Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carmen Cuadrado | + 2697 word(s) | 2697 | 2021-08-13 08:15:08 | | | |
| 2 | Camila Xu | + 448 word(s) | 3145 | 2021-09-06 10:57:20 | | | | |
| 3 | Camila Xu | -211 word(s) | 2486 | 2021-09-06 11:00:24 | | | | |
| 4 | Camila Xu | -211 word(s) | 2486 | 2021-09-06 11:01:27 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cuadrado, C. Nut Allergenicity. Encyclopedia. Available online: https://encyclopedia.pub/entry/13917 (accessed on 07 February 2026).
Cuadrado C. Nut Allergenicity. Encyclopedia. Available at: https://encyclopedia.pub/entry/13917. Accessed February 07, 2026.
Cuadrado, Carmen. "Nut Allergenicity" Encyclopedia, https://encyclopedia.pub/entry/13917 (accessed February 07, 2026).
Cuadrado, C. (2021, September 06). Nut Allergenicity. In Encyclopedia. https://encyclopedia.pub/entry/13917
Cuadrado, Carmen. "Nut Allergenicity." Encyclopedia. Web. 06 September, 2021.
Copy Citation
Nut allergenic proteins are characterized by their resistance to denaturation and proteolysis. Food processing is proposed as a tool to modify the allergenicity of nuts, to ensure their safety and to improve their organoleptic properties. The effect of processing (conventional and novel methods) on nut allergenicity is variable by abolishing existing epitopes or generating neoallergens.
allergens
food hypersensitivity
nuts
thermal processing
pressure processing
enzymatic digestion
1. Introduction
Currently, the main cause of anaphylactic reactions in Western countries is food allergies. It has been estimated that food allergies affect between 1 and 3% of the general population and up to 8% of children. Food allergies cause more than 30,000 anaphylactic reactions in the US [1]. In Europe, food allergies are the leading cause of anaphylaxis, and between 10 and 18% of anaphylactic reactions occur at school [2]. The list containing the 14 most allergenic foods in the European Union includes peanuts and tree nuts. In accordance with Regulation (EU) No. 1169/2011, the presence of nuts must be indicated on food labels [3]. After fruit, peanuts and tree nuts are the most prevalent cause of allergic reactions in Spain (26%) [4]. However, nuts are increasingly consumed in the last years due to their health benefits, which are attributable to their high content of protein, unsaturated fatty acids, vitamins and antioxidants [5].
Nut allergen proteins belong to seed storage proteins, such as legumin (11–13S globulin composed of acidic subunits of 30–40 kDa and basic 15–20 kDa), vicilin (7S globulin of approximately 50–60 kDa) and 2S albumin (15 kDa) [6]. Nut allergenic proteins are characterized by their resistance to denaturation and proteolysis [7]. Other nut allergens, such as pathogenesis-related (PR) proteins, profilins and lipid transfer proteins (LTP), are considered to be panallergens because they contribute to the allergenicity of a large group of seeds, pollen, nuts, fruit and other plants [8].
Food processing is used in the industry to ensure safety and to enhance organoleptic properties, in addition to altering food allergenicity. Food processing can modify the structure, properties and function of proteins, and as a result, the IgE-binding capacity of allergens can be affected. As some processing treatments have been shown to decrease the allergenicity of certain foods, food processing may play an important role in developing hypoallergenic foods and using them for food tolerance induction. Other processes, however, can increase the allergenicity of some foods [9]. Heat treatment modifies the structure of proteins, and therefore, epitopes and their immunogenic potential can be affected. This effects depends on both technological parameters and the type of matrix [10][11]. The effect of processing on allergenicity is variable and, as such, new allergenic compounds can be generated, while existing reactive epitopes can also be damaged or destroyed [12][13][14][15]. The structural changes produced using treatments such as boiling, microwave heating and pressure-cooking, and their effects on legume and nut allergenicity, have been evaluated. Importantly, findings indicate that heat- or pressure-based processing reduces IgE-binding capacity [12][13][14][15][16][17][18].
2. Most Prevalent Allergenic Nuts and Their Predominant Allergens
Nuts are a rich source of protein and other nutritional compounds with functional properties. This promotes their presence in manufactured foods. In Europe, tree nut allergies are common [19], with a hazelnut allergy being the most prevalent tree nut allergy. In the US, peanuts and tree nuts, such as almonds, walnuts or cashews, seem to be more common allergenic sources [20].
