
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Akhtar Ali | + 5806 word(s) | 5806 | 2021-07-26 10:50:19 | | | |
| 2 | Camila Xu | -322 word(s) | 5484 | 2021-07-26 12:11:50 | | |
Video Upload Options
Plant bioactive compounds, such as phytochemicals, in poultry diets, are gaining popularity due to their potential antioxidant and anti-microbial activities. Phytogenic feed additives (PFAs) have emerged as natural alternatives to antibiotic growth promotors and have great potential in the poultry industry. In recent years, cinnamon (one of the most widely used spices) has attracted attention from researchers as a natural product with numerous health benefits for poultry. The essential oils in cinnamon, in particular, are of interest because of their antioxidant, anti-microbial, anti-inflammatory, antifungal, and hypocholesterolaemic effects, in addition to their ability to stimulate digestive enzymes in the gut. This review mainly emphasizes the potential impact of cinnamon as a natural feed additive on overall gut health, nutrient digestibility, blood biochemical profile, gene expression, gut microbiota and immune response.
1. Introduction
2. Cinnamon
Phytochemistry of Cinnamon
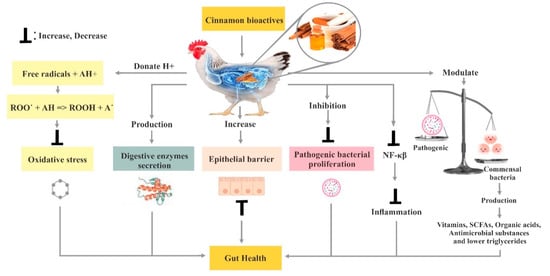
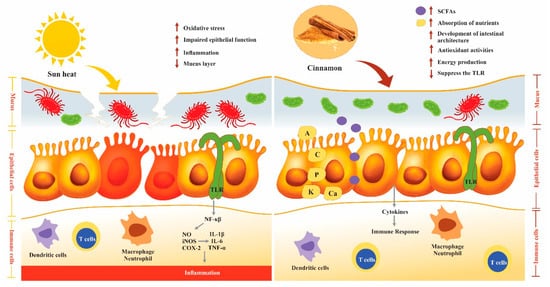
3. Poultry Gut Health
3.1. Utilization of Cinnamon in Poultry Feed
3.2. Impact of Cinnamon on the Digestibility of Nutrients
3.3. Cinnamon and Blood Biochemical Profile
3.4. Cinnamon and Gene Expression in Poultry
3.5. Effect of Cinnamon on the Gut Microbiota
| Feed Composition | Bird Type | Feed Level | Gut Microbiota | Gut Part | Ref. |
|---|---|---|---|---|---|
| * Cinnamon powder | Broiler | 10% cinnamon | Total counts of Enterococcus spp. and Lactobacillus spp. ↑ Campylobacter spp. and E. coli ↓ | Ileum and cecum | [1] |
| CNO | Broiler | 100 mg/kg | Lactobacillus and Bifidobacterium ↑ E. coli ↓ |
Cecum | [4] |
| CNO | Broiler | 300 mg/kg | No change in Lactobacillus spp.; E. coli and Clostridium spp. ↓ | Cecum | [37] |
| CNO | Japanese quail | 200 mg/kg | Lactobacillus ↑ Coliforms count ↓ | intestine | [50] |
| * CNO | Broiler | 500 mg/kg | No effect on total bacterial counts, E. coli and Lactobacillus; Clostridium and Salmonella counts ↓ | Ileum and cecum | [74] |
| * Cinnamaldehyde | Ross broiler | 5 g + 15 g/tonne | E. coli ↓ | Cecum | [75] |
| * Cinnamaldehyde | Hubbard broiler | 100 mg/kg | Lactobacillus counts ↑, E. coli and Clostridium perfringens↓ | Ileum and cecum | [77] |
3.6. Effect of Cinnamon on the Immune System
References
- Rashid, Z.; Mirani, Z.A.; Zehra, S.; Gilani, S.M.H.; Ashraf, A.; Azhar, A.; Al-Ghanim, K.A.; Al-Misned, F.; Al-Mulahim, N.; Mahboob, S. Enhanced modulation of gut microbial dynamics affecting body weight in birds triggered by natural growth promoters administered in conventional feed. Saudi J. Biol. Sci. 2020, 27, 2747–2755.
