Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sylvie Babajko | + 3523 word(s) | 3523 | 2021-05-25 08:33:27 | | | |
| 2 | Dean Liu | -10 word(s) | 3513 | 2021-07-28 02:37:44 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Babajko, S. GH/IGF Axis. Encyclopedia. Available online: https://encyclopedia.pub/entry/12507 (accessed on 07 February 2026).
Babajko S. GH/IGF Axis. Encyclopedia. Available at: https://encyclopedia.pub/entry/12507. Accessed February 07, 2026.
Babajko, Sylvie. "GH/IGF Axis" Encyclopedia, https://encyclopedia.pub/entry/12507 (accessed February 07, 2026).
Babajko, S. (2021, July 27). GH/IGF Axis. In Encyclopedia. https://encyclopedia.pub/entry/12507
Babajko, Sylvie. "GH/IGF Axis." Encyclopedia. Web. 27 July, 2021.
Copy Citation
The GH/IGF axis is a major regulator of bone formation and resorption and is essential to the achievement of normal skeleton growth and homeostasis.
GH
IGF
IGFBP
animal models
age-related pathologies
tooth
enamel
dentin
dental ligament
alveolar bone
mandible
oral tissue engineering
dentin repair
orthodontic treatment
periodontal regeneration
osseointegration
bone repair
1. Introduction
GH/IGF axis or somatotropic axis consists of growth hormone (GH), insulin-like growth factors (IGFs), their receptors (GHR and IGF-1R, respectively), and IGF binding proteins (IGFBPs). GH is secreted by the anterior pituitary gland and acts directly on target tissues by its specific receptor or indirectly by up-regulating the production of IGF1 in the liver and in other target tissues. IGFs transmit their effects by IGF-1R, which is expressed in most tissues including bone and dental cells. The somatotropic axis is known for its crucial role in postnatal growth and development. More specifically, the GH/IGF axis has a strong influence on the growth and metabolism of craniofacial bones and dental tissues reviewed here.
Clinical observations report that many patients with GH dysregulations who are suffering from dwarfism or acromegaly, also have tooth and cranio-facial bone dysmorphology underlying a particular role of the GH/IGF axis in facial mineralized tissues (see [1] for review). Analysis of GHR variant phenotypes showed an association between P561T variant and the mandibular length as well as a lower face height supporting the GHR that might be a candidate gene for mandibular morphogenesis [2]. While GH/IGF/IGFBP endocrine and paracrine actions in bone are well documented for growth and ageing in axial and appendicular skeleton, the effects of these molecules have been much less investigated in the cranio-facial bones, and even less in the dento-alveolar complex where dental and bone cells are interacting (see [1][3][4][5][6] for reviews). Indeed, bone cells present specificities depending on their localization site and embryonic origin, as already reported by Kasperk et al., who showed higher mRNA levels for IGF2 in human mandibular osteoblastic cells compared to iliac osteoblastic cells [7].
Expression of the GHR by most mineralizing cells in cranio-facial bones and dental cells argue for a direct physiological role of GH in these tissues without excluding some IGF effects transmitted by the widely expressed IGF-1R and modulated by IGFBPs, that may also have additional IGF-independent effects. Among the various hormones and growth factors investigated, GH appears to be one of the most closely associated with dental maturity [8]. Recent studies have shown that GHR variants (rs6184 and rs6180) are associated with specific tooth and root dimensions [9][10], as well as with mandibular morphology [11]. Genetic polymorphisms in GHR (rs1509460) are also associated with developmental defects of enamel arguing for the GHR contribution to dental enamel synthesis and structure [12].
These data demonstrate the involvement of GH and GHR in physiological development of the dento-alveolar complex in addition to their well-known consequences when GH levels are deregulated notably on mandibular growth (prognathism or retrognathism). However, the beneficial or detrimental consequences of deregulated GH secretion on dento-alveolar complex are controversial, as reported recently for acromegaly, which does not appear to induce generalized hypertrophy of the gingiva or hypercementosis, but could protect from a severe periodontal disease by conferring more robust periodontal tissues [10][13] due to an increase in the levels of protective molecules such as bone morphogenetic protein 2 (BMP2) [14].