Table 1 summarizes the main nut allergens. Several hazelnut proteins have been described as allergens: Cor a 1 (Bet v 1 homologue), Cor a 2 (profilin), Cor a 8 (lipid transfer protein (LPT)), Cor a 9 (11S legumin), Cor a 11 (7S vicilin), Cor a 14 (2S albumin) and the oleosins Cor a 12, Cor a 13 and Cora a 15 [21]. Ana o 1 (7S vicilin) [22], Ana o 2 (11S legumin) [23] and Ana o 3 (2S albumin) have been identified and characterized as cashew allergens [24]. Pistachios are also well characterized for their allergenic potential and display high cross-reactivity with cashews and mangoes [25]. The five major allergens in pistachios are one 2S albumin (Pis v 1), two 11S legumins (Pis v 2 and 5), one 7S vicilin (Pis v 3) and one superoxide dismutase (Pis v 4) [25][26][27][28]. Most of the epitopic regions of Pis v 1 and Pis v 3 showed a high degree of similarity with the Ana o 1, Ana o 2 and Ana o 3 epitopes. This is considered to be the molecular basis for the IgE-binding cross-reactivity observed between pistachios and cashews [29]. In almonds, six allergenic proteins have been characterized: Pru du 3 (LTP), Pru du 4 (profilin), Pru du 5 (60S ribosomal protein), Pru du 6 (11S legumin), Pru du 8 (antimicrobial seed storage protein) and Pru du 10 (mandelonitrile lyase 2) [30]. Thus far, five allergenic proteins have been identified in walnuts: Jug r 1 (2S albumin), Jug r 2 (7S vicilin), Jug r 3 (LTP), Jug r 4 (11S legumin) and Jug r 5 (profilin) [31]. The major allergen in walnuts is Jug r 4, which is highly homologous with other 11S globulin allergens, such as Cor a 9 (hazelnut), Ana o 2 (cashew) and Ara h 3 (peanut), explaining their IgE cross-reactivity [31].
Table 1. Allergens in nuts (WHO/IUIS Allergen Nomenclature Subcommittee).
| Source | Allergen | Protein Family | MW* (kDa) |
|---|---|---|---|
| Hazelnut (Corylus avellana) |
Cor a 1 Cor a 2 Cor a 8 Cor a 9 Cor a 11 Cor a 12 Cor a 13 Cor a 14 Cor a 15 |
Pathogen-related protein (PR10) Profilin Non-specific lipid transfer protein (LTP) 11S globulin/legumin 7S globulin/vicilin Oleosin Oleosin 2S albumin Oleosin |
17 14 9 40 47 17 14–16 16 17 |
| Cashew (Anacardium occidentale) |
Ana o 1 Ana o 2 Ana o 3 |
Vicilin-like protein Legumin-like protein 2S albumin |
50 55 14 |
| Pistachio (Pistachia vera) |
Pis v 1 Pis v 2 Pis v 3 Pis v 4 Pis v 5 |
2S albumin 11S globulin/legumin 7S globulin/vicilin Manganese Superoxide dismutase 11S globulin/legumin |
7 32 55 25.7 36 |
| Almond (Prunus dulcis) |
Pru du 3 Pru du 4 Pru du 5 Pru du 6 Pru du 8 Pru du 10 |
Non-specific lipid transfer protein 1(LTP) Profilin 60S acidic ribosomal protein P2 11S globulin/legumin Antimicrobial seed storage protein Mandelonitrile lyase 2 |
9 14 10 60 31 60 |
| Walnut (Juglans regia) |
Jug r 1 Jug r 2 Jug r 3 Jug r 4 Jug r 5 |
2S albumin 7S globulin/vicilinNon-specific lipid transfer protein (LTP) 11S globulin/legumin Profilin |
15 44 9 36 20 |
| Brazil nut (Bertholletia excelsa) |
Ber e 1 Ber e 2 |
2S sulfur-rich albumin 11S globulin |
9 29 |
| Chestnut (Castanea sativa) |
Cas s 5 Cas s 8 Cas s 9 |
Chitinase Non-specific lipid transfer protein 1 Cytosolic class I small heat shock protein |
12–13 17 |
MW*: molecular weight.
