| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tomasz Andrzej Pawłowski | + 2319 word(s) | 2319 | 2021-02-04 10:24:15 | | | |
| 2 | Karina Chen | Meta information modification | 2319 | 2021-02-06 06:42:33 | | |
Video Upload Options
Environmental conditions are the basis of plant reproduction and are the critical factors controlling seed dormancy and germination. Global climate change is currently affecting environmental conditions and changing the reproduction of plants from seeds. Disturbances in germination will cause disturbances in the diversity of plant communities. Models developed for climate change scenarios show that some species will face a significant decrease in suitable habitat area. Dormancy is an adaptive mechanism that affects the probability of survival of a species. The ability of seeds of many plant species to survive until dormancy recedes and meet the requirements for germination is an adaptive strategy that can act as a buffer against the negative effects of environmental heterogeneity. The influence of temperature and humidity on seed dormancy status underlines the need to understand how changing environmental conditions will affect seed germination patterns.
1. Adaptations of Seed Germination to the Changing Environment
Climate and geographical changes in the history of a population are important indicators of the adaptive evolution of seed germination [1][2]. A meta-analysis of 3164 plant species showed that plants in environments associated with frost and/or drought are more likely to have some form of dormancy [3]. The environmental variability (seasonality) is crucial to explaining the existence or absence of dormancy and evolutionary transitions between these states [4]. In addition to the genetic background of this adaptation, environmental factors can shape the depth of dormancy and seed germination [5]. Models developed for climate change scenarios show that many species would face a significant decrease in suitable habitat area [6], also because of disturbances in the reproduction process (Figure 1). For example, in species with physiological dormancies, germination will be delayed with global warming, if the current length of the stratification period approximates its minimum requirement, as shortened winters will not adequately overcome dormancy. On the other hand, germination will be earlier if the current length of stratification greatly exceeds the minimum required, as premature spring warm-up accelerates germination. Simulation of the germination response to diurnally alternating temperatures under climate change scenarios showed that increasing temperatures decreased the base temperature for seed germination and the thermal time required for germination [7]. The effect of higher temperatures increased germination under future climate scenarios, but this seems to be only to a limited extent—temperature will also have inhibitory effects [8][9]. The germination variability, however, complicates generalizing the impact of climate change.
The number of studies conducted on the ecophysiology of seed dormancy and germination with regard to climate change is now increasing [10] Changes in temperature and water availability affect the seed germination and survival of plants [11][12]. Germination responses to temperature differ among species but also within species across their latitudinal and altitudinal ranges [13]. In response to environmental factors, seed dormancy and germination characteristics may vary within one species. Many authors have demonstrated variability of dormancy among seed collections from different places and years [14][15][16][17]. Variability of dormancy shows latitudinal differences on a wide geographical scale [18][19][20], and there is also a positive correlation between dormancy and population altitude [21][22][23]. Variability of dormancy has also been detected among seed collections from different environments—e.g., the negative influence of winter temperatures on seed dormancy in a wide gradient along western North America has been described for Artemisia tridentata [24]. Differences in the germination ability of Thymelaea hirsuta (L.) Endl. seeds collected from six different desert habitats has been established, with lower germination in seeds from more extreme sites [25]. Seed dormancy responses to temperature in Patagonian Nothofagus species are related to distribution and determines temporal patterns of germination across altitudes [26]. This phenomenon may influence the maintenance of vegetation patterns in these ecosystems by placing germinated seeds in a favorable environment for growth. It is presumed that the mere presence of intraspecies variability shows the enormous potential of physiological dormancy in adaptation to rapid environmental changes [27].