2. Expression and Action of GH/IGF Axis in the Dento-Alveolar Complex
First, a brief introduction of all the tissues of the dento-alveolar complex will help to better understand the interactions between the different types of cells involving GH and IGFs/IGFBPs. The dento-alveolar complex is constituted by the teeth attached to the underlying alveolar bone through the cement and the periodontal ligament (PDL) (Figure 1). The teeth are formed after initial epithelial cell proliferation and interaction with the oral mesenchyme in the place of dental lamina that determines the number of teeth. Dental development is characterized by specific stages called placodes, bud, cap, and bell (Figure 1A) that finally form the teeth with the crown corresponding to the erupted part, and the root anchored in the alveolar bone. The most external layer of the crown is the enamel that is the most mineralized tissue in living organisms, consisting of 96–97% apatite crystals and only 3% of organic matter and water. It is an avascular and non-innervated matrix. Enamel is synthesized by ameloblasts, which are epithelial cells able to change their shape and functions during the process of amelogenesis (Figure 1B) (see [15] for review). Despite important differences in dentition in mammals, the amelogenesis process is very similar with the specificity of rodent continually growing incisor that concomitantly exhibits all the stages and thus constitutes a model of choice for studies of amelogenesis (Figure 1B). First, precursor cells are proliferating in the cervical loop, then are committed and differentiate into secreting ameloblasts that secrete Enamel Matrix Proteins (EMPs), mainly amelogenins and enamelin, crucial for enamel mineralization initiation (Figure 1B and 1C). Contrary to bone mineralization, collagen 1α1 is not involved in enamel mineralization, and EMPs are degraded by proteases, mainly MMP20 and KLK4, for complete enamel mineralization (see [15][16] for reviews). The degradation of EMPs and the enamel terminal mineralization are achieved by maturation-stage ameloblasts that secrete high levels of proteases, present high levels of ion pumps and transporters at their apical side with a fine-tuned pH regulation that allow the assembly of apatite crystals for complete enamel mineralization [15]. The two main stages of amelogenesis, secretion- and maturation-stages, are characterized by specific ameloblasts with particular cell features that allow them to change their shape and function during the short and brutal transition stage. At the end of amelogenesis, ameloblasts are lost, leading to an acellular and irreparable enamel matrix. Epithelial cells localized in the radical part of the teeth form the Hertwig’s epithelial root sheath (HERS)/Malassez rest and contribute to the formation of the periodontal ligament with the cementoblasts that participate to anchor the tooth to the alveolar bone (Figure 1). Alveolar bone presents many specificities that distinguish it from all other bones (see [17] for review). Its formation is tightly associated with dental development and eruption, which means that its size, structure, and shape are dependent on teeth [18] and it may disappear when the teeth are lost. The alveolar bone protects the dental root whose development begins after the crown formation. The dental root elongation tightly involves the osteoblasts and osteoclasts of the alveolar bone, the odontoblasts and the dental pulp cells. Odontoblasts are cells in charge of the dentin formation, which underlies the enamel in the coronal part and the cement in the radicular part. The dentin protects the pulp, which ensures the vitality of the dental organ. Odontoblasts are mesenchymal cells whose differentiation is coordinated with that of ameloblasts during tooth formation to ensure coordinated dentin and enamel formation (Figure 1) (see [19] for review). Contrary to enamel forming cells that are lost after tooth eruption, the odontoblasts are present throughout life and may synthesize tertiary dentin matrix (reactionary or reparative) beneath the site of injury. Of note, the dental pulp contains the dental pulp stem cells (DPSCs), as well as the apical zone of the formed dental root contains the stem cells from apical papilla (SCAPs) (see [20] for review). Together, DPSCs and SCAPs constitute an important reservoir of stem cells in living teeth with promising properties in tissue repair and regeneration due to their ability to differentiate into epithelial and mesenchymal cells [21][22][23][24]).
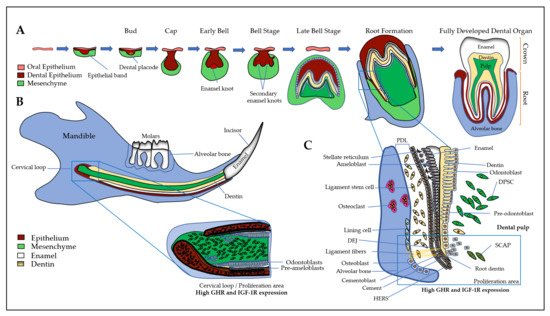
Figure 1. The dento-alveolar development. (A) Epithelial cells (in brown) interact with ecto-mesenchymal cells (in green) forming the bud, cap, and bell. The final erupted tooth is formed with the crown and the root anchored in the alveolar bone. The most external layer of the crown is the enamel (in white), which is synthesized by ameloblasts. The dentin (in yellow) is synthesized by the odontoblasts and the pulp contains DPCs, DPSCs, nerves, and vessels; (B) The rodent continually growing incisor summarizes the whole process of odontogenesis. First, precursor cells (expressing high levels of GHR and IGF-1R) are proliferating in the cervical loop, then cells differentiate either into ameloblasts that secrete enamel matrix proteins (in white), or into odontoblasts that synthesize the dentin (in yellow). At the end of amelogenesis, ameloblasts are lost, leading to an acellular and irreparable enamel matrix; (C) In human and rodent molars, the precursor cells are localized near the forming root involving odontoblasts, HERS, osteoblasts, and osteoclasts of the alveolar bone. During enamel synthesis, ameloblasts differentiate and change their shape and function. During dentin synthesis, odontoblast body cells move away from the DEJ, thus reducing the volume of the pulp chamber. The space between the dental root and the alveolar bone is formed by the fibroblasts, PDLCs and the cementoblasts lining the tooth, forming ligament fibers that attach the tooth to the bone. SCAPs are associated to the apex of a developing root, they may be recruited in case of necrotic pulp in order to complete root development and apexogenesis. DEJ: dentin- enamel junction, DPCs: dental pulp cells, DPSCs: dental pulp stem cells, GHR: growth hormone receptor, HERS: Hertwig epithelial root sheath, IGF-1R: insulin-like growth factor 1 (IGF1) receptor, PDLCs: periodontal ligament cells, SCAPs: Stem cells from the apical papilla.