A study reported that the prevalence of tree nut allergies in the US was greater than 1.1% and was more common in individuals under 18 years of age [20]. In 2005, the European Commission funded a large Europe-wide research project (EuroPrevall) designed to evaluate and provide a broad overview of the prevalence, basis and cost of food allergies. For this project, 56 partners from 19 European countries collaborated [32]. Studies involved community surveys, birth cohort studies and clinical studies using double-blind placebo-controlled food challenges (DBPCFC) and SPT. The project provided knowledge about the prevalence of food allergies, as well as the ranking of allergenic foods (food groups) as a function of the number of reactions they provoke both in the overall population and in specific population groups (regarding age and geographic location). In this context, Lyons et al. [33] reported that the highest prevalence of nut allergies was estimated for hazelnut (4%) in accordance with challenge tests and sensitization assessed by SPT. Nut allergies appear to affect adults and adolescents more, probably due to their late introduction into the diet [34]. Hazelnuts are widely consumed in Europe and presented a high prevalence of positive reactions in a double blind, placebo-controlled food challenge (DBPCFC) [19]. In the US, 7.5% of the total of 188 participants were found to be allergic to hazelnuts in a 11-year follow-up study [35]. The effects of variety showed no influence on the allergenicity of hazelnuts, with Cor a 9 and Cor a 1 being the predominant IgE-binding proteins in 13 European varieties [36]. Cashews are the second most allergic nut and a significant health problem in the US [37]. The anaphylactic reactions to cashew are, often, severe clinical manifestations, and even more dangerous than with peanuts. Cashew nuts are consumed all over the world due to their beneficial effects on human health, but they have also been reported to cause allergic reactions in sensitized patients [38]. In a study by Rance et al. [39], it was concluded that 2-year-old infants are more at risk among children sensitive to cashew nuts. The reactions were triggered in three-quarters of cases at first exposure. A possible explanation for this finding is that there is a correlation between earlier exposure to cashew nuts and a greater cashew nut allergy [40]. The symptoms upon the ingestion of cashews were also reported to be more severe compared to those caused due to exposure to other food allergens, such as nuts and peanuts [38]. Pistachio nuts are widely consumed all over the world and primarily produced in Iran and the United States. However, they have also been observed to cause significant allergic reactions in people sensitive to the allergens Pis v 1, Pis v 2 and Pis v 3 [41]. Sicherer et al. [35] estimated an allergy to pistachios in approximately 10% of the individuals evaluated. Almonds can provide many health benefits due to their low glycemic index and being a source of vitamin E and energy, manganese, fiber, protein and various polyphenolic components [5]. Almonds are ranked third after walnuts and cashews in eliciting allergic reactions [35], although some almond-allergic patients tend to pass oral food challenges, probably because many profilin-sensitized patients do not exhibit symptoms [30]. Amandin, or Almond Major Protein, is primarily responsible for IgE-mediated immunoreactivity. Walnuts are considered to be responsible for the highest number of allergic reactions in sensitized subjects [31], but they have health benefits, such as reducing the risk of diabetes and cardio-vascular diseases [5].
3. Food Processing
The reasons for consuming processed foods include ensuring preservation and safety; improving quality, such as flavor, color and taste; convenience; variety; out of season availability; and lack of equipment, time or skills needed for home food preparation of certain foods. A significant quantity of food is processed at home by consumers, in addition to industrial food processing and in institutional settings. The type of processing method can be chosen by product type, scale of processing, available infrastructure, consumer preferences and product sensory qualities. These reasons explain that the same raw food can be often processed differently [42]. As the majority of foods are usually consumed after processing, it was also relevant to understand the protein characteristics which are influenced by processing, such as stability against heat and pressure, as well as mechanical and chemical activities [43]. Thermal processing methods, such as boiling, frying, roasting, baking, pressure cooking or microwave heating, are applied to certain food products before consumption to improve their suitability for specific applications [44]. Non-thermal food processing, such as high hydrostatic pressure (HHP) or enzymatic treatment, can be applied to some foods. These processing treatments can modify the biochemical characteristics of proteins or generate chemical reactions within the food matrix components.
4. Effect of Thermal Processing on Food Allergens
Thermal and non-thermal processing methods are applied to foods to improve their preservation, quality, safety and suitability for specific product applications. The processing can affect the solubility, digestibility and other related parameters. Through thermal treatments, proteins can form oligomers, or become aggregated, denatured, fragmented or re-assembled, and these modifications can produce a decreased solubility [45]. The overall IgE-binding capacity of a particular extract can be more or less antigenic or result in new allergens (neoallergens) as a consequence of heat processing [46]. Thermal processing analysis is, therefore, necessary for assessing the allergenicity of existing and newly introduced foods [11]. The influence of heat processing mainly depends on the temperature and time conditions used. In addition, interactions with other food matrix constituents can affect the structure of a protein. Generally, the loss of a secondary structure occurs when the temperature is around 70–80 °C, while at 80–90 °C, rearrangements of disulfide bonds and the formation of new bonds take place. Aggregation occurs at higher temperatures (90–100 °C) [47].