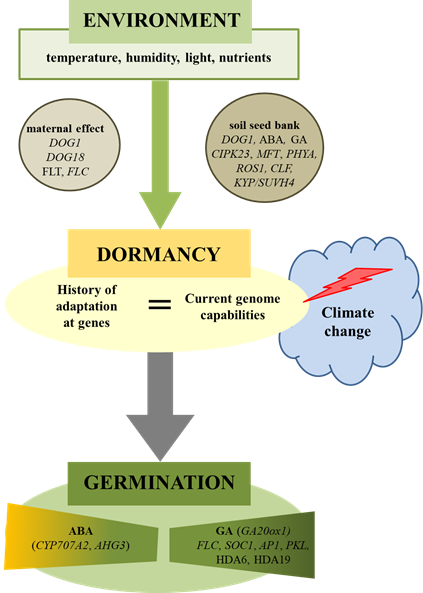
Figure 1. Environmental regulation of plant seed dormancy and germination. Seed germination is dependent on environmental conditions acting during maturation (maternal effect) as well as during storage (soil seed bank). Climate change can cause disturbances in the seed germination if the history of species (genetics) as well as the present (ecology) will not match. Physiological and molecular factors were involved in this regulation. ABA-hypersensitive Germination 3 (AHG3), abscisic Acid (ABA), Apetala1 (AP1), Cbl-Interacting Protein Kinase 23 (CIPK23), Curly Leaf (CLF), Cytochrome P450 Monooxygenase (CYP707A2), Delay OF Germination 1 (DOG1), Flowering Locus T (FLT), Flowering Locus C (FLC), Giberellic Acid (GA), Gibberellin 20-Oxidase 1 (GA20ox1), Histone Deacetylase 6 (HDA6), Kryptonite (KYP/SUVH4), Mother OF Flowering Time (MFT), Nitrite Reductase 1 (NRT1.1), Pickle (PKL), Phytochrome A (PHYA), Repressor OF Silencing1 (ROS1), Suppressor of Overexpression of Constans 1 (SOC1).
Understanding the sensitivity of a given species to changes in climate requires determining its germination temperature thresholds, including the variations among populations along the climate gradient they populate [13]. The responses are usually idiosyncratic and related to the local climate of the population. For instance, in studies along latitudinal gradients, in some cases, an inverse relationship between latitude and germination temperature has been documented, whereby populations at higher latitudes have increased germination rates at warmer temperatures, while populations at lower latitudes have high germination rates at colder temperatures. However, other works show the opposite pattern or the lack of a clear relationship between latitude and germination temperature [13]. Germination of western Iberia Erica australis and E. umbellata seeds showed that rising temperatures will affect these species, particularly at their northern ranges, where many seeds will remain dormant during warmer winters [28]. This study proved that models aiming at assessing climate change impacts in the species need to include the variability across latitudes. The examination of the species from arid Australia showed that some of the studied species had significantly greater levels of germination after exposure to predicted increased soil temperatures, in addition to another species displaying a dramatic decrease in seed viability [10]. Additionally, Dwyer and Erickson [29] indicate that most of the Australian (the Mediterranean climate) winter annual species studied will germinate higher fractions of seeds under future climate conditions due to the cumulative effects of warmer maternal, after-ripening, and germination environments. The analysis of 55 cactus species from the Americas, reflecting the broad environmental envelope of the family, indicated that 25% of species will have reduced germination performances, whilst the remainder will have efficiency gains by the end of the 21st century [30]. In the example of species from a Brazilian tropical dry forest which is tolerant to extreme temperature and water deficits, in future climate scenarios, rainfall rather than temperature will be extremely limiting for seed germination [31]. The same was showed for savanna species Acacia nigrescens and Colophospermum mopane, where higher future temperatures will not limit germination directly, but they will reduce the number of germination events by reducing the time window of suitable available soil water [32].
Studies on Atlantic–European Hypericum elodes L., characterized by physiological dormancy, showed that the populations did not respond equally to stratification, as the benefit of cold stratification for the southern population was lower [33]. The seed dormancy was clearly correlated with the maturation environment: higher temperatures in the summer and winter precipitation predicted poor and rapid loss of dormancy, respectively. Research on the impact of autumn and spring heat waves on the seed germination of alpine plants showed that, in the absence of heat waves, germination took place mainly in spring, but in autumn, it was the opposite—germination increased significantly after heat waves in half of the tested species [34]. The study showed that heat waves can affect germination time, and warming can lead to germination transition mostly from spring to autumn [35], especially among nondormant or conditionally dormant seeds . Vitis vinifera subsp. sylvestris was investigated in four Sardinian populations over the full altitudinal range of the species [36]. Under the simulated climate warming scenarios, an altitude-related risk from climate warming was identified, with lowland populations being more threatened due to a compromised seed dormancy release and a narrowed seed germination window. Daws et al. [37] observed differences in the dormancy breakage and germination of seeds collected from sycamore trees growing in Europe. Batches of seeds from the south germinated under the influence of higher temperatures than those from the north, and batches of seeds from northern Europe required low temperature stratification. The effect on the germination had the sum of heat to which the trees were exposed during seed maturation.
