morris; Colin D. Gause; Pam K. Gillin; Stephen Crawford; Andrew W. Zimmerman; Antibodies against fetal brain in sera of mothers with autistic children. Journal of Neuroimmunology 2008, 194, 165-172, 10.1016/j.jneuroim.2007.11.004.
- Daniel Braunschweig; Paul Duncanson; Robert Boyce; Robin Hansen; Paul Ashwood; Isaac N. Pessah; Irva Hertz-Picciotto; Judy Van De Water; Behavioral Correlates of Maternal Antibody Status Among Children with Autism. Journal of Autism and Developmental Disorders 2011, 42, 1435-1445, 10.1007/s10803-011-1378-7.
- D Braunschweig; P Krakowiak; P Duncanson; R Boyce; R L Hansen; P Ashwood; Irva Hertz-Picciotto; I N Pessah; Judy Van De Water; Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Translational Psychiatry 2013, 3, e277-e277, 10.1038/tp.2013.50.
- L Brimberg; A Sadiq; P K Gregersen; B Diamond; Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder. Molecular Psychiatry 2013, 18, 1171-1177, 10.1038/mp.2013.101.
- L Brimberg; Simone Mader; V Jeganathan; R Berlin; Thomas R. Coleman; P K Gregersen; P T Huerta; Bruce Volpe; Betty Diamond; Caspr2-reactive antibody cloned from a mother of an ASD child mediates an ASD-like phenotype in mice. Molecular Psychiatry 2016, 21, 1663-1671, 10.1038/mp.2016.165.
- Ester Coutinho; Leslie Jacobson; Marianne Giørtz Pedersen; Michael Eriksen Benros; Bent Nørgaard-Pedersen; Preben Bo Mortensen; Paul J Harrison; Angela Vincent; CASPR2 autoantibodies are raised during pregnancy in mothers of children with mental retardation and disorders of psychological development but not autism. Journal of Neurology, Neurosurgery & Psychiatry 2017, 88, 718-721, 10.1136/jnnp-2016-315251.
- Betty Jurek; Mariya Chayka; Jakob Kreye; Katharina Lang; Larissa Kraus; Pawel Fidzinski; Hans‐Christian Kornau; Le‐Minh Dao; Nina K. Wenke; Melissa Long; et al.Marion RivalanYork WinterJonas LeubnerJulia HerkenSimone MayerSusanne MuellerPhilipp Boehm‐SturmUlrich DirnaglDietmar SchmitzMichael KölchHarald Prüss Human gestational N ‐methyl‐ d ‐aspartate receptor autoantibodies impair neonatal murine brain function. Annals of Neurology 2019, 86, 656-670, 10.1002/ana.25552.
- Leslie Jacobson; Agata Polizzi; Gillian Morriss-Kay; Angela Vincent; Plasma from human mothers of fetuses with severe arthrogryposis multiplex congenita causes deformities in mice. Journal of Clinical Investigation 1999, 103, 1031-1038, 10.1172/jci5943.
- Paola Dalton; Robert Deacon; Andrew M Blamire; Michael Pike; Ian McKinlay; John Stein; Peter Styles; Angela Vincent; Maternal neuronal antibodies associated with autism and a language disorder. Annals of Neurology 2003, 53, 533-537, 10.1002/ana.10557.
- Loren A. Martin; Paul Ashwood; Daniel Braunschweig; Maricel Cabanlit; Judy Van De Water; David G. Amaral; Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism.. Brain, Behavior, and Immunity 2008, 22, 806-816, 10.1016/j.bbi.2007.12.007.
- Ji Y. Lee; Patricio T. Huerta; Jie Zhang; Czeslawa Kowal; Eva Bertini; Bruce T. Volpe; Betty Diamond; Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nature Medicine 2008, 15, 91-96, 10.1038/nm.1892.
- Harvey S. Singer; Christina Morris; Colin Gause; Matthew Pollard; Andrew W. Zimmerman; Mikhail Pletnikov; Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. Journal of Neuroimmunology 2009, 211, 39-48, 10.1016/j.jneuroim.2009.03.011.
- Daniel Braunschweig; Mari S. Golub; Claire M. Koenig; Lihong Qi; Isaac N. Pessah; Judy Van De Water; Robert F. Berman; Maternal autism-associated IgG antibodies delay development and produce anxiety in a mouse gestational transfer model. Journal of Neuroimmunology 2012, 252, 56-65, 10.1016/j.jneuroim.2012.08.002.
- Melissa D. Bauman; Ana-Maria Iosif; P Ashwood; D Braunschweig; A Lee; C M Schumann; J Van De Water; D G Amaral; Maternal antibodies from mothers of children with autism alter brain growth and social behavior development in the rhesus monkey. Translational Psychiatry 2013, 3, e278-e278, 10.1038/tp.2013.47.
- Nordahl, C.W.; Lange, N.; Li, D.D.; Barnett, L.A.; Lee, A.; Buonocore, M.H.; Simon, T.J.; Rogers, S.; Ozonoff, S.; Amaral, D.G. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc. Natl. Acad. Sci. USA 2011, 108, 20195–20200.