The influence of a wide variety of treatments on the allergenicity of nuts has been studied (Figure 1, Table 2) [44][48][49]. Thermal treatments in walnuts [17]; HHP in hazelnuts [50]; and roasting, blanching, autoclaving and microwave heating in almonds [51] have been analyzed, and the results differ depending on the material and the conditions of the treatments studied. Studies with walnuts have indicated that the digestibility of protein probably increases after heat treatment, so the absorption of the protein can also increase in the gastrointestinal tract, and due to this, the possibility of allergens eliciting an allergenic response decreases [52]. However, in some cases, thermal processing may cause some neoantigens that were not originally present to form or the digestibility of a particular allergen may be reduced. These neoantigens may present an additional problem, and these facts can enhance the allergenic manifestation in sensitized patients. The formation of some neoantigens can be produced by the Maillard reaction when sugar residues interact with proteins during heating, generating sugar-conjugated protein derivatives, which enhance the allergenicity of some proteins, such as 2S albumins [53]. However, glycation and aggregation from the Maillard reaction reduced the Ig E binding capacity of legumins and did not affect the IgE-binding capacity of vicilins and nsLTP [43]
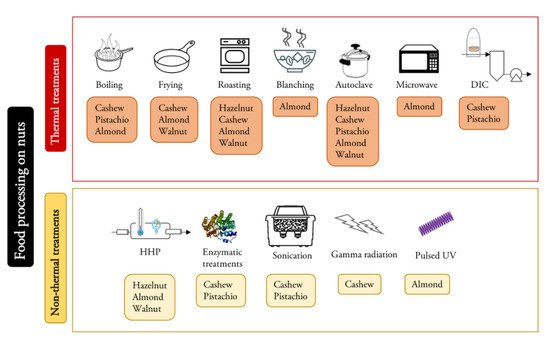
Figure 1. Summary of food processing methods and their applications in the reviewed nuts.
Table 2. Effects of processing on IgE immunoreactivity of nut allergens.
| Source | Processing Conditions | IgE Reactivity * | Reference |
|---|---|---|---|
| Hazelnut | Roasting 140 °C, 40 min | ↓ | Hansen et al. [54] |
| Roasting 144 °C, time not indicated | ↓ | Worm et al. [55] | |
| Autoclaving 138 °C, 15 and 30 min | ↓↓ | Lopez et al. [56]; Cuadrado et al. [57] | |
| HHP 300–600 MPa, 15 min | = | Prieto et al. [50]; Cuadrado et al. [57] | |
| Cashew | Boiling, 100 °C, 30 and 60 min | = (~↓) | Cuadrado et al. [14]; Sanchiz et al. [15] |
| Boiling, 100 °C, 15 min + sodium sulfite | ~↓ | Mattison et al. [58] | |
| Frying, 191 °C, 1 min | = (~↓) | Su et al. [59] | |
| Roasting 200 °C, 15 min | ~↑ | Venkatachalam et al. [60] | |
| Autoclaving 138 °C, 15 and 30 min | ↓ | Cuadrado et al. [14]; Sanchiz et al. [15] | |
| Autoclaving 138 °C, 30 min + Amano 3DS 120 min | ↓↓ | Cuadrado et al. [14] | |
| DIC 7 bar, 2 min | ↓↓ | Vicente et al. [61] | |
| Pistachio | Boiling, 100 °C, 30 and 60 min | = (~↓) | Cuadrado et al. [14]; Sanchiz et al. [15] |
| Steaming | ~↓ | Noorbakhsh et al. [62]; | |
| Autoclaving 138 °C, 15 and 30 min | ↓ | Cuadrado et al. [14]; Sanchiz et al. [15] | |
| Autoclaving 138 °C, 30 min + Amano SD 60 min | ↓↓ | Cuadrado et al. [14] | |
| DIC 7 bar, 2 min | ↓↓ | Vicente et al. [61] | |
| Almond | Boiling, 100 °C, 5 and 10 min | = | Su et al. [59] |
| Frying, 191 °C, 1 min | = (~↓) | Su et al. [59] | |
| Roasting 180 °C, 15 min | = | Su et al. [59] | |
| Autoclaving 121 °C, 30 min | = | Venkatachalam et al. [51] | |
| Autoclaving 138 °C, 15 and 30 min | ↓↓ | Cuadrado et al. [57] | |
| HHP 300–600 MPa, 15 min | = | Cuadrado et al. [57] | |
| Walnut | Frying, 191°C, 1 min | = (~↓) | Su et al. [59] |
| Roasting 160 °C, 30 min; 177 °C, 12 min | = | Su et al. [59] | |
| Autoclaving 138 °C, 15 and 30 min | ↓↓ | Cabanillas et al. [17]. | |
| HHP 300–600 MPa, 15 min | = | Cabanillas et al. [17]. |
* IgE reactivity determined by different techniques and conditions. Pictography: =, ↓, ↑, ~, are a symbolic representation of the global effect of the specific treatment on the IgE reactivity of a given food (=, similar; ↑, increase; ↓, decrease; ~ ↑, slight increase; ~↓, slight decrease).