Turkish pine (Pinus brutia Ten.) seeds growing in a cold climate germinated only under the influence of low temperatures, whereas seeds of trees growing in a dry, warm climate germinated at a wide temperature range [19]. Their adaptation strategy (survival of young seedlings) assumes the germination of seeds in the spring (cold climate) or in autumn (hot and dry climate), or both seasons (in intermediate conditions). Gosling et al. [38], who studied black alder [Alnus glutinosa (L.) Gaertn.] seeds, showed that cold stratification improves seed germination in a wider temperature range. This feature promotes germination in the autumn and stimulates earlier and more synchronous seed emergence in a wider range of temperatures in the next spring. This also shows how the population can survive climate changes. If climate change brings a longer and warmer autumn, more seeds will germinate before winter. If the winter will be warm and/or short, those seedlings will develop well until the spring. Even if frost kills these seedlings, some seeds will remain dormant until the spring. These experiments showed the adaptive potential of black alder to the changing climate.
Research conducted on the Mediterranean genus Romulea shows that phylogenetically closely related species show differences in ecophysiological traits, such as dormancy and germination characteristics, thus reflecting the different habitats and bioclimatic areas in which they occur [39]. The authors stated that the seed maturation environment may play an even greater role than genetic variation in explaining the processes of dormancy [40]. Vidigal et al. [39] linked the seed dormancy of A. thaliana (from different stands in Spain) with altitude and climate. They indicated that deep dormancy is associated with high temperature, low rainfall, and high sunlight. Escudero et al. [41] studied different pine species and found intraspecific and intrapopulation variability in seed germination. The authors concluded that the differences between populations are not a consequence of different ecotypes, but are the result of the environment of the mother plant and the maternal genotype. Variability in seed germination may increase the chances of survival of the species under changing climatic conditions [42]. Another study on A. thaliana showed that both the origin of the population and the temperature during seed maturation affect the level of dormancy and the expression of the genes controlling dormancy variability [43]. A study of the dormancy of Centaurium somedanum Lainz. seeds showed that populations growing at lower altitudes in a generally milder climate benefited from a longer growing season and produced seeds that would sprout earlier [27]. Plants from higher heights, where the winters are sharper, produced seeds that would not germinate until the end of the unfavorable season. The authors also proved that dormancy has a genetic basis, but may show significant adaptive changes in response to short-term climate changes [27].
Climate change may be beneficial for some plant species, enabling them to find new ecological niches, which have had so far unfavorable conditions for the production and germination of seeds. Global warming may increase germination capacity and seed survival of species characterized by high plasticity. It seems, however, that along with the deepening of the climate changes, as a result of exceeding the tolerance barriers for a given species, the developed adaptive mechanisms related to reproduction of plants may fail, consequently leading to the disappearance of species in a given area. From a practical point of view, it seems necessary to conduct new experiments in natural conditions on the influence of climate on the germination of seeds, which will enable the determination of the relationship between the reproduction of plants from seeds and climate warming and facilitate the selection of appropriate, more plastic populations and species.
2. Mechanisms of Adaptation of Seed Germination to the Changing Environment
A variety of dormancy mechanisms have been observed, in line with the diversity of climates and habitats that various plant species have been able to colonize [44][45]. Physiological dormancy is the most commonly occurring form across all major angiosperm clades and is the class present in most seed model species [45][46][47]. It may facilitate the colonization of new environments by allowing species to adjust germination time in variable or new habitat schemes [48]. Thanks to laboratory research, much of which includes the analysis of mutants and changes in their dormancy and germination, the basic mechanisms responsible for the regulation of dormancy and germination are well known [48][49][50][51]. The mechanisms that regulate these processes are mainly based on maintaining a dynamic balance between abscisic acid (ABA) and gibberellins (GAs) and a set of many genes that regulate their metabolism, perception, and sensitivity through signaling networks [52][53][54][55][56][57]. Important for these processes are genes of the DOG (Delay Of Germinatio) family[58][59][60]. Seed dormancy-specific loci, including the DOG genes, were identified by analyzing the quantitative trait locus (QTL) using the natural variability of Arabidopsis [61][62][63]. One of the DOG family genes (DOG1) has been characterized in detail, and its genetic role in seed dormancy and the importance of gene expression in the sensing and adaptation of the environment have been well documented [64][65]. However, we do not know everything about this gene yet [60]. Transcription of DOG1 is initially low in developing seeds; however, it increases with dormancy acquisition and disappears completely in the initial germination phase [65]. Consequently, the expression of DOG1 shows a high correlation with the depth of seed dormancy [65]. For DOG1 protein, its chemical property, rather than quantity, is critical for maintaining seed dormancy, and its change into the nonfunctional form during maturation allows seed germination [66]. DOG1 protein, however, loses its function in completely nondormant seeds, which may be affected by the post-translational modification (PTM) of the protein [66]. The expression of DOG1 is regulated by ABA [67] and both are necessary for determining primary seed dormancy [66][68]. However, DOG1 may act independently of ABA to delay the germination of dormant seeds [69].
