
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alfredo Cassano | + 1756 word(s) | 1756 | 2021-05-28 09:47:35 | | | |
| 2 | Ron Wang | Meta information modification | 1756 | 2021-06-03 04:20:44 | | |
Video Upload Options
In any membrane filtration, the prediction of permeate flux is critical to calculate the membrane surface required, which is an essential parameter for scaling-up, equipment sizing, and cost determination. Permeate flux prediction is an essential parameter in membrane performance evaluation and the projections for scaling-up from laboratory to the pilot plant or the industrial scale.
1. Introduction
2. Theory
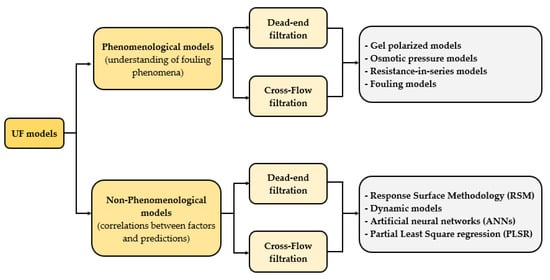
3. Analysis of Model Goodness-of-Fit
- (i) Type of configuration: models tested or developed for cross-flow filtration of fruit juices were selected.
- (ii) Validation: models with more than one validation were considered.
- (iii) The number of citations: models with a high number of citations were selected in order to take into account the scientific impact of each model.
- (iv) Membrane module: models tested or developed in fruit juice processing with hollow fiber and tubular membranes were selected.
- (v) Mathematical complexity: Considering the easy application of the models, the most straightforward models were preferred.
| Bergamot | Kiwi Fruit | Pomegranate | Reference | |
|---|---|---|---|---|
| DCQ II-006C | Koch Series-Cor TM HFM 251 | FUC 1582 | ||
| Membrane characteristics and operation | ||||
| Membrane material | Polysulfone (PS) | Polyvinylidene fluoride (PVDF) | Triacetate cellulose (CTA) | - |
| Configuration | Hollow Fiber | Tubular | Hollow Fiber | - |
| Area (m2) | 0.16 | 0.23 | 0.26 | - |
| MWCO (kDa) | 100 | 100 | 150 | - |
| ΔP (bar) | 1 | 0.85 | 0.6 | - |
| Temperature (°C) | 20 | 25 | 25 | - |
| Flow (Lh−1) | 114 | 800 | 400 | - |
| Porosity (dimensionless) | 0.0057 | 1.1 | 0.0007 | |
| Tortuosity (dimensionless) | 3 | 3 | 0.03 | - |
| Membrane thickness (m) | 4.7 × 10−7 | 2.0 × 10−6 | 0.00023 | [34] |
| Pore density, N (number of pores m−1) |
6.0 × 1012 | 4.0 × 1016 | 1.0 × 1013 | [58] |
| Module length, L (mm) | 330 | 406 | 136 | [59] |
| Module diameter (m) | 0.0021 | 0.025 | 0.0008 | [30][58][60] |
| Hydraulic resistance (m−1) | 3.6 × 1012 | 1.6 × 1012 | 2.1 × 1012 | - |
| Hydraulic permeability (mPa−1s−1) | 2.7 × 10−10 | 5.9 × 10−10 | 4.6 × 10−10 | - |
| Fruit juices characteristics | ||||
| Total soluble solids (°Brix) | 9.4 | 12.6 | 18.7 | [30][38][43][61] |
| Titratable Acidity | 53.86 (gL−1) | - | 1.04 (% citric acid) | [30][38][43][61] |
| pH | 2.40 | 3.19 | 3.61 | [30][38][43][61] |
| Total phenolic compounds | 660 (mg/L) | 421.6 (mg/L) | 1930 (mg GAE/100 L) | [30][38][43][61] |
| Turbidity (%) | 33.67 | - | [30][38][43][61] | |
| Feed density, ρ (kgm−3) | 1091 | 1070 | 1131 | [62][63] |
| Feed viscosity, μ (Pa s) | 0.0019 | 0.0014 | 0.0017 | [31][64] |
| Concentration in food (%) | 12 | 10.08 | 4.9 | [27][33][36] |
The determination of the quality of fit for the selected models was performed using the root mean square error (RMSE), the mean absolute percentage error (MAPE), and the percentage of variability explained (R2) at 95% confidence level. In addition, a validation procedure was carried out using residual analysis. The analysis of residuals, intended as the difference between the observed and predicted value, is fundamental for validating any model. The residuals represent the prediction error: they must have a random distribution and they must be unpredictable, which means that they must follow a normal distribution. In cases where the residuals do not have a normal distribution, the constants and predictors included in the model are intended not to be enough to predict the response. In this sense, two statistics, such as the Shapiro–Wilks (S-W) and Kolmogorov–Smirnov tests (KS), were used for determining the normal distribution of the residues for the analyzed models. Thus, it is expected that a valid model must demonstrate a normal distribution in at least one of the statistics used. All the computations were performed in Statgraphics Centurion XVI (Statgraphics Technologies, The Plains, VA, USA) and Excel 2010 (Microsoft, Redmond, WA, USA).