- Diaz Carrasco, J.M.; Casanova, N.A.; Fernández Miyakawa, M.E. Microbiota, Gut Health and Chicken Productivity: What Is the Connection? Microorganisms 2019, 7, 374.
- Cottrell, J.J.; Le, H.H.; Artaiz, O.; Iqbal, Y.; Suleria, H.A.; Ali, A.; Celi, P.; Dunshea, F.R. Recent advances in the use of phytochemicals to manage gastrointestinal oxidative stress in poultry and pigs. Anim. Prod. Sci. 2021, 10, 1071.
- Yang, Y.-F.; Zhao, L.-L.; Shao, Y.-X.; Liao, X.-D.; Zhang, L.-Y.; Lu, L.; Luo, X.-G. Effects of dietary graded levels of cinnamon essential oil and its combination with bamboo leaf flavonoid on immune function, antioxidative ability and intestinal microbiota of broilers. J. Integ. Agri. 2019, 18, 2123–2132.
- Saeed, M.; Abbas, G.; Alagawany, M.; Kamboh, A.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Chao, S. Heat stress management in poultry farms: A comprehensive overview. J. Therm. Biol. 2019, 84, 414–425.
- Jayaprakasha, G.K.; Rao, L.J. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit. Rev. Food Sci. Nutr. 2011, 51, 547–562.
- Paranagama, P.A.; Dayananda, K.R.; Hewage, J.W. Chemistry and Bioactive Compounds of Cinnamomum zeylanicum Blume. In Cinnamon, 1st ed.; Senaratne, R., Pathirana, R., Eds.; Springer: Cham, Switzerland, 2020; pp. 251–271.
- Bandaranayake, P.C.G.; Pushpakumara, D. Genetics and Molecular Characterization of Genus Cinnamomum. In Cinnamon, 1st ed.; Senaratne, R., Pathirana, R., Eds.; Springer: Cham, Switzerland, 2020; pp. 119–146.
- Sharifi-Rad, J.; Song, S.; Ali, A.; Subbiah, V.; Taheri, Y.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS characterization of phenolic compounds from Pyracantha coccinea M. Roem. and their antioxidant capacity. Cellu. Molec. Biol. 2021, 67, 201–211.
- Chou, O.; Ali, A.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterisation of Phenolics in Herbal Tea Infusion and Their Antioxidant Potential. Fermentation 2021, 7, 73.
- Hashemi, S.R.; Davoodi, H. Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet. Res. Commun. 2011, 35, 169–180.
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive Profiling of Most Widely Used Spices for Their Phenolic Compounds through LC-ESI-QTOF-MS2 and Their Antioxidant Potential. Antioxidants 2021, 10, 721.
- Li, Y.; Kong, D.; Lin, X.; Xie, Z.; Bai, M.; Huang, S.; Nian, H.; Wu, H. Quality evaluation for essential oil of Cinnamomum verum leaves at different growth stages based on GC–MS, FTIR and microscopy. Food Anal. Methods 2016, 9, 202–212.
- Liyanage, N.M.N.; Bandusekara, B.S.; Kanchanamala, R.W.M.K.; Hathurusinghe, H.A.B.M.; Dilhan, A.M.R.W.S.; Pushpakumara, D.K.N.G.; Samita, S.; Wijesinghe, K.G.G.; Jayasinghe, G.G.; Liyanage, W.K. Identification of superior Cinnamomum zeylanicum Blume germplasm for future true cinnamon breeding in the world. J. Food Comp. Anal. 2021, 96, 103747.
- Ribeiro-Santos, R.; Andrade, M.; Madella, D.; Martinazzo, A.P.; Moura, L.d.A.G.; de Melo, N.R.; Sanches-Silva, A. Revisiting an ancient spice with medicinal purposes: Cinnamon. Trends Food Sci. Technol. 2017, 62, 154–169.
- Geng, S.; Cui, Z.; Huang, X.; Chen, Y.; Xu, D.; Xiong, P. Variations in essential oil yield and composition during Cinnamomum cassia bark growth. Ind. Crops Prod. 2011, 33, 248–252.