The IgE recognizes and interacts with epitopes belonging to allergenic proteins.
Two types of IgE binding epitopes are possible, either linear or conformational ones. In linear epitopes, amino acid is arranged in linear order in the polypeptide chain, while in the conformational epitopes, amino acids are far apart in the primary sequence but may come together during the folding of the polypeptide chain. Linear epitopes may be more problematic as compared to the conformational ones, as they are mostly resistant to heat treatment. Thermal processing mainly affects conformational epitopes as the bonds can be broken down due to heat. Refolding allows the formation of native conformational epitopes, but few new allergens may be formed, which requires further efforts to minimize the risk associated with neoantigens [63]. Thus, thermal processing can strongly alter the structure, function and allergenicity of foods.
References
- Jerschow, E.; Lin, R.Y.; Scaperotti, M.M.; McGinn, A.P. Fatal anaphylaxis in the United States, 1999–2010: Temporal patterns and demographic associations. J. Allergy Clin. Immunol. 2014, 134, 1318–1328.e7.
- Muraro, A.; Agache, I.; Clark, A.; Sheikh, A.; Roberts, G.; Akdis, C.A.; Borrego, L.M.; Higgs, J.; Hourihane, J.O.; Jorgensen, P.; et al. EAACI food allergy and anaphylaxis guidelines: Managing patients with food allergy in the community. Allergy 2014, 69, 1046–1057.
- Regulation (EU) 1169/2011EC of the European Parliament and of the Council of 25 October 2011on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council; Official Journal of the European Union: Bruxelles, Belgium, 2011; pp. 18–43.
- Fernandez Rivas, M. Food allergy in Alergologica-2005. J. Investig. Allergol. Clin. Immunol. 2009, 2, 37–44.
- Ros, E. Health benefits of nut consumption. Nutrients 2010, 2, 652–682.
- Crespo, J.F.; James, J.M.; Fernandez-Rodriguez, C.; Rodriguez, J. Food allergy: Nuts and tree nuts. Br. J. Nutr. 2006, 96, S95–S102.
- Roux, K.H.; Teuber, S.S.; Sathe, S.K. Tree nut allergens. Int. Arch. Allergy Immunol. 2003, 131, 234–244.
- Radauer, C.; Breiteneder, H. Evolutionary biology of plant food allergens. J. Allergy Clin. Immunol. 2007, 120, 518.
- Cabanillas, B.; Novak, N. Effects of daily food processing on allergenicity. Crit. Rev. Food Sci. Nutr. 2019, 59, 31–42.
- Mills, C.; Johnson, P.E.; Zuidmeer-Jongejan, L.; Critenden, R.; Wal, J.M.; Asero, R. Effect of Processing on the Allergenicity of Foods. In Risk Management for Food Allergy; Elsevier: Amsterdam, The Netherlands, 2013; pp. 227–251.
- Wal, J.M. Thermal processing and allergenicity of foods. Allergy 2003, 58, 727–729.
- Álvarez-Álvarez, J.; Guillamón, E.; Crespo, J.F.; Cuadrado, C.; Burbano, C.; Rodríguez, J.; Fernández, C.; Muzquiz, M. Effects of extrusion, boiling, autoclaving, and microwave heating on lupine allergenicity. J. Agric. Food Chem. 2005, 53, 1294–1298.
- Cuadrado, C.; Cabanillas, B.; Pedrosa, M.M.; Varela, A.; Guillamon, E.; Muzquiz, M.; Crespo, J.F.; Rodriguez, J.; Burbano, C. Influence of thermal processing on IgE reactivity to lentil and chickpea proteins. Mol. Nutr. Food Res. 2009, 53, 1462–1468.
- Cuadrado, C.; Cheng, H.; Sanchiz, A.; Ballesteros, I.; Easson, M.; Grimm, C.C.; Dieguez, M.C.; Linacero, R.; Burbano, C.; Maleki, S.J. Influence of enzymatic hydrolysis on the allergenic reactivity of processed cashew and pistachio. Food Chem. 2018, 241, 372–379.
- Sanchiz, A.; Cuadrado, C.; Dieguez, M.C.; Ballesteros, I.; Rodriguez, J.; Crespo, J.F.; Cuevas, N.L.; Rueda, J.; Linacero, R.; Cabanillas, B.; et al. Thermal processing effects on the IgE-reactivity of cashew and pistachio. Food Chem. 2018, 245, 595–602.