Results of statistically validated models showed high variability in the prediction capacity by phenomenological models for the studied juices. In particular, phenomenological models present a capacity of prediction ranging from 75.91 to 99.78% (R-squares), whereas the Mean Absolute Percentage Error (MAPE) ranged from 3.14 to 51.69, and Root Mean Square Error (RMSE) from 0.22 to 2.01. Non-phenomenological models showed a better prediction of permeate flux with R-squares higher than 97% and lowered MAPE (0.25–2.03) and RMSE (3.74–28.91) in comparison with phenomenological models. However, these models do not provide information related to the effect of different parameters on the permeate flux, a crucial point for the system scaling-up. On the contrary, phenomenological models are still a proper method for scaling-up purposes, mainly for research in the understanding of the UF process. Therefore, the challenge herein is the development of new phenomenological models with assumptions that include the different phenomena occurring in the filtration of complex matrices in order to improve the capacity of prediction of permeate flux in long-term operation.
References
- Van den Berg, G.B.; Smolders, C.A. Flux decline in ultrafiltration processes. Desalination 1990.
- Blatt, W.F.; Dravid, A.; Michaels, A.S.; Nelsen, L. Solute Polarization and Cake Formation in Membrane Ultrafiltration: Causes, Consequences, and Control Techniques. Membr. Sci. Technol. 1970, 47–97.
- Rodgers, V.G.J. Membrane Processes, by R. Rautenbach and R. Albrecht, John Wiley & Sons, UK (1989, reprinted 1994). 459 pages. ISBN 0-47-191-1100. Dev. Chem. Eng. Miner. Process. 2008.
- Lipnizki, F.; Ruby-Figueroa, R. Membrane operations in the brewing and sugar production. In Integrated Membrane Operations in the Food Production; Cassano, A., Drioli, E., Eds.; Verlag Walter de Gruyter & Co.: Berlin, Germany, 2013; pp. 163–195. ISBN 3110284677.
- Salahi, A.; Abbasi, M.; Mohammadi, T. Permeate flux decline during UF of oily wastewater: Experimental and modeling. Desalination 2010, 251, 153–160.
- Ochando-Pulido, J.M.; Verardo, V.; Segura-Carretero, A.; Martinez-Ferez, A. Technical optimization of an integrated UF/NF pilot plant for conjoint batch treatment of two-phase olives and olive oil washing wastewaters. Desalination 2015, 364, 82–89.
- Salahi, A.; Mohammadi, T.; Mosayebi Behbahani, R.; Hemmati, M. Asymmetric polyethersulfone ultrafiltration membranes for oily wastewater treatment: Synthesis, characterization, ANFIS modeling, and performance. J. Environ. Chem. Eng. 2015, 3, 170–178.
- Zhu, Y.; Zhang, X.; Zhang, H. System dynamics modeling and simulation of a coagulation–ultrafiltration process for the treatment of drinking water. Desalin. Water Treat. 2016, 57, 505–517.
- Sousa, M.R.S.; Lora-Garcia, J.; López-Pérez, M.F. Modelling approach to an ultrafiltration process for the removal of dissolved and colloidal substances from treated wastewater for reuse in recycled paper manufacturing. J. Water Process Eng. 2018, 21, 96–106.
- Torkamanzadeh, M.; Jahanshahi, M.; Peyravi, M.; Rad, A.S. Comparative experimental study on fouling mechanisms in nano-porous membrane: Cheese whey ultrafiltration as a case study. Water Sci. Technol. 2016, 74, 2737–2750.
- Marchese, J.; Ochoa, N.A.; Pagliero, C.; Almandoz, C. Pilot-scale ultrafiltration of an emulsified oil wastewater. Environ. Sci. Technol. 2000.