- Hamidpour, R.; Hamidpour, M.; Hamidpour, S.; Shahlari, M. Cinnamon from the selection of traditional applications to its novel effects on the inhibition of angiogenesis in cancer cells and prevention of Alzheimer’s disease, and a series of functions such as antioxidant, anticholesterol, antidiabetes, antibacterial, antifungal, nematicidal, acaracidal, and repellent activities. J. Tradit. Complement. Med. 2015, 5, 66–70.
- Kim, Y.-G.; Lee, J.-H.; Kim, S.-I.; Baek, K.-H.; Lee, J. Cinnamon bark oil and its components inhibit biofilm formation and toxin production. Int. J. Food Microbiol. 2015, 195, 30–39.
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid. Based Complement. Alternat Med. 2014, 2014, 642942.
- Namazi, N.; Khodamoradi, K.; Khamechi, S.P.; Heshmati, J.; Ayati, M.H.; Larijani, B. The impact of cinnamon on anthropometric indices and glycemic status in patients with type 2 diabetes: A systematic review and meta-analysis of clinical trials. Complement. Ther. Med. 2019, 43, 92–101.
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Antibacterial properties and major bioactive components of cinnamon stick (Cinnamomum burmannii): Activity against foodborne pathogenic bacteria. J. Agric. Food Chem. 2007, 55, 5484–5490.
- Nawab, A.; Ibtisham, F.; Li, G.; Kieser, B.; Wu, J.; Liu, W.; Zhao, Y.; Nawab, Y.; Li, K.; Xiao, M. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018, 78, 131–139.
- Iqbal, Y.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Gut Microbiota-Polyphenol Interactions in Chicken: A Review. Animals 2020, 10, 1391.
- Zhu, C.; Yan, H.; Zheng, Y.; Santos, H.O.; Macit, M.S.; Zhao, K. Impact of Cinnamon Supplementation on cardiometabolic Biomarkers of Inflammation and Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2020, 53, 102517.
- Lin, C.C.; Wu, S.J.; Chang, C.H.; Ng, L.T. Antioxidant activity of Cinnamomum cassia. Phytother. Res. 2003, 17, 726–730.
- Wenk, C. Herbs and botanicals as feed additives in monogastric animals. Asian-Australas J. Anim. Sci. 2003, 16, 282–289.
- O’Bryan, C.A.; Pendleton, S.J.; Crandall, P.G.; Ricke, S.C. Potential of plant essential oils and their components in animal agriculture–in vitro studies on antibacterial mode of action. Front. Vet. Sci. 2015, 2, 35.
- Bento, M.; Ouwehand, A.; Tiihonen, K.; Lahtinen, S.; Nurminen, P.; Saarinen, M.; Schulze, H.; Mygind, T.; Fischer, J. Essential oils and their use in animal feeds for monogastric animals--Effects on feed quality, gut microbiota, growth performance and food safety: A review. Vet. Med. 2013, 58, 449–458.
- Lee, K.-W.; Everts, H.; Kappert, H.; Frehner, M.; Losa, R.; Beynen, A. Effects of dietary essential oil components on growth performance, digestive enzymes and lipid metabolism in female broiler chickens. Br. Poult. Sci. 2003, 44, 450–457.
- Jang, I.; Ko, Y.; Kang, S.; Lee, C. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim. Feed Sci. Technol. 2007, 134, 304–315.
- Jamroz, D.; Wertelecki, T.; Houszka, M.; Kamel, C. Influence of diet type on the inclusion of plant origin active substances on morphological and histochemical characteristics of the stomach and jejunum walls in chicken. J. Anim. Physiol. Anim. Nutr. 2006, 90, 255–268.
- Basmacioğlu Malayoğlu, H.; Baysal, Ş.; Misirlioğlu, Z.; Polat, M.; Yilmaz, H.; Turan, N. Effects of oregano essential oil with or without feed enzymes on growth performance, digestive enzyme, nutrient digestibility, lipid metabolism and immune response of broilers fed on wheat–soybean meal diets. Br. Poult. Sci. 2010, 51, 67–80.
- Garcia, V.; Catala-Gregori, P.; Hernandez, F.; Megias, M.; Madrid, J. Effect of formic acid and plant extracts on growth, nutrient digestibility, intestine mucosa morphology, and meat yield of broilers. J. Appl. Poult. Res. 2007, 16, 555–562.
- Zhai, H.; Liu, H.; Wang, S.; Wu, J.; Kluenter, A.-M. Potential of essential oils for poultry and pigs. Anim. Nutr. 2018, 4, 179–186.