Interestingly, studies report that IGF2 secreted by mesenchymal cells induces the expression of IGF-1R in mouse epithelial cell 3D cultures [25], thus contributing to the coordinated differentiation process of odontoblasts and ameloblasts. The microdissected medial part of the mouse mandibular arch of E10.5 embryos showed that IGF1 was highly expressed in the mesenchyme, IGF2 and IGF-1R were expressed in both the midline epithelium and surrounding mesenchyme, and IGFBP5 was highly expressed in the epithelium [26].
The knowledge of factors driving the development of the dento-alveolar complex and being involved in cell differentiation is crucial for the development of innovative therapeutic strategies. Among all the factors involved, the GH/IGF axis seems to play an important role in all tissues of the dento-alveolar complex.
3.1. Dental Epithelium and Enamel
GHR and IGF1 proteins are detected in the incisor and first molar of rat embryos with high signals in dental epithelial cells, suggesting the involvement of GH/IGF axis in dental development [27][28]. In addition, GH treatments enhance IGF1 expression in dental epithelial cells demonstrating their responsiveness to this hormone [29]. IGF1 and IGF-1R involvement in amelogenesis was confirmed when Joseph et al. showed a relationship between a decrease in IGF-1R expression and ameloblasts apoptosis during two key steps of amelogenesis, the transition period when ameloblasts suddenly change their shape and their function, between the secretion- and the maturation-stages, and at the end of the maturation-stage just before tooth eruption [30] (Figure 2A,B).
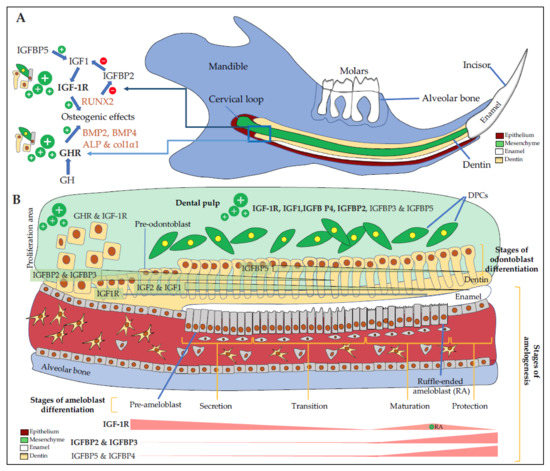
Figure 2. Expression of IGF components in the dento-alveolar complex. (A) Schematic representation of a sagittal section of a rodent hemi-mandible with the bone in blue, the teeth (3 molars and the growing incisor in white), the dental epithelium in red, the dental mesenchyme in green, and the dentin in yellow. There are many immature proliferating cells in the selected area especially in the cervical loop with cells expressing high levels of GHR and IGF-1R. Osteoblasts, odontoblasts, cementoblasts, periodontal ligament cells are responsive to GH, which increases the level of differentiation markers such as BMP2, BMP4, ALP, col1α1, and IGF1. IGFBP5 promotes IGF1 activity, which up-regulates RUNX2 expression. RUNX2 decreases IGFBP2 expression, thus promoting IGF1 action also; (B) Schematic magnification of odontogenesis. DPCs (in green) express GHR, IGF-1R, IGF1, and mostly IGFBP4 and IGFBP5 in addition to IGFBP3 and IGFBP2. In the CL, immature proliferating cells express high levels of GHR and IGF-1R. During odontoblast differentiation (yellow cells), GHR and IGF-1R, as well as IGF2 and IGF1, are decreasingly expressed during the whole differentiation process with first IGFBP2 and IGFBP3, then mainly IGFBP5. During amelogenesis, pre-ameloblasts express high levels of GHR and IGF-1R, whose expression decreases during the secretion and the transition stages. During the maturation, corresponding to enamel terminal mineralization, IGF-1R is highly expressed in RA, as well as IGFBP2 and IGFBP3, and to as lesser extend IGFBP5 and IGFBP4. IGFBP6, which inhibits IGF2 action, seems expressed in HERS only (not shown here). ALP: alkaline phosphatase, BMP2, 4: bone morphogenetic protein 2, 4, CL: cervical loop, Col1α1: Collagen 1α1, DPCs: dental pulp cells, GHR: growth hormone (GH) receptor, HERS: Hertwig epithelial root sheath, IGFBP2, 3, 4, 5: insulin-like growth factor-binding protein 2, 3, 4, 5, IGF-1R: insulin-like growth factor 1 (IGF1) receptor RA: ruffle-ended ameloblasts, RUNX2: Runt-related transcription factor 2.
In the continuously erupting rat incisor, IGF1, IGF2, IGF-1R proteins and mRNAs are detected in ameloblasts with strong signal in undifferentiated cells of the cervical loop, lower signals in secreting ameloblasts, and higher again in the maturation-stage ameloblasts in charge of enamel terminal mineralization [31]. More precisely, IGF1 and IGF2 mRNAs are detected preferentially in maturation-stage ameloblasts, with a preferential expression in ruffle-ended ameloblasts that are involved in calcium transport and pH regulation, two important parameters for enamel mineralization (see [15] for review). Similarly, in mouse, the first mandibular molars were dissected from E16 and E17 mouse embryos and placed in organ culture. Most mRNAs coding IGF system elements except IGFBP6 and probably IGFBP1 were detected by RT-PCR [32]. In this model, IGF1 treatment induces enamel matrix proteins, amelogenins, and enamelin responsible for the depth of enamel.