- Díaz, V.H.G.; Prado-Rubio, O.A.; Willis, M.J.; von Stosch, M. Dynamic Hybrid Model for Ultrafiltration Membrane Processes; Elsevier Masson SAS: Amsterdam, The Netherlands, 2017; Volume 40, ISBN 9780444639653.
- Salahi, A.; Mohammadi, T.; Behbahani, R.M.; Hemati, M. PES and PES/PAN Blend Ultrafiltration Hollow Fiber Membranes for Oily Wastewater Treatment: Preparation, Experimental Investigation, Fouling, and Modeling. Adv. Polym. Technol. 2015, 34.
- Kurada, K.V.; De Tanmay, S. Modeling of cross flow hollow fiber ultrafiltration for treatment of effluent from Railway Workshop. J. Memb. Sci. 2018, 551, 223–233.
- Klimkiewicz, A.; Cervera-Padrell, A.E.; van den Berg, F.W.J. Multilevel Modeling for Data Mining of Downstream Bio-Industrial Processes. Chemom. Intell. Lab. Syst. 2016, 154, 62–71.
- Roa, R.; Zholkovskiy, E.K.; Nägele, G. Ultrafiltration modeling of non-ionic microgels. Soft Matter 2015, 11, 4106–4122.
- Vincent Vela, M.C.; Álvarez Blanco, S.; Lora García, J.; Gozálvez-Zafrilla, J.M.; Bergantiños Rodríguez, E. Modelling of flux decline in crossflow ultrafiltration of macromolecules: Comparison between predicted and experimental results. Desalination 2007, 204, 328–334.
- Astudillo-Castro, C.L. Limiting flux and critical transmembrane pressure determination using an exponential model: The effect of concentration factor, temperature, and cross-flow velocity during casein micelle concentration by microfiltration. Ind. Eng. Chem. Res. 2015, 54, 414–425.
- Chamberland, J.; Bouyer, A.; Benoit, S.; Provault, C.; Bérubé, A.; Doyen, A.; Pouliot, Y. Efficiency assessment of water reclamation processes in milk protein concentrate manufacturing plants: A predictive analysis. J. Food Eng. 2020.
- Ng, K.S.Y.; Haribabu, M.; Harvie, D.J.E.; Dunstan, D.E.; Martin, G.J.O. Mechanisms of flux decline in skim milk ultrafiltration: A review. J. Memb. Sci. 2017, 523, 144–162.
- Bhattacharya, P.K.; Agarwal, S.; De, S.; Rama Gopal, U.V.S. Ultrafiltration of sugar cane juice for recovery of sugar: Analysis of flux and retention. Sep. Purif. Technol. 2001, 21, 247–259.
- Vu, T.; LeBlanc, J.; Chou, C.C. Clarification of sugarcane juice by ultrafiltration membrane: Toward the direct production of refined cane sugar. J. Food Eng. 2020.
- Krishna Kumar, N.S.; Yea, M.K.; Cheryan, M. Ultrafiltration of soy protein concentrate: Performance and modelling of spiral and tubular polymeric modules. J. Memb. Sci. 2004, 244, 235–242.
- Bacchin, P.; Aimar, P.; Sanchez, V. Influence of surface interaction on transfer during colloid ultrafiltration. J. Memb. Sci. 1996, 115, 49–63.
- Bhattacharjee, C.; Bhattacharya, P.K. Prediction of limiting flux in ultrafiltration of kraft black liquor. J. Memb. Sci. 1992, 72, 137–147.
- Cassano, A.; Conidi, C.; Drioli, E. A Membrane-Based Process for the Valorization of the Bergamot Juice. Sep. Sci. Technol. 2013, 48, 537–546.
- Conidi, C.; Cassano, A.; Drioli, E. A membrane-based study for the recovery of polyphenols from bergamot juice. J. Memb. Sci. 2011, 375, 182–190.
- Jiraratananon, R.; Chanachai, A. A study of fouling in the ultrafiltration of passion fruit juice. J. Memb. Sci. 1996, 111, 39–48.
- Rai, P.; Majumdar, G.C.; Sharma, G.; Das Gupta, S.; De, S. Effect of Various Cutoff Membranes on Permeate Flux and Quality During Filtration of Mosambi (Citrus Sinensis (L.) Osbeck) Juice. Food Bioprod. Process. 2006, 84, 213–219.