- Mehdi, Y.; Létourneau-Montminy, M.-P.; Gaucher, M.-L.; Chorfi, Y.; Suresh, G.; Rouissi, T.; Brar, S.K.; Côté, C.; Ramirez, A.A.; Godbout, S. Use of antibiotics in broiler production: Global impacts and alternatives. Anim. Nutr. 2018, 4, 170–178.
- Devi, P.C.; Samanta, A.K.; Das, B.; Kalita, G.; Behera, P.S.; Barman, S. Effect of plant extracts and essential oil blend as alternatives to antibiotic growth promoters on growth performance, nutrient utilization and carcass characteristics of broiler chicken. Indian J. Anim. Nutri 2018, 35, 421–427.
- Chowdhury, S.; Mandal, G.P.; Patra, A.K.; Kumar, P.; Samanta, I.; Pradhan, S.; Samanta, A.K. Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim. Feed Sci. Technol. 2018, 236, 39–47.
- Windisch, W.; Schedle, K.; Plitzner, C.; Kroismayr, A. Use of phytogenic products as feed additives for swine and poultry. Anim. Sci. J. 2008, 86, E140–E148.
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7.
- Chowdhury, S.; Mandal, G.P.; Patra, A.K. Different essential oils in diets of chickens: 1. Growth performance, nutrient utilisation, nitrogen excretion, carcass traits and chemical composition of meat. Anim. Feed. Sci. Technol. 2018, 236, 86–97.
- Redondo, L.M.; Chacana, P.A.; Dominguez, J.E.; Fernandez Miyakawa, M.E. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front. Microbiol. 2014, 5, 118.
- Cross, D.E.; McDevitt, R.M.; Acamovic, T. Herbs, thyme essential oil and condensed tannin extracts as dietary supplements for broilers, and their effects on performance, digestibility, volatile fatty acids and organoleptic properties. Br. Poult. Sci. 2011, 52, 227–237.
- Jamroz, D.; Orda, J.; Kamel, C.; Wiliczkiewicz, A.; Wertelecki, T.; Skorupinska, J. The influence of phytogenic extracts on performance, nutrient digestibility, carcass characteristics, and gut microbial status in broiler chickens. J. Anim. Feed Sci. 2003, 12, 583–596.
- Mountzouris, K.; Paraskevas, V.; Tsirtsikos, P.; Palamidi, I.; Steiner, T.; Schatzmayr, G.; Fegeros, K. Assessment of a phytogenic feed additive effect on broiler growth performance, nutrient digestibility and caecal microflora composition. Anim. Feed Sci. Technol. 2011, 168, 223–231.
- Platel, K.; Srinivasan, K. Digestive stimulant action of spices: A myth or reality? Ind. J. Med. Res. 2004, 119, 167.
- Maenner, K.; Vahjen, W.; Simon, O. Studies on the effects of essential-oil-based feed additives on performance, ileal nutrient digestibility, and selected bacterial groups in the gastrointestinal tract of piglets. Anim. Sci. J. 2011, 89, 2106–2112.
- Kettunen, H.; Ouwehand, A.; Schulze, H.; Rautonen, N. Dietary essential oil supplementation enhanced intestinal immunocompetence in young broiler chick. Reprod. Nutr. Dev. 2006, 46, S101.
- Al-Kassie, G.A. Influence of two plant extracts derived from thyme and cinnamon on broiler performance. Pak. Vet. J. 2009, 29, 169–173.
- Ciftci, M.; Simsek, U.G.; Yuce, A.; Yilmaz, O.; Dalkilic, B. Effects of dietary antibiotic and cinnamon oil supplementation on antioxidant enzyme activities, cholesterol levels and fatty acid compositions of serum and meat in broiler chickens. Acta. Vet. Brno. 2010, 79, 33–40.
- Mehdipour, Z.; Afsharmanesh, M. Evaluation of synbiotic and cinnamon (Cinnamomum verum) as antibiotic growth promoter substitutions on growth performance, intestinal microbial populations and blood parameters in Japanese quail. J. Livest. Sci. Technol. 2018, 6, 1–8.