IGFs and IGF-1R expression during the development of human incisor tooth germs between the 7th and the 20th week of development was also investigated [33]. IGF2 is highly expressed mostly in the cervical loop of the enamel organ in highly proliferative cells and in differentiating pre-ameloblasts and pre-odontoblasts of the cusp tip region during the early and late bell stages when enamel organ acquires definitive shape. Expression patterns of investigated IGF system elements are time- and space-dependent indicators of the importance of these factors in crown morphogenesis of human incisors.
2.2. Dental Mesenchyme and Dentin
In various mouse transgenic models, GH status was found to influence the crown width, the root length, and the dentin thickness [34]. This is concordant with the expression of paracrine GH and GH receptors during tooth bud morphogenesis, and of GH receptors in the enamel organ, dental papilla, and Hertwig’s epithelial root sheath (HERS) during dentinogenesis. Based on prior studies carried out in mineralizing cells, these GH morphogenetic actions may be mediated by the induction of both bone morphogenetic proteins (essentially BMP2 and BMP4) and IGFs.
IGFBP2 is expressed in mouse dental mesenchyme but not in epithelial cells. IGFBP2, whose expression is down-regulated by the Runt-related transcription factor 2 (RUNX2) master gene, is proposed to actively contribute to the pathophysiology of odontoblasts and pulp cells [35]. RUNX2 expression is upregulated by IGF1 in osteoblasts and odontoblasts [36]. In addition to IGFBP2 and IGF1, predentin and odontoblastic processes are also stained for IGFBP3 [37]. The detailed expression patterns of IGF1, IGF-1R, IGFBP3, and IGFBP5 were examined in the mouse incisor mesenchyme [38]. IGF1 and IGF-1R are found mainly in undifferentiated dental papilla cells and preodontoblasts whereas IGFBP3 and IGFBP5 are expressed in more differentiated odontoblasts (Figure 2B). The authors concluded that IGFBP3 regulates the transition from the proliferative to differentiation stage by inhibiting the action of IGF1 on the proliferation of dental papilla cells, and that IGFBP5 plays an important role in the maintenance of the differentiated odontoblasts during tooth development.
2.3. Dental Pulp
The dental pulp contains several types of cells (e.g., fibroblasts, immune cells, DPSCs) embedded in the extracellular matrix (ECM) containing IGF system components. Human and rodent dental pulp cells (DPCs) have been shown to express all elements of the IGF system (Figure 2B).
In freshly extracted human third molars, IGF-1R presents a greater expression in pulp cells of teeth having incomplete root development [39], whereas IGF1 is preferentially expressed in dental pulp of teeth with complete root development [40]. Authors hypothesized that pulp cell proliferation is constant and not dependent on the root development stage to maintain the tolerance and the capacity of the pulp to respond to physical, chemical or mechanical aggression. This proliferative ability allows pulp cells to constantly renew themselves, maintain tissue homeostasis, or form new hard tissue as a defense mechanism.
IGFBPs are secreted by DPCs as shown by Götz et al., who detected all IGF components including IGFBP1 in human teeth [37]. This was not completely confirmed by Al-Khafaji et al., who did not detect IGFBP1, but found IGFBP4 as one of the main IGFBP expressed by DPCs [41]. Others found that DPCs express IGF1, IGFBP1, IGFBP3, IGFBP5, and IGFBP6 [42].
IGFBP3, whose expression and secretion are coordinated with that of IGFBP2, regulates the transition from the proliferative to the differentiation stage by inhibiting the action of IGF1 on the proliferation of human DPCs [43]. IGFBP5 plays an important role in the maintenance of the differentiated odontoblasts [38]. IGF-independent action of IGFBP5 might play a key role in the regulation of cell survival and apoptosis in dental pulp stem/progenitor cells following tooth injury [44]. However, the precise variations of each IGFBP levels and their corresponding roles in pathophysiological processes remain discussed as IGFBP may have IGF-dependent and -independent functions. Moreover, their limited proteolysis may either enhance or inhibit IGF functions leading to apparent controversial conclusions depending on the studies. Thus, further investigations are required to understand their roles and their possible use in innovative therapeutic strategies.
2.4. Cement and Periodontal Ligament
The periodontal ligament is constituted by different types of cells, cementoblasts sharing many features with odontoblasts and osteoblasts, epithelial rests of Malassez forming the Hertwig’s epithelial root sheath (HERS), fibroblasts, and periodontal ligament stem cells (PDLSCs) (Figure 1). HERS and the apical papilla are two embryonic structures that coordinate the entire radicular development through multiple epithelial-mesenchymal interactions. Although cementum has been poorly studied until now, the similarities between cementoblasts/cementocytes and osteoblasts/osteocytes and the numerous stresses it undergoes (mechanical forces, orthodontic forces or periapical periodontitis) lead to hypothesizing an active remodeling activity in cementum that may share similar cellular mechanisms with the Bone Remodeling Compartment (BRC). In that context, Brochado Martins et al. recently provided histological evidence of a specialized remodeling compartment in root cementum [45].