References
- Donohue, K.; Casas, R.R. de; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, Postgermination Adaptation, and Species Ecological Ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319, doi:10.1146/annurev-ecolsys-102209-144715.
- Williams, J.T.; Harper, J.L. Seed Polymorphism and Germination. Weed Res. 1965, 5, 141–150, doi:10.1111/j.1365-3180.1965.tb00337.x.
- Jurado, E.; Flores, J. Is Seed Dormancy under Environmental Control or Bound to Plant Traits? J. Veg. Sci. 2005, 16, 559–564, doi:10.1111/j.1654-1103.2005.tb02396.x.
- Rubio de Casas, R.; Willis, C.G.; Pearse, W.D.; Baskin, C.C.; Baskin, J.M.; Cavender-Bares, J. Global Biogeography of Seed Dormancy Is Determined by Seasonality and Seed Size: A Case Study in the Legumes. New Phytol. 2017, 214, 1527–1536, doi:10.1111/nph.14498.
- Pawłowski, T.A.; Klupczyńska, E.A.; Staszak, A.M.; Suszka, J. Proteomic Analysis of Black poplar (Populus Nigra L.) Seed Storability. Ann. For. Sci. 2019, 76, 104, doi:10.1007/s13595-019-0887-y.
- Dyderski, M.K.; Paz, S.; Frelich, L.E.; Jagodzinski, A.M. How Much Does Climate Change Threaten European Forest Tree Species Distributions? Glob. Chang. Biol. 2018, 24, 1150–1163, doi:10.1111/gcb.13925.
- Fernández-Pascual, E.; Seal, C.E.; Pritchard, H.W. Simulating the Germination Response to Diurnally Alternating Tempera-tures under Climate Change Scenarios: Comparative Studies on Carex diandra Seeds. Ann. Bot. 2015, 115, 201–209, doi:10.1093/aob/mcu234.
- Pawłowski, T.A.; Bujarska-Borkowska, B.; Suszka, J.; Tylkowski, T.; Chmielarz, P.; Klupczyńska, E.A.; Staszak, A.M. Tem-perature Regulation of Primary and Secondary Seed Dormancy in Rosa canina L.: Findings from Proteomic Analysis. Int. J. Mol. Sci. 2020, 21, 7008, doi:10.3390/ijms21197008.
- Gareca, E.E.; Vandelook, F.; Fernández, M.; Hermy, M.; Honnay, O. Seed Germination, Hydrothermal Time Models and the Effects of Global Warming on a Threatened High Andean Tree Species. Seed Sci. Res. 2012, 22, 287–298, doi:10.1017/S0960258512000189.
- Ooi, M.K.J.; Auld, T.D.; Denham, A.J. Climate Change and Bet-Hedging: Interactions between Increased Soil Temperatures and Seed Bank Persistence. Glob. Chang. Biol. 2009, 15, 2375–2386, doi:10.1111/j.1365-2486.2009.01887.x.
- Lippmann, R.; Babben, S.; Menger, A.; Delker, C.; Quint, M. Development of Wild and Cultivated Plants under Global Warming Conditions. Curr. Biol. 2019, 29, R1326–R1338, doi:10.1016/j.cub.2019.10.016.
- Penfield, S.; MacGregor, D.R. Effects of Environmental Variation during Seed Production on Seed Dormancy and Germina-tion. J. Exp. Bot. 2017, 68, 819–825, doi:10.1093/jxb/erw436.