- Kanani, P.B.; Daneshyar, M.; Najafi, R. Effects of cinnamon (Cinnamomum zeylanicum) and turmeric (Curcuma longa) powders on performance, enzyme activity, and blood parameters of broiler chickens under heat stress. Poult. Sci. J. 2016, 4, 47–53.
- Naderi, M.; Akbari, M.; Asadi-Khoshoei, E.; Khaksar, K.; Khajali, F. Effects of Dietary Inclusion of Turmeric (Curcuma longa) and Cinnamon (Cinnamomum verum) Powders on Performance, Organs Relative Weight and Some Immune System Parameters in Broiler Chickens. Poult. Sci. J. 2014, 2, 153–163.
- Toghyani, M.; Toghyani, M.; Gheisari, A.; Ghalamkari, G.; Eghbalsaied, S. Evaluation of cinnamon and garlic as antibiotic growth promoter substitutions on performance, immune responses, serum biochemical and haematological parameters in broiler chicks. Livest. Sci. 2011, 138, 167–173.
- Faghani, M.; Rahimian, Y.; Rafiee, A.; Namjoo, A.R. Effect of garlic and cinnamon in comparison to virginiamycin on performance and some haematological parameters in broiler chicks. Res. Opin. Anim. Vet. Sci. 2014, 4, 504–507.
- Najafi, S.; Taherpour, K. Effects of dietary ginger (Zingiber Ofjicinale), cinnamon (Cinnamomum), synbiotic and antibiotic supplementation on performance of broilers. J. Anim. Sci. Adv. 2014, 4, 658–667.
- Hossain, M.; Howlader, A.; Islam, M.; Beg, M. Evaluation of locally available herbs and spices on physical, biochemical and economical parameters on broiler production. IJPAES 2014, 4, 317–323.
- Torki, M.; Akbari, M.; Kaviani, K. Single and combined effects of zinc and cinnamon essential oil in diet on productive performance, egg quality traits, and blood parameters of laying hens reared under cold stress condition. Int. J. Biometeorol. 2015, 59, 1169–1177.
- Shirzadegan, K. Reactions of Modern Broiler Chickens to Administration of Cinnamon Powder in the Diet. Iran. J. Appl. Anim. Sci. 2014, 4, 367–371.
- Sang-Oh, P.; Chae-Min, R.; Byung-Sung, P.; Jong, H. The meat quality and growth performance in broiler chickens fed diet with cinnamon powder. J. Environ. Biol. 2013, 34, 127–133.
- Abudabos, A.M.; Alyemni, A.H.; Dafalla, Y.M.; Khan, R.U. The effect of phytogenics on growth traits, blood biochemical and intestinal histology in broiler chickens exposed to Clostridium perfringens challenge. J. Appl. Anim. Res. 2018, 46, 691–695.
- Izadnia, H.R.; Tahmoorespur, M.; Bakhtiarizadeh, M.R.; Nassiri, M.; Esmaeilkhanien, S. Gene expression profile analysis of residual feed intake for Isfahan native chickens using RNA-SEQ data. Italian J. Anim. Sci. 2019, 18, 246–260.
- Zhuo, Z.; Lamont, S.J.; Lee, W.R.; Abasht, B. RNA-Seq Analysis of Abdominal Fat Reveals Differences between Modern Commercial Broiler Chickens with High and Low Feed Efficiencies. PLoS ONE 2015, 10, e0135810.
- Tabatabaei, S.M.; Badalzadeh, R.; Mohammadnezhad, G.-R.; Balaei, R. Effects of Cinnamon extract on biochemical enzymes, TNF-± and NF-ºB gene expression levels in liver of broiler chickens inoculated with Escherichia coli. Pesqui. Veterinária Bras. 2015, 35, 781–787.
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169.
- Mandal, R.S.; Saha, S.; Das, S. Metagenomic Surveys of Gut Microbiota. Genom. Proteom. Bioinform. 2015, 13, 148–158.
- Mueller, K.; Blum, N.M.; Kluge, H.; Mueller, A.S. Influence of broccoli extract and various essential oils on performance and expression of xenobiotic-and antioxidant enzymes in broiler chickens. Br. J. Nutr. 2012, 108, 588–602.
- Lee, S.A.; Apajalahti, J.; Vienola, K.; González-Ortiz, G.; Fontes, C.M.G.A.; Bedford, M.R. Age and dietary xylanase supplementation affects ileal sugar residues and short chain fatty acid concentration in the ileum and caecum of broiler chickens. Anim. Feed Sci. Technol. 2017, 234, 29–42.