In rats, cementoblasts and odontoblasts localized at sites of new matrix formation show intense GHR immunoreactivity, whereas mature cementoblasts and odontoblasts at later stages of tooth development are nonreactive [46]. These patterns of GHR expression during odontogenesis suggest that GH may promote the functional state of these cells. The importance of GH on cellular cementum length was confirmed by Smid et al., who proposed therapeutic applications of GH to help regeneration of the periodontium based on its high responsiveness to GH [47]. The cementoblasts, osteoblasts, and PDL cells (PDLCs) responded to GH by expressing osteogenic markers, BMP2 and BMP4, BMPR1A, alkaline phosphatase (ALP), osteocalcin (OCN), and osteopontin (OPN), and by increasing the numbers of PDLCs [48]. However, while long-term treatment with GH may promote mineralization of human PDLCs and alveolar bone cells, short-term treatment does not promote proliferation of osteoblast precursors nor induce expression of late osteogenic markers [49]. IGF1, for its part, promotes not only proliferation, but also differentiation of human PDLSCs, by up-regulating the expression of RUNX2, SP7, and OCN, and by activating the phosphorylation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) arguing for large IGF1 effects transmitted by IGF-1R [50].
In human premolars, IGF-1R is expressed by periodontal fibroblasts. IGF1, IGF2, and the six IGFBPs could be detected by immunohistochemistry in the ECM of the adhering PDLCs, whereas only IGF2 could be detected in the acellular cementum [42][51]. Also of note, outer cementum layers with inserting Sharpey’s fibers reacted with all antibodies against the IGF system components except for IGFBP4 and IGFBP6. IGFBP6 and, to a lesser extent, IGFBP4 are expressed by epithelial rests of Malassez located in the periodontal ligament [51]. IGF1 was shown to induce elongation of HERS and increase cell proliferation in its outer layer illustrating IGF1 involvement in early root formation [52]. Considering the high affinity of IGFBP6 for IGF2, IGFBP6 may inhibit the mitogenic activity of IGFs present in the PDL on Malassez cells.
2.5. Alveolar Bone
In the alveolar bone of rats, osteoblasts engaged in intramembranous ossification and osteoclasts localized at sites of bone remodeling resorption are immunopositive for GHR, while osteocytes and endosteal cells are immunonegative [46]. Joseph et al. also reported a high IGF1 signal in osteoblasts and osteoclasts from rat alveolar bone [28]. These findings with those described above reporting high expression of IGFs in PDL and HERS support the notion of paracrine or autocrine functions of IGF1 in dental root development [52].
During bone formation, growth factors (including IGF1) released from the bone matrix during osteoclastic bone resorption stimulate osteoblast differentiation, thus bone remodeling. Molar root formation and tooth eruption are dependent on both (anabolic) osteoblast and (catabolic) osteoclast activities controlled by receptor activator of NF-κB ligand (RANKL) as demonstrated in RANKL -/- mice [55][56]. In these mutants, the IGF signaling pathway is down-regulated in cells involved in root elongation and this impairment is rescued by the addition of IGF1, demonstrating that dental root and eruption defect in RANKL mutant mice may result from failure of IGF1 release from bone matrix through osteoclast bone resorption.
References
- Young, W.G. Growth Hormone and Insulin-like Growth Factor-I in Odontogenesis. Int. J. Dev. Biol. 1995, 39, 263–272.
- Bayram, S.; Basciftci, F.A.; Kurar, E. Relationship between P561T and C422F Polymorphisms in Growth Hormone Receptor Gene and Mandibular Prognathism. Angle Orthod. 2014, 84, 803–809.
- Werner, H.; Katz, J. The Emerging Role of the Insulin-like Growth Factors in Oral Biology. J. Dent. Res. 2004, 83, 832–836.
- Litsas, G. Growth Hormone Therapy and Craniofacial Bones: A Comprehensive Review. Oral Dis. 2013, 19, 559–567.
- Al-Kharobi, H.; El-Gendy, R.; Devine, D.A.; Beattie, J. The Role of the Insulin-like Growth Factor (IGF) Axis in Osteogenic and Odontogenic Differentiation. Cell Mol. Life Sci. 2014, 71, 1469–1476.
- Yakar, S.; Isaksson, O. Regulation of Skeletal Growth and Mineral Acquisition by the GH/IGF-1 Axis: Lessons from Mouse Models. Growth Horm. IGF Res. 2016, 28, 26–42.
- Kasperk, C.; Wergedal, J.; Strong, D.; Farley, J.; Wangerin, K.; Gropp, H.; Ziegler, R.; Baylink, D.J. Human Bone Cell Phenotypes Differ Depending on Their Skeletal Site of Origin. J. Clin. Endocrinol. Metab. 1995, 80, 2511–2517.