- Conidi, C.; Cassano, A. Recovery of phenolic compounds from bergamot juice by nanofiltration membranes. Desalin. Water Treat. 2015, 56, 3510–3518.
- Cassano, A.; Donato, L.; Drioli, E. Ultrafiltration of kiwifruit juice: Operating parameters, juice quality and membrane fouling. J. Food Eng. 2007, 79, 613–621.
- Cassano, A.; Jiao, B.; Drioli, E. Production of concentrated kiwifruit juice by integrated membrane process. Food Res. Int. 2004, 37, 139–148.
- Cassano, A.; Donato, L.; Conidi, C.; Drioli, E. Recovery of bioactive compounds in kiwifruit juice by ultrafiltration. Innov. Food Sci. Emerg. Technol. 2008, 9, 556–562.
- Cassano, A.; Conidi, C.; Drioli, E. Clarification and concentration of pomegranate juice (Punica granatum L.) using membrane processes. J. Food Eng. 2011, 107, 366–373.
- Conidi, C.; Cassano, A.; Caiazzo, F.; Drioli, E. Separation and purification of phenolic compounds from pomegranate juice by ultrafiltration and nanofiltration membranes. J. Food Eng. 2017, 195, 1–13.
- Cassano, A.; Conidi, C.; Tasselli, F. Clarification of pomegranate juice (Punica granatum L.) by hollow fibre membranes: Analyses of membrane fouling and performance. J. Chem. Technol. Biotechnol. 2015, 90, 859–866.
- Vladisavljević, G.T.; Vukosavljević, P.; Bukvić, B. Permeate flux and fouling resistance in ultrafiltration of depectinized apple juice using ceramic membranes. J. Food Eng. 2003, 60, 241–247.
- Giuffrè, A.M. Bergamot (Citrus bergamia, Risso): The effects of cultivar and harvest date on functional properties of juice and cloudy juice. Antioxidants 2019, 8, 221.
- Tsiokanos, E.; Tsafantakis, N.; Termentzi, A.; Aligiannis, N.; Skaltsounis, L.A.; Fokialakis, N. Phytochemical characteristics of bergamot oranges from the Ionian islands of Greece: A multi-analytical approach with emphasis in the distribution of neohesperidose flavanones. Food Chem. 2021, 343, 128400.
- Cautela, D.; Vella, F.M.; Laratta, B. The effect of processing methods on phytochemical composition in bergamot juice. Foods 2019, 8, 474.
- Hashemi, S.M.B.; Jafarpour, D. Fermentation of bergamot juice with Lactobacillus plantarum strains in pure and mixed fermentations: Chemical composition, antioxidant activity and sensorial properties. LWT 2020, 131, 109803.
- Cassano, A.; Figoli, A.; Tagarelli, A.; Sindona, G.; Drioli, E. Integrated membrane process for the production of highly nutritional kiwifruit juice. Desalination 2006, 189, 21–30.
- Conidi, C.; Drioli, E.; Cassano, A. Perspective of membrane technology in pomegranate juice processing: A review. Foods 2020, 9, 889.
- Putnik, P.; Kresoja, Ž.; Bosiljkov, T.; Režek Jambrak, A.; Barba, F.J.; Lorenzo, J.M.; Roohinejad, S.; Granato, D.; Žuntar, I.; Bursać Kovačević, D. Comparing the effects of thermal and non-thermal technologies on pomegranate juice quality: A review. Food Chem. 2019, 279, 150–161.
- Vela, M.C.V.; Blanco, S.Á.; García, J.L.; Rodríguez, E.B. Permeate flux decline prediction in the ultrafiltration of macromolecules with empirical estimation of the gel layer concentration. Desalination 2008, 221, 390–394.
- Carman, P. Fluid flow through a granular bed. Trans. Inst. Chem. Eng J. 1937, 15, 150–156.
- Carman, P. Fundamental principles of industrial filtration. Trans. Inst. Chem. Eng J. 1938, 16, 168–188.
- Li, W.; Xing, W.; Xu, N. Modeling of relationship between water permeability and microstructure parameters of ceramic membranes. Desalination 2006, 192, 340–345.
- Scott, K.; Hughes, R.; Staude, E. Industrial Membrane Separation Technology; Blackie Academic and Professional: london, UK, 1997; Volume 130, ISBN 0751403385.