- Chamorro, D.; Luna, B.; Moreno, J.M. Germination Responses to Current and Future Temperatures of Four Seeder Shrubs across a Latitudinal Gradient in Western Iberia. Am. J. Bot. 2017, 104, 83–91, doi:10.3732/ajb.1600278.
- Herranz, J.M.; Copete, M.Á.; Ferrandis, P.; Copete, E. Intermediate Complex Morphophysiological Dormancy in the Endemic Iberian Aconitum napellus Subsp. castellanum (Ranunculaceae). Seed Sci. Res. 2010, 20, 109–121, doi:10.1017/S0960258510000048.
- Koutecká, E., Lepš, J. Effect of Light and Moisture Conditions and Seed Age on Germination of Three Closely Related Myoso-tis Species. Folia Geobot. 2009, 44, 109, doi:10.1007/s12224-009-9038-9.
- Schütz, W.; Rave, G. Variation in Seed Dormancy of the Wetland Sedge, Carex Elongata, between Populations and Individu-als in Two Consecutive Years. Seed Sci. Res. 2003, 13, 315–322.
- Andersson, L.; Milberg, P. Variation in Seed Dormancy among Mother Plants, Populations and Years of Seed Collection. Seed Sci. Res. 1998, 8, 29–38, doi:10.1017/S0960258500003883.
- Wagmann, K.; Hautekèete, N.-C.; Piquot, Y.; Meunier, C.; Schmitt, S.E.; Van Dijk, H. Seed Dormancy Distribution: Explana-tory Ecological Factors. Ann. Bot. 2012, 110, 1205–1219, doi:10.1093/aob/mcs194.
- Skordilis, A.; Thanos, C.A. Seed Stratification and Germination Strategy in the Mediterranean Pines Pinus brutia and P. halepensis. Seed Sci. Res. 1995, 5, 151–160, doi:10.1017/S0960258500002774.
- Ren, Z.; Abbott, R.J. Seed Dormancy in Mediterranean Senecio Vulgaris L. New Phytol. 1991, 117, 673–678, doi:10.1111/j.1469-8137.1991.tb00972.x.
- Cavieres, L.A.; Arroyo, M.T.K. Seed Germination Response to Cold Stratification Period and Thermal Regime in Phacelia Secunda (Hydrophyllaceae)—Altitudinal Variation in the Mediterranean Andes of Central Chile. Plant Ecol. 2000, 149, 1–8, doi:10.1023/A:1009802806674.
- Holm, S.-O. Reproductive Patterns of Betula Pendula and B. Pubescens Coll. along a Regional Altitudinal Gradient in North-ern Sweden. Ecography 1994, 17, 60–72, doi:10.1111/j.1600-0587.1994.tb00077.x.
- Beardsell, D.; Mullet, J. Seed Generation of Eucalyptus Pauciflora Sieb. Ex Spreng. From Low and High Altitude Populations in Victoria. Aust. J. Bot. 1984, 32, 475–480, doi:10.1071/bt9840475.
- Meyer, S.E. (Intermountain R.S.; Monsen, S.B. Habitat-Correlated Variation in Mountain Big Sagebrush (Artemisia tridentata Ssp. Vaseyana) Seed Germination Patterns. Ecol. A Publ. Ecol. Soc. Am. 1991, 72, 739–742.
- El-Keblawy, A.A.; Shaltout, K.H.; Doust, J.L.; Doust, L.L. Maternal Effects on Progeny in Thymelaea hirsuta. New Phytol. 1996, 132, 77–85, doi:10.1111/j.1469-8137.1996.tb04511.x.
- Arana, M.V.; Gonzalez‐Polo, M.; Martinez‐Meier, A.; Gallo, L.A.; Benech‐Arnold, R.L.; Sánchez, R.A.; Batlla, D. Seed Dor-mancy Responses to Temperature Relate to Nothofagus Species Distribution and Determine Temporal Patterns of Germination across Altitudes in Patagonia. New Phytol. 2016, 209, 507–520, doi:10.1111/nph.13606.
- Fernandez-Pascual, E.; Jimenez-Alfaro, B.; Caujape-Castells, J.; Jaen-Molina, R.; Emilio Diaz, T. A Local Dormancy Cline Is Related to the Seed Maturation Environment, Population Genetic Composition and Climate. Ann. Bot. 2013, 112, 937–945, doi:10.1093/aob/mct154.