- Krekmanova, L.; Carlstedt-Duke, J.; Brönnegård, M.; Marcus, C.; Gröndahl, E.; Modéer, T.; Dahllöf, G. Dental Maturity in Children of Short Stature, with or without Growth Hormone Deficiency. Eur. J. Oral Sci. 1997, 105, 551–556.
- Hikita, Y.; Yamaguchi, T.; Tomita, D.; Adel, M.; Nakawaki, T.; Katayama, K.; Maki, K.; Kimura, R. Growth Hormone Receptor Gene Is Related to Root Length and Tooth Length in Human Teeth. Angle Orthod. 2018, 88, 575–581.
- Shinde, G.R.; Mhaisekar, R.D.; Chaube, S.H.; Barad, A.N.; Bhadange, S.; Patel, H.J. Assessment of Correlation of Growth Hormone Receptor Gene with Tooth Dimensions: A CBCT and Genotyping Study. J. Pharm. Bioallied Sci. 2019, 11, S457–S462.
- Nakawaki, T.; Yamaguchi, T.; Isa, M.; Kawaguchi, A.; Tomita, D.; Hikita, Y.; Suzuki-Tomoyasu, Y.; Adel, M.; Ishida, H.; Maki, K.; et al. Growth Hormone Receptor Gene Variant and Three-Dimensional Mandibular Morphology. Angle Orthod. 2017, 87, 68–73.
- Arid, J.; Oliveira, D.B.; Evangelista, S.S.; Vasconcelos, K.R.F.; Dutra, A.L.T.; de Oliveira, S.S.; de Queiroz, A.M.; Nelson-Filho, P.; Vieira, A.R.; Küchler, E.C. Oestrogen Receptor Alpha, Growth Hormone Receptor, and Developmental Defect of Enamel. Int. J. Paediatr. Dent. 2019, 29, 29–35.
- Roumeau, S.; Thevenon, J.; Ouchchane, L.; Maqdasy, S.; Batisse-Lignier, M.; Duale, C.; Pham Dang, N.; Caron, P.; Tauveron, I.; Devoize, L. Assessment of Oro-Dental Manifestations in a Series of Acromegalic Patients, the AcroDent Study. Endocr. Connect 2020, 9, 824–833.
- BaŞÇil, S.; Turhan İyİdİr, Ö.; Bayraktar, N.; ErtÖrer, M.E.; BaŞÇil TÜtÜncÜ, N. Severe Chronic Periodontitis Is Not Common in Acromegaly: Potential Protective Role of Gingival BMP-2. Turk. J. Med. Sci. 2021.
- Lacruz, R.S.; Habelitz, S.; Wright, J.T.; Paine, M.L. Dental Enamel Formation and Implications for Oral Health and Disease. Physiol. Rev. 2017, 97, 939–993.
- Lignon, G.; de la Dure-Molla, M.; Dessombz, A.; Berdal, A.; Babajko, S. Enamel: A unique self-assembling in mineral world. Med. Sci. 2015, 31, 515–521.
- Hathaway-Schrader, J.D.; Novince, C.M. Maintaining Homeostatic Control of Periodontal Bone Tissue. Periodontol 2000 2021.
- Nassif, A.; Senussi, I.; Meary, F.; Loiodice, S.; Hotton, D.; Robert, B.; Bensidhoum, M.; Berdal, A.; Babajko, S. Msx1 Role in Craniofacial Bone Morphogenesis. Bone 2014, 66, 96–104.
- Yu, T.; Klein, O.D. Molecular and Cellular Mechanisms of Tooth Development, Homeostasis and Repair. Development 2020, 147.
- Nuti, N.; Corallo, C.; Chan, B.M.F.; Ferrari, M.; Gerami-Naini, B. Multipotent Differentiation of Human Dental Pulp Stem Cells: A Literature Review. Stem Cell Rev. Rep. 2016, 12, 511–523.
- Palma, P.J.; Martins, J.; Diogo, P.; Sequeira, D.; Ramos, J.C.; Diogenes, A.; Santos, J.M. Does Apical Papilla Survive and Develop in Apical Periodontitis Presence after Regenerative Endodontic Procedures? Appl. Sci. 2019, 9, 3942.
- Aydin, S.; Şahin, F. Stem Cells Derived from Dental Tissues. Adv. Exp. Med. Biol. 2019, 1144, 123–132.
- Diana, R.; Ardhani, R.; Kristanti, Y.; Santosa, P. Dental Pulp Stem Cells Response on the Nanotopography of Scaffold to Regenerate Dentin-Pulp Complex Tissue. Regen. Ther. 2020, 15, 243–250.
- Kang, J.; Fan, W.; Deng, Q.; He, H.; Huang, F. Stem Cells from the Apical Papilla: A Promising Source for Stem Cell-Based Therapy. Biomed. Res. Int. 2019, 2019, 6104738.
- Matsumoto, A.; Harada, H.; Saito, M.; Taniguchi, A. Induction of Insulin-like Growth Factor 2 Expression in a Mesenchymal Cell Line Co-Cultured with an Ameloblast Cell Line. In Vitro Cell Dev. Biol. Anim. 2011, 47, 675–680.