- Cabanillas, B.; Maleki, S.J.; Rodriguez, J.; Burbano, C.; Muzquiz, M.; Aranzazu Jimenez, M.; Pedrosa, M.M.; Cuadrado, C.; Crespo, J.F. Heat and pressure treatments effects on peanut allergenicity. Food Chem. 2012, 132, 360–366.
- Cabanillas, B.; Maleki, S.J.; Rodriguez, J.; Cheng, H.; Teuber, S.S.; Wallowitz, M.L.; Muzquiz, M.; Pedrosa, M.M.; Linacero, R.; Burbano, C.; et al. Allergenic properties and differential response of walnut subjected to processing treatments. Food Chem. 2014, 157, 141–147.
- Cabanillas, B.; Pedrosa, M.M.; Rodriguez, J.; Muzquiz, M.; Maleki, S.J.; Cuadrado, C.; Burbano, C.; Crespo, J.F. Influence of Enzymatic Hydrolysis on the Allergenicity of Roasted Peanut Protein Extract. Int. Arch. Allergy Immunol. 2012, 157, 41–50.
- Ortolani, C.; Ballmer-Weber, B.K.; Hansen, K.S.; Ispano, M.; Wüthrich, B.; Bindslev-Jensen, C. Hazelnut allergy: A double- blind, placebo-controlled food challenge multicenter study. J. Allergy Clin. Immunol. 2000, 105, 577–581.
- Weinberger, T.; Sicherer, S. Current perspectives on tree nut allergy: A review. J. Asthma Allergy 2018, 11, 41–51.
- Costa, J.; Mafra, I.; Carrapatoso, I.; Oliveira, M.B.P.P. Hazelnut Allergens: Molecular Characterization, Detection, and Clinical Relevance. Crit. Rev. Food Sci. Nutr. 2016, 56, 2579–2605.
- Wang, F.; Robotham, J.M.; Teuber, S.S.; Tawde, P.; Sathe, S.K.; Roux, K.H. Ana o 1, a cashew (Anacardium occidentale L.) allergen of the vicilin seed storage protein family. J. Allergy Clin. Immunol. 2002, 110, 160–166.
- Wang, F.; Robotham, J.M.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Ana o 2, a major cashew (Anacardium occidentale L.) nut allergen of the legumin family. Int. Arch. Allergy Immunol. 2003, 132, 27–39.
- Robotham, J.M.; Wang, F.; Seamon, V.; Teuber, S.S.; Sathe, S.K.; Sampson, H.A.; Beyer, K.; Seavy, M.; Roux, K.H. Ana o 3, an important cashew nut (Anacardium occidentale L.) allergenof the 2S albumin family. J. Allergy Clin. Immunol. 2005, 115, 1284–1290.
- Noorbakhsh, R.; Mortazavi, S.A.; Sankian, M.; Shahidi, F.; Tehrani, M.; Azad, F.J.; Behmanesh, F.; Varasteh, A. Pistachio allergy-prevalence and in vitro cross-reactivity with other nuts. Allergol. Int. 2011, 60, 425–432.
- Ahn, K.; Bardina, L.; Grishina, G.; Beyer, K.; Sampson, H.A. Identification of two pistachio allergens, Pis v 1 and Pis v 2, belonging to the 2S albumin and 11S globulin family. Clin. Exp. Allergy 2009, 39, 926–934.
- Ayuso, R.; Grishina, G.; Ahn, K. Identification of a MnSOD-like protein as a new major pistachio allergen. J. Allergy Clin. Immunol. 2007, 119, s115.
- Willison, L.N.; Tawde, P.; Robotham, J.M.; Penney, R.M.; Teuber, S.S.; Sathe, S.K.; Roux, K.H. Pistachio vicilin, Pis v 3, is immunoglobulin E-reactive and cross-reacts with the homologous cashew allergen, Ana o 1. Clin. Exp. Allergy 2008, 38, 1229–1238.
- Barre, A.; Nguyen, C.; Granier, C.; Benoist, H.; Rougé, P. IgE-Binding Epitopes of Pis v 1, Pis v 2 and Pis v 3, the Pistachio (Pistacia vera) Seed Allergens. Allergies 2021, 1, 63–91.
- Costa, J.; Mafra, I.; Carrapatoso, I.; Oliveira, M.B. Almond allergens: Molecular characterization, detection, and clinical relevance. J. Agric. Food Chem. 2012, 60, 1337–1349.