- Yin, Y.; Lei, F.; Zhu, L.; Li, S.; Wu, Z.; Zhang, R.; Gao, G.F.; Zhu, B.; Wang, X. Exposure of different bacterial inocula to newborn chicken affects gut microbiota development and ileum gene expression. ISME J. 2010, 4, 367–376.
- Bogusławska-Tryk, M.; Szymeczko, R.; Piotrowska, A.; Burlikowska, K.; Śliżewska, K. Ileal and cecal microbial population and short-chain fatty acid profile in broiler chickens fed diets supplemented with lignocellulose. Pak. Vet. J. 2015, 35, 212–216.
- Chang, S.-T.; Chen, P.-F.; Chang, S.-C. Antibacterial activity of leaf essential oils and their constituents from Cinnamomum osmophloeum. J. Ethnopharmacol. 2001, 77, 123–127.
- Chao, S.C.; Young, D.G.; Oberg, C.J. Screening for Inhibitory Activity of Essential Oils on Selected Bacteria, Fungi and Viruses. J. Essent. Oil Res. 2000, 12, 639–649.
- Zhao, L.; Liao, X.; Zhang, L.; Luo, X.; Lu, L. Bacteriostatic effects of plant extracts and their compounds on chicken pathogenic bacteria in vitro. Chin. J. Anim. Nutr. 2017, 29, 3277–3286.
- Gupta, C.; Garg, A.P.; Uniyal, R.C.; Kumari, A. Comparative analysis of the antimicrobial activity of cinnamon oil and cinnamon extract on some food-borne microbes. Afr. J. Microbiol. Res. 2008, 2, 247–251.
- Pathak, M.; Mandal, G.P.; Patra, A.K.; Samanta, I.; Pradhan, S.; Haldar, S. Effects of dietary supplementation of cinnamaldehyde and formic acid on growth performance, intestinal microbiota and immune response in broiler chickens. Anim. Prod. Sci. 2017, 57, 821–827.
- Tiihonen, K.; Kettunen, H.; Bento, M.H.; Saarinen, M.; Lahtinen, S.; Ouwehand, A.C.; Schulze, H.; Rautonen, N. The effect of feeding essential oils on broiler performance and gut microbiota. Br. Poult. Sci. 2010, 51, 381–392.
- Rehman, H.U.; Vahjen, W.; Awad, W.A.; Zentek, J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch. Anim. Nutr. 2007, 61, 319–335.
- Jamroz, D.; Wiliczkiewicz, A.; Wertelecki, T.; Orda, J.; Skorupińska, J. Use of active substances of plant origin in chicken diets based on maize and locally grown cereals. Br. Poult. Sci. 2005, 46, 485–493.
- Johny, A.K.; Darre, M.; Donoghue, A.; Donoghue, D.; Venkitanarayanan, K. Antibacterial effect of trans-cinnamaldehyde, eugenol, carvacrol, and thymol on Salmonella Enteritidis and Campylobacter jejuni in chicken cecal contents in vitro. J. Appl. Poult. Res. 2010, 19, 237–244.
- Si, W.; Gong, J.; Tsao, R.; Zhou, T.; Yu, H.; Poppe, C.; Johnson, R.; Du, Z. Antimicrobial activity of essential oils and structurally related synthetic food additives towards selected pathogenic and beneficial gut bacteria. J. Appl. Microbiol. 2006, 100, 296–305.
- Hernandez, F.; Madrid, J.; Garcia, V.; Orengo, J.; Megias, M. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult. Sci. 2004, 83, 169–174.
- Lambert, R.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462.
- Faix, Š.; Faixová, Z.; Plachá, I.; Koppel, J. Effect of Cinnamomum zeylanicum essential oil on antioxidative status in broiler chickens. Acta. Vet. Brno. 2009, 78, 411–417.
- Atiphasaworn, P.; Monggoot, S.; Pripdeevech, P. Chemical composition, antibacterial and antifungal activities of Cinamomum bejolghota bark oil from Thailand. J. Appl. Pharm. Sci. 2017, 7, 069–073.