- Fujita, K.; Taya, Y.; Shimazu, Y.; Aoba, T.; Soeno, Y. Molecular Signaling at the Fusion Stage of the Mouse Mandibular Arch: Involvement of Insulin-like Growth Factor Family. Int. J. Dev. Biol. 2013, 57, 399–406.
- Joseph, B.K.; Savage, N.W.; Young, W.G.; Waters, M.J. Prenatal Expression of Growth Hormone Receptor/Binding Protein and Insulin-like Growth Factor-I (IGF-I) in the Enamel Organ. Role for Growth Hormone and IGF-I in Cellular Differentiation during Early Tooth Formation? Anat. Embryol. 1994, 189, 489–494.
- Joseph, B.K.; Savage, N.W.; Daley, T.J.; Young, W.G. In Situ Hybridization Evidence for a Paracrine/Autocrine Role for Insulin-like Growth Factor-I in Tooth Development. Growth Factors 1996, 13, 11–17.
- Joseph, B.K.; Savage, N.W.; Young, W.G.; Gupta, G.S.; Breier, B.H.; Waters, M.J. Expression and Regulation of Insulin-like Growth Factor-I in the Rat Incisor. Growth Factors 1993, 8, 267–275.
- Joseph, B.K.; Harbrow, D.J.; Sugerman, P.B.; Smid, J.R.; Savage, N.W.; Young, W.G. Ameloblast Apoptosis and IGF-1 Receptor Expression in the Continuously Erupting Rat Incisor Model. Apoptosis 1999, 4, 441–447.
- Yamamoto, T.; Oida, S.; Inage, T. Gene Expression and Localization of Insulin-like Growth Factors and Their Receptors throughout Amelogenesis in Rat Incisors. J. Histochem. Cytochem. 2006, 54, 243–252.
- Catón, J.; Bringas, P.; Zeichner-David, M. IGFs Increase Enamel Formation by Inducing Expression of Enamel Mineralizing Specific Genes. Arch Oral Biol. 2005, 50, 123–129.
- Kero, D.; Cigic, L.; Medvedec Mikic, I.; Galic, T.; Cubela, M.; Vukojevic, K.; Saraga-Babic, M. Involvement of IGF-2, IGF-1R, IGF-2R and PTEN in Development of Human Tooth Germ-an Immunohistochemical Study. Organogenesis 2016, 12, 152–167.
- Smid, J.R.; Rowland, J.E.; Young, W.G.; Coschigano, K.T.; Kopchick, J.J.; Waters, M.J. Mouse Molar Dentin Size/Shape Is Dependent on Growth Hormone Status. J. Dent. Res. 2007, 86, 463–468.
- Greene, S.L.; Mamaeva, O.; Crossman, D.K.; Lu, C.; MacDougall, M. Gene-Expression Analysis Identifies IGFBP2 Dysregulation in Dental Pulp Cells From Human Cleidocranial Dysplasia. Front Genet 2018, 9, 178.
- Yu, Y.; Mu, J.; Fan, Z.; Lei, G.; Yan, M.; Wang, S.; Tang, C.; Wang, Z.; Yu, J.; Zhang, G. Insulin-like Growth Factor 1 Enhances the Proliferation and Osteogenic Differentiation of Human Periodontal Ligament Stem Cells via ERK and JNK MAPK Pathways. Histochem. Cell Biol. 2012, 137, 513–525.
- Götz, W.; Heinen, M.; Lossdörfer, S.; Jäger, A. Immunohistochemical Localization of Components of the Insulin-like Growth Factor System in Human Permanent Teeth. Arch Oral Biol. 2006, 51, 387–395.
- Aizawa, C.; Saito, K.; Ohshima, H. Regulation of IGF-I by IGFBP3 and IGFBP5 during Odontoblast Differentiation in Mice. J. Oral Biosci. 2019, 61, 157–162.
- Caviedes-Bucheli, J.; Muñoz, H.R.; Rodríguez, C.E.; Lorenzana, T.C.; Moreno, G.C.; Lombana, N. Expression of Insulin-like Growth Factor-1 Receptor in Human Pulp Tissue. J. Endod. 2004, 30, 767–769.
- Caviedes-Bucheli, J.; Canales-Sánchez, P.; Castrillón-Sarria, N.; Jovel-Garcia, J.; Alvarez-Vásquez, J.; Rivero, C.; Azuero-Holguín, M.M.; Diaz, E.; Munoz, H.R. Expression of Insulin-like Growth Factor-1 and Proliferating Cell Nuclear Antigen in Human Pulp Cells of Teeth with Complete and Incomplete Root Development. Int. Endod. J. 2009, 42, 686–693.
- Al-Khafaji, H.; Noer, P.R.; Alkharobi, H.; Alhodhodi, A.; Meade, J.; El-Gendy, R.; Oxvig, C.; Beattie, J. A Characteristic Signature of Insulin-like Growth Factor (IGF) Axis Expression during Osteogenic Differentiation of Human Dental Pulp Cells (HDPCs): Potential Co-Ordinated Regulation of IGF Action. Growth Horm IGF Res. 2018, 42–43, 14–21.