- Costa, J.; Carrapatoso, I.; Oliveira, M.B.; Mafra, I. Walnut allergens: Molecular characterization, detection and clinical relevance. Clin. Exp. Allergy 2014, 44, 319–341.
- Mills, C.; Mackie, A.; Burney, P.; Beyer, K.; Frewer, L.; Madsen, C.; Botjes, E.; Crevel, R.; van Ree, R. The prevalence, cost and basis of food allergy across Europe. Allergy 2007, 62, 717–722.
- Lyons, S.; Clausen, M.; Knulst, A.; Ballmer-Weber, B.; Fernandez-Rivas, M.; Barreales, L.; Bieli, C.; Dubakiene, R.; Pérez, C.; Jędrzejczak-Czechowicz, M.; et al. Prevalence of Food Sensitization and Food Allergy in Children Across Europe. J. Allergy Clin. Immunol. Pract. 2020, 8, 2736–2746.
- Zuidmeer, L.; Goldhahn, K.; Rona, R.J.; Gislason, D.; Madsen, C.; Summers, C.; Sodergren, E.; Dahlstrom, J.; Lindner, T.; Sigurdardottir, S.T.; et al. The prevalence of plant food allergies: A systematic review. J. Allergy Clin. Immunol. 2008, 121, 1210–1218.e4.
- Sicherer, S.H.; Munoz-Furlong, A.; Godbold, J.H.; Sampson, H.A. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J. Allergy Clin. Immunol. 2012, 125, 1322–1326.
- Ribeiro, M.; Costa, J.; Mafra, I.; Cabo, S.; Silva, A.P.; Gonçalves, B.; Hillion, M.; Hébraud, M.; Igrejas, G. Natural Variation of Hazelnut Allergenicity: Is There Any Potential for Selecting Hypoallergenic Varieties? Nutrients 2020, 12, 2100.
- Clark, A.T.; Anagnostou, K.; Ewan, P.W. Cashew nut causes more severe reactions than peanut: Case matched comparison in 141 children. Allergy 2007, 62, 913–916.
- Mendes, C.; Costa, J.; Vicente, A.A.; Oliveira, M.B.; Mafra, I. Cashew Nut Allergy: Clinical Relevance and Allergen Characterisation. Clin. Rev. Allergy Immunol. 2019, 57, 1–22.
- Rance, F.; Bidat, E.; Bourrier, T.; Sabouraud, D. Cashew allergy: Observations of 42 children without associated peanut allergy. Allergy 2003, 58, 1311–1314.
- van der Valk, J.P.; Dubois, A.E.; Gerth van Wijk, R.; Wichers, H.J.; de Jong, N.W. Systematic review on cashew nut allergy. Allergy 2014, 69, 692–698.
- Costa, J.; Silva, I.; Vicente, A.A.; Oliveira, M.B.P.P.; Mafra, I. Pistachio nut allergy: An updated overview. Crit. Rev. Food Sci. Nutr. 2019, 59, 546–562.
- Cuadrado, C.; Pedrosa, M. Legume Allergenicity: The effect of food processing In Legumes for Global Food Security; Jimenez-Lopez, J.C., Clemente, A., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 223–248.
- Costa, J.; Bavaro, S.; Benedé, S.; Diaz-Perales, A.; Bueno-Diaz, C.; Gelencser, E.; Klüber, J.; Larre, C.; Lozano-Ojalvo, D.; Lupi, R.; et al. Are Physicochemical Properties Shaping the Allergenic Potency of Plant Allergens? Clin. Rev. Allergy Immunol. 2020, 1–36.
- Masthoff, L.J.; Hoff, R.; Verhoeckx, K.C.; van Os-Medendorp, H.; Michelsen-Huisman, A.; Baumert, J.L.; Pasmans, S.G.; Meijer, Y.; Knulst, A.C. A systematic review of the effect of thermal processing on the allergenicity of tree nuts. Allergy 2013, 68, 983–993.
- Maleki, S.J. Food processing: Effects on allergenicity. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 241–245.
- Schmitt, D.A.; Nesbit, J.B.; Hurlburt, B.K.; Cheng, H.; Maleki, S.J. Processing can alter the properties of peanut extract preparations. J. Agric. Food Chem. 2010, 58, 1138–1143.
- Davis, P.; Williams, S. Protein modification by thermal processing. Allergy 1998, 53, 102–105.
- Jiménez-Saiz, R.; Benedé, S.; Molina, E.; López-Expósito, I. Effect of Processing Technologies on the Allergenicity of Food Products. Crit. Rev. Food Sci. Nutr. 2015, 55, 1902–1917.