- Courchesne, E.; Karns, C.M.; Davis, H.R.; Ziccardi, R.; Carper, R.A.; Tigue, Z.D.; Chisum, H.J.; Moses, P.; Pierce, K.; Lord, C.; et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology 2001, 57, 245–254.
- Schumann, C.M.; Bloss, C.S.; Barnes, C.C.; Wideman, G.M.; Carper, R.A.; Akshoomoff, N.; Pierce, K.; Hagler, D.; Schork, N.; Lord, C.; et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J. Neurosci. 2010, 30, 4419–4427.
- Hazlett, H.C.; Poe, M.; Gerig, G.; Smith, R.G.; Provenzale, J.; Ross, A.; Gilmore, J.; Piven, J. Magnetic resonance imaging and head circumference study of brain size in autism: Birth through age 2 years. Arch. Gen. Psychiatry 2005, 62, 1366–1376.
- Hazlett, H.C.; Poe, M.D.; Gerig, G.; Styner, M.; Chappell, C.; Smith, R.G.; Vachet, C.; Piven, J. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch. Gen. Psychiatry 2011, 68, 467–476.
- Herbert, M.R.; Ziegler, D.A.; Deutsch, C.K.; O’Brien, L.M.; Lange, N.; Bakardjiev, A.; Hodgson, J.; Adrien, K.T.; Steele, S.; Makris, N.; et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain 2003, 126, 1182–1192.
- Herbert, M.R.; Ziegler, D.A.; Makris, N.; Filipek, P.A.; Kemper, T.L.; Normandin, J.J.; Sanders, H.A.; Kennedy, D.N.; Caviness, V.S., Jr. Localization of white matter volume increase in autism and developmental language disorder. Ann. Neurol. 2004, 55, 530–540.
- Bigler, E.D.; Abildskov, T.J.; Petrie, J.A.; Johnson, M.; Lange, N.; Chipman, J.; Lu, J.; McMahon, W.; Lainhart, J.E. Volumetric and voxel-based morphometry findings in autism subjects with and without macrocephaly. Dev. Neuropsychol. 2010, 35, 278–295.
- Christine Wu Nordahl; Daniel Braunschweig; Ana-Maria Iosif; Aaron Lee; Sally Rogers; Paul Ashwood; David G. Amaral; Judy Van De Water; Maternal autoantibodies are associated with abnormal brain enlargement in a subgroup of children with autism spectrum disorder. Brain, Behavior, and Immunity 2013, 30, 61-65, 10.1016/j.bbi.2013.01.084.
- Verónica Martínez-Cerdeño; Jasmin Camacho; Elizabeth Fox; Elaine Miller; Jeanelle Ariza; Devon Kienzle; Kaela Plank; Stephen C. Noctor; Judy Van De Water; Prenatal Exposure to Autism-Specific Maternal Autoantibodies Alters Proliferation of Cortical Neural Precursor Cells, Enlarges Brain, and Increases Neuronal Size in Adult Animals.. Cerebral Cortex 2014, 26, 374-383, 10.1093/cercor/bhu291.
- Jeanelle Ariza; Jesus Hurtado; Haille Rogers; Raymond Ikeda; Michael Dill; Craig Steward; Donnay Creary; Judy Van De Water; Verónica Martínez-Cerdeño; Maternal autoimmune antibodies alter the dendritic arbor and spine numbers in the infragranular layers of the cortex. PLoS ONE 2017, 12, e0183443, 10.1371/journal.pone.0183443.
- Ester Coutinho; David A. Menassa; Leslie Jacobson; Steven J. West; Joana Domingos; Teresa C. Moloney; Bethan Lang; Paul J. Harrison; David L.H. Bennett; David M Bannerman; et al.Angela Vincent Persistent microglial activation and synaptic loss with behavioral abnormalities in mouse offspring exposed to CASPR2-antibodies in utero.. Acta Neuropathologica 2017, 134, 567-583, 10.1007/s00401-017-1751-5.
- Karen L. Jones; Michael C. Pride; Elizabeth Edmiston; Mu Yang; Jill L. Silverman; Jacqueline N. Crawley; Judy Van De Water; Autism-specific maternal autoantibodies produce behavioral abnormalities in an endogenous antigen-driven mouse model of autism. Molecular Psychiatry 2018, null, 1, 10.1038/s41380-018-0126-1.
- Noel R. Rose; Constantin Bona; Defining criteria for autoimmune diseases (Witebsky's postulates revisited). Immunology Today 1993, 14, 426-430, 10.1016/0167-5699(93)90244-f.
- David R. Hampson; Gene J. Blatt; Autism spectrum disorders and neuropathology of the cerebellum. Frontiers in Neuroscience 2015, 9, 420, 10.3389/fnins.2015.00420.
- Gillian Baird; Emily Simonoff; Andrew Pickles; Susie Chandler; Tom Loucas; David Meldrum; Tony Charman; Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP). The Lancet 2006, 368, 210-215, 10.1016/s0140-6736(06)69041-7.