- Götz, W.; Kunert, D.; Zhang, D.; Kawarizadeh, A.; Lossdörfer, S.; Jäger, A. Insulin-like Growth Factor System Components in the Periodontium during Tooth Root Resorption and Early Repair Processes in the Rat. Eur. J. Oral Sci. 2006, 114, 318–327.
- Alkharobi, H.; Alhodhodi, A.; Hawsawi, Y.; Alkafaji, H.; Devine, D.; El-Gendy, R.; Beattie, J. IGFBP-2 and -3 Co-Ordinately Regulate IGF1 Induced Matrix Mineralisation of Differentiating Human Dental Pulp Cells. Stem Cell Res. 2016, 17, 517–522.
- Saito, K.; Ohshima, H. The Putative Role of Insulin-like Growth Factor (IGF)-Binding Protein 5 Independent of IGF in the Maintenance of Pulpal Homeostasis in Mice. Regen. Ther. 2019, 11, 217–224.
- Brochado Martins, J.F.; Rodrigues, C.F.D.; Nunes, P.D.; Paulo, S.; Palma, P.J.; do Vale, F.F. Remodelling Compartment in Root Cementum. Folia Morphol. 2020.
- Zhang, C.Z.; Young, W.G.; Li, H.; Clayden, A.M.; Garcia-Aragon, J.; Waters, M.J. Expression of Growth Hormone Receptor by Immunocytochemistry in Rat Molar Root Formation and Alveolar Bone Remodeling. Calcif. Tissue Int. 1992, 50, 541–546.
- Smid, J.R.; Rowland, J.E.; Young, W.G.; Daley, T.J.; Coschigano, K.T.; Kopchick, J.J.; Waters, M.J. Mouse Cellular Cementum Is Highly Dependent on Growth Hormone Status. J. Dent. Res. 2004, 83, 35–39.
- Li, H.; Bartold, P.M.; Young, W.G.; Xiao, Y.; Waters, M.J. Growth Hormone Induces Bone Morphogenetic Proteins and Bone-Related Proteins in the Developing Rat Periodontium. J. Bone Miner Res. 2001, 16, 1068–1076.
- Haase, H.R.; Ivanovski, S.; Waters, M.J.; Bartold, P.M. Growth Hormone Regulates Osteogenic Marker MRNA Expression in Human Periodontal Fibroblasts and Alveolar Bone-Derived Cells. J. Periodont. Res. 2003, 38, 366–374.
- Li, X.; Yao, J.; Wu, J.; Du, X.; Jing, W.; Liu, L. Roles of PRF and IGF-1 in Promoting Alveolar Osteoblast Growth and Proliferation and Molecular Mechanism. Int. J. Clin. Exp. Pathol. 2018, 11, 3294–3301.
- Götz, W.; Lossdörfer, S.; Krüger, U.; Braumann, B.; Jäger, A. Immunohistochemical Localization of Insulin-like Growth Factor-II and Its Binding Protein-6 in Human Epithelial Cells of Malassez. Eur. J. Oral Sci. 2003, 111, 26–33.
- Fujiwara, N.; Tabata, M.J.; Endoh, M.; Ishizeki, K.; Nawa, T. Insulin-like Growth Factor-I Stimulates Cell Proliferation in the Outer Layer of Hertwig’s Epithelial Root Sheath and Elongation of the Tooth Root in Mouse Molars in Vitro. Cell Tissue Res. 2005, 320, 69–75.
- Liu, D.; Wang, Y.; Jia, Z.; Wang, L.; Wang, J.; Yang, D.; Song, J.; Wang, S.; Fan, Z. Demethylation of IGFBP5 by Histone Demethylase KDM6B Promotes Mesenchymal Stem Cell-Mediated Periodontal Tissue Regeneration by Enhancing Osteogenic Differentiation and Anti-Inflammation Potentials. Stem Cells 2015, 33, 2523–2536.
- Han, N.; Zhang, F.; Li, G.; Zhang, X.; Lin, X.; Yang, H.; Wang, L.; Cao, Y.; Du, J.; Fan, Z. Local Application of IGFBP5 Protein Enhanced Periodontal Tissue Regeneration via Increasing the Migration, Cell Proliferation and Osteo/Dentinogenic Differentiation of Mesenchymal Stem Cells in an Inflammatory Niche. Stem Cell Res. Ther. 2017, 8, 210.
- Huang, H.; Wang, J.; Zhang, Y.; Zhu, G.; Li, Y.-P.; Ping, J.; Chen, W. Bone Resorption Deficiency Affects Tooth Root Development in RANKL Mutant Mice Due to Attenuated IGF-1 Signaling in Radicular Odontoblasts. Bone 2018, 114, 161–171.
- Gama, A.; Vargas-Franco, J.W.; Sánchez Mesa, D.C.; Restrepo Bedoya, E.; Amiaud, J.; Babajko, S.; Berdal, A.; Acevedo, A.C.; Heymann, D.; Lézot, F.; et al. Origins of Alterations to Rankl Null Mutant Mouse Dental Root Development. Int. J. Mol. Sci. 2020, 21, 2201.
More
Information
Subjects:
Cell Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
846
Revisions:
2 times
(View History)
Update Date:
28 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