- Vanga, S.K.; Raghavan, V. Processing Effects On Tree Nut Allergens: A Review. Crit. Rev. Food Sci. Nutr. 2016, 57, 2077–2094.
- Prieto, N.; Burbano, C.; Iniesto, E.; Rodriguez, J.; Cabanillas, B.; Crespo, J.; Pedrosa, M.; Muzquiz, M.; del Pozo, J.; Linacero, R.; et al. A Novel Proteomic Analysis of the Modifications Induced by High Hydrostatic Pressure on Hazelnut Water-Soluble Proteins. Foods 2014, 3, 279–289.
- Venkatachalam, M.; Teuber, S.S.; Roux, K.H.; Sathe, S.K. Effects of roasting, blanching, autoclaving, and microwave heating on antigenicity of almond (Prunus dulcis L.) proteins. J. Agric. Food Chem. 2002, 50, 3544–3548.
- Downs, M.L.; Simpson, A.; Custovic, A.; Semic-Jusufagic, A.; Bartra, J.; Fernandez-Rivas, M.; Taylor, S.L.; Baumert, J.L.; Mills, E.N.C. Insoluble and soluble roasted walnut proteins retain antibody reactivity. Food Chem. 2016, 194, 1013–1021.
- Maleki, S.J.; Chung, S.Y.; Champagne, E.T.; Raufman, J.P. The effects of roasting on the allergenic properties of peanut proteins. J. Allergy Clin. Immunol. 2000, 106, 763–768.
- Hansen, K.S.; Ballmer-Weber, B.K.; Leuttkopf, D.; Skov, P.S.; Weuthrich, B.; Bindslev-Jensen, C.; Vieths, S.; Poulsen, L.K. Roasted hazelnuts—Allergenic activity evaluated by double-blind, placebo-controlled food challenge. Allergy Eur. J. Allergy Clin. Immunol. 2003, 58, 132–138.
- Worm, M.; Hompes, S.; Fiedler, E.M.; Illner, A.K.; Zuberbier, T.; Vieths, S. Impact of native, heat-processed and encapsulated hazelnuts on the allergic response in hazelnut-allergic patients. Clin. Exp. Allergy 2009, 39, 159–166.
- Lopez, E.; Cuadrado, C.; Burbano, C.; Jimenez, M.A.; Rodriguez, J.; Crespo, J.F. Effects of autoclaving and high pressure on allergenicity of hazelnut proteins. J. Clin. Bioinform. 2012, 2, 1–13.
- Cuadrado, C.; Sanchiz, A.; Vicente, F.; Ballesteros, I.; Linacero, R. Changes Induced by Pressure Processing on Immunoreactive Proteins of Tree Nuts. Molecules 2020, 25, 954.
- Mattison, C.P.; Desormeaux, W.A.; Wasserman, R.L.; Yoshioka-Tarver, M.; Condon, B.; Grimm, C.C. Decreased immunoglobulin E (IgE) binding to cashew allergens following sodium sulfite treatment and heating. J. Agric. Food Chem. 2014, 62, 6746–6755.
- Su, M.; Venkatachalam, M.; Teuber, S.S.; Roux, K.H.; Sathe, S.K. Impact of γ-irradiation and thermal processing on the antigenicity of almond, cashew nut and walnut proteins. J. Sci. Food Agric. 2004, 84, 1119–1125.
- Venkatachalam, M.; Monaghan, E.K.; Kshirsagar, H.H.; Robotham, J.M.; O’Donnell, S.E.; Gerber, M.S.; Roux, K.H.; Sathe, S.K. Effects of processing on immunoreactivity of cashew nut (Anacardium occidentale L.) seed flour proteins. J. Agric. Food Chem. 2008, 56, 8998–9005.
- Vicente, F.; Sanchiz, A.; Rodriguez-Perez, R.; Pedrosa, M.; Quirce, S.; Haddad, J.; Besombes, C.; Linacero, R.; Allaf, K.; Cuadrado, C. Influence of Instant Controlled Pressure Drop (DIC) on Allergenic Potential of Tree Nuts. Molecules 2020, 25, 1742.
- Noorbakhsh, R.; Mortazavi, S.A.; Sankian, M.; Shahidi, F.; Maleki, S.J.; Nasiraii, L.R.; Falak, R.; Sima, H.R.; Varasteh, A. Influence of processing on the allergenic properties of pistachio nut assessed in vitro. J. Agric. Food Chem. 2010, 58, 10231–10235.
- Sathe, S.K.; Sharma, G.M. Effects of food processing on food allergens. Mol. Nutr. Food Res. 2009, 53, 970–978.
More
Information
Subjects:
Food Science & Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
4 times
(View History)
Update Date:
06 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