- Cheng, T.W.; Wu, J.G. Modified boundary layer resistance model for membrane ultrafiltration. Tamkang J. Sci. Eng. 2001, 4, 111–117.
- Ohanessian, K.; Monnot, M.; Moulin, P.; Ferrasse, J.H.; Barca, C.; Soric, A.; Boutin, O. Dead-end and crossflow ultrafiltration process modelling: Application on chemical mechanical polishing wastewaters. Chem. Eng. Res. Des. 2020, 158, 164–176.
- Cheng, T.W.; Yeh, H.M. Complete momentum-balance analysis of permeate flux for ultrafiltration in hollow-fiber modules. Tamkang J. Sci. Eng. 2008, 11, 239–246.
- Beicha, A.; Zaamouch, R.; Sulaiman, N.M. Permeate Flux in Ultrafiltration Membrane: A Review. J. Appl. Membr. Sci. Technol. 2017, 14.
- Bhattacharjee, C.; Datta, S. Simulation of continuous stirred ultrafiltration process: An approach based on analytical solution coupled with turbulent back transport. J. Chem. Technol. Biotechnol. 2003, 78, 1135–1141.
- Davis, R. Modeling of fouling of crossflow microfiltration membranes. Sep. Purif. Rev. 1992, 21, 75–126.
- Kedem, O.; Katchalsky, A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim. Biophys. Acta 1958, 27, 229–246.
- Wijmans, J.G.; Nakao, S.; Smolders, C.A. Flux limitation in ultrafiltration: Osmotic pressure model and gel layer model. J. Memb. Sci. 1984, 20, 115–124.
- De, S.; Bhattacharya, P.K. Modeling of ultrafiltration process for a two-component aqueous solution of low and high (gel-forming) molecular weight solutes. J. Memb. Sci. 1997, 136, 57–69.
- Ho, C.C.; Zydney, A.L. A combined pore blockage and cake filtration model for protein fouling during microfiltration. J. Colloid Interface Sci. 2000, 232, 389–399.
- Mondal, S.; De, S. Generalized criteria for identification of fouling mechanism under steady state membrane filtration. J. Memb. Sci. 2009, 344, 6–13.
- Song, L. Flux decline in crossflow microfiltration and ultrafiltration: Mechanisms and modeling of membrane fouling. J. Memb. Sci. 1998, 139, 183–200.
- Yee, K.W.K.; Wiley, D.E.; Bao, J. A unified model of the time dependence of flux decline for the long-term ultrafiltration of whey. J. Memb. Sci. 2009, 332, 69–80.
- Ruby-Figueroa, R.; Saavedra, J.; Bahamonde, N.; Cassano, A. Permeate flux prediction in the ultrafiltration of fruit juices by ARIMA models. J. Memb. Sci. 2017, 524, 108–116.
- Cheryan, M. Ultrafiltration and Microfiltration Handbook; CRC Press: Boca Raton, FL, USA, 1998; ISBN 9781566765985.
- Mondal, S.; Cassano, A.; Conidi, C.; De, S. Modeling of gel layer transport during ultrafiltration of fruit juice with non-Newtonian fluid rheology. Food Bioprod. Process. 2016, 100, 72–84.
- Clever, M.; Jordt, F.; Knauf, R.; Räbiger, N.R.; Rtidebusch, M.; Hilker-Scheibel, R. Process water production from river water by ultrafiltration and reverse osmosis. Desalination 2000, 131, 325–336.
- Qin, G.; Lü, X.; Wei, W.; Li, J.; Cui, R.; Hu, S. Microfiltration of kiwifruit juice and fouling mechanism using fly-ash-based ceramic membranes. Food Bioprod. Process. 2015, 96, 278–284.
- Riyahi, R.; Rafiee, S.; Dalvand, M.J.; Keyhani, A. Some physical characteristics of pomegranate, seeds and arils. J. Agric. Technol. 2011, 7, 1523–1537.
- Tapia Duran, M.P. Estudio de Factores Fisiólogicos del Kiwi (Actinidia Deliciosa) Variedad Hayward, y sus Efectos en la Textura Durante el Almacenamiento. Bachelor’s Thesis, Universidad de Chile, Santiago, Chile, 2012.
- Magerramov, M.A.; Abdulagatov, A.I.; Azizov, N.D.; Abdulagatov, I.M. Effect of temperature, concentration, and pressure on the viscosity of pomegranate and pear juice concentrates. J. Food Eng. 2007, 80, 476–489.