- Orengo, J.; Buendía, A.; Ruiz-Ibáñez, M.; Madrid, J.; Del Río, L.; Catalá-Gregori, P.; García, V.; Hernández, F. Evaluating the efficacy of cinnamaldehyde and Echinacea purpurea plant extract in broilers against Eimeria acervulina. Vet. Parasitol. 2012, 185, 158–163.
- Naidoo, V.; McGaw, L.J.; Bisschop, S.; Duncan, N.; Eloff, J.N. The value of plant extracts with antioxidant activity in attenuating coccidiosis in broiler chickens. Vet. Parasitol. 2008, 153, 214–219.
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Alvarenga, J.F.R.; Leal, L.N.; Lamuela-Raventos, R.M. A comprehensive study on the phenolic profile of widely used culinary herbs and spices: Rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014, 154, 299–307.
- Dvorackova, E.; Snoblova, M.; Chromcova, L.; Hrdlicka, P. Effects of extraction methods on the phenolic compounds contents and antioxidant capacities of cinnamon extracts. Food Sci. Biotechnol. 2015, 24, 1201–1207.
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52.
- Teixeira, S. Bioflavonoids: Proanthocyanidins and quercetin and their potential roles in treating musculoskeletal conditions. J. Orthop. Sports Phys. Ther. 2002, 32, 357–363.
- Forester, S.C.; Waterhouse, A.L. Metabolites are key to understanding health effects of wine polyphenolics. J. Nutr. 2009, 139, 1824S–1831S.
- Diarra, M.S.; Malouin, F. Antibiotics in Canadian poultry productions and anticipated alternatives. Front. Microbiol. 2014, 5, 282.
- Anderson, R.C.; Vodovnik, M.; Min, B.R.; Pinchak, W.E.; Krueger, N.A.; Harvey, R.B.; Nisbet, D.J. Bactericidal effect of hydrolysable and condensed tannin extracts on Campylobacter jejuni in vitro. Folia Microbiol. 2012, 57, 253–258.
- Bonilla, J.; Sobral, P.J.d.A. Antioxidant and antimicrobial properties of ethanolic extracts of guarana, boldo, rosemary and cinnamon. Braz. J. Food Technol. 2017, 20, 1–8.
- Kikusato, M. Phytobiotics to improve health and production of broiler chickens: Functions beyond the antioxidant activity. Anim. Biosci. 2021, 34, 345.
- Jha, R.; Fouhse, J.M.; Tiwari, U.P.; Li, L.; Willing, B.P. Dietary Fiber and Intestinal Health of Monogastric Animals. Front. Vet. Sci. 2019, 6, 48.
- Choct, M. Managing gut health through nutrition. Br. Poult. Sci. 2009, 50, 9–15.
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Front. Immunol 2020, 11, 2054.
- Hansson, G.C. Role of mucus layers in gut infection and inflammation. Curr. Opin. Microbiol 2012, 15, 57–62.
- Şimşek, Ü.G.; Ciftci, M.; Doğan, G.; Özçelik, M. Antioxidant activity of cinnamon bark oil (Cinnamomum zeylanicum L.) in Japanese quails under thermo neutral and heat stressed conditions. Kafkas Univ. Vet. Fak. Derg. 2013, 19, 889–894.
- Dawson, J.; Miltz, W.; Mir, A.K.; Wiessner, C. Targeting monocyte chemoattractant protein-1 signalling in disease. Expert. Opin. Ther. Targets 2003, 7, 35–48.
- Pannee, C.; Chandhanee, I.; Wacharee, L. Antiinflammatory effects of essential oil from the leaves of Cinnamomum cassia and cinnamaldehyde on lipopolysaccharide-stimulated J774A.1 cells. J. Adv. Pharm. Technol. Res. 2014, 5, 164–170.
- Youn, H.S.; Lee, J.K.; Choi, Y.J.; Saitoh, S.I.; Miyake, K.; Hwang, D.H.; Lee, J.Y. Cinnamaldehyde suppresses toll-like receptor 4 activation mediated through the inhibition of receptor oligomerization. Biochem. Pharmacol. 2008, 75, 494–502.
- Lillehoj, H.S.; Kim, D.K.; Bravo, D.M.; Lee, S.H. Effects of dietary plant-derived phytonutrients on the genome-wide profiles and coccidiosis resistance in the broiler chickens. BMC Proc. 2011, 5 (Suppl. 4), 1–8.