- Chamorro, D.; Luna, B.; Moreno, J.M. Local Climate Controls Among-Population Variation in Germination Patterns in Two Erica Species across Western Iberia. Seed Sci. Res. 2018, 28, 112–122, doi:10.1017/S0960258518000041.
- Dwyer, J.M.; Erickson, T.E. Warmer Seed Environments Increase Germination Fractions in Australian Winter Annual Plant Species. Ecosphere 2016, 7, e01497, doi:10.1002/ecs2.1497.
- Seal, C.E.; Daws, M.I.; Flores, J.; Ortega‐Baes, P.; Galíndez, G.; León‐Lobos, P.; Sandoval, A.; Stuva, A.C.; Bullón, N.R.; Dávila‐Aranda, P.; et al. Thermal Buffering Capacity of the Germination Phenotype across the Environmental Envelope of the Cactaceae. Glob. Chang. Biol. 2017, 23, 5309–5317, doi:10.1111/gcb.13796.
- Dantas, B.F.; Moura, M.S.B.; Pelacani, C.R.; Angelotti, F.; Taura, T.A.; Oliveira, G.M.; Bispo, J.S.; Matias, J.R.; Silva, F.F.S.; Pritchard, H.W.; et al. Rainfall, Not Soil Temperature, Will Limit the Seed Germination of Dry Forest Species with Climate Change. Oecologia 2020, 192, 529–541, doi:10.1007/s00442-019-04575-x.
- tevens, N.; Seal, C.E.; Archibald, S.; Bond, W. Increasing Temperatures Can Improve Seedling Establishment in Ar-id-Adapted Savanna Trees. Oecologia 2014, 175, 1029–1040, doi:10.1007/s00442-014-2958-y.
- Carta, A.; Probert, R.; Puglia, G.; Peruzzi, L.; Bedini, G. Local Climate Explains Degree of Seed Dormancy in Hypericum elodes L. (Hypericaceae). Plant Biol. 2016, 18 (Suppl. S1), 76–82, doi:10.1111/plb.12310.
- Orsenigo, S.; Abeli, T.; Rossi, G.; Bonasoni, P.; Pasquaretta, C.; Gandini, M.; Mondoni, A. Effects of Autumn and Spring Heat Waves on Seed Germination of High Mountain Plants. PLoS ONE 2015, 10, e0133626, doi:10.1371/journal.pone.0133626.
- Mondoni, A.; Rossi, G.; Orsenigo, S.; Probert, R.J. Climate Warming Could Shift the Timing of Seed Germination in Alpine Plants. Ann. Bot. 2012, 110, 155–164, doi:10.1093/aob/mcs097.
- Orrù, M.; Mattana, E.; Pritchard, H.W.; Bacchetta, G. Thermal Thresholds as Predictors of Seed Dormancy Release and Ger-mination Timing: Altitude-Related Risks from Climate Warming for the Wild Grapevine Vitis Vinifera Subsp. Sylvestris. Ann. Bot. 2012, 110, 1651–1660, doi:10.1093/aob/mcs218.
- Daws, M.I.; Cleland, H.; Chmielarz, P.; Gorian, F.; Leprince, O.; Mullins, C.E.; Thanos, C.A.; Vandvik, V.; Pritchard, H.W. Variable Desiccation Tolerance in Acer pseudoplatanus Seeds in Relation to Developmental Conditions: A Case of Phenotypic Recalcitrance? Funct. Plant Biol. 2006, 33, 59–66, doi:10.1071/FP04206.
- Gosling, P.G.; McCartan, S.A.; Peace, A.J. Seed Dormancy and Germination Characteristics of Common Alder (Alnus glutino-sa L.) Indicate Some Potential to Adapt to Climate Change in Britain. Forestry 2009, 82, 573–582, doi:10.1093/forestry/cpp024.
- Vidigal, D.S.; Marques, A.C.S.S.; Willems, L.A.J.; Buijs, G.; Méndez-Vigo, B.; Hilhorst, H.W.M.; Bentsink, L.; Picó, F.X.; Alonso-Blanco, C. Altitudinal and Climatic Associations of Seed Dormancy and Flowering Traits Evidence Adaptation of Annual Life Cycle Timing in Arabidopsis thaliana. Plant Cell Environ. 2016, 39, 1737–1748, doi:10.1111/pce.12734.
- Carta, A.; Hanson, S.; Müller, J.V. Plant Regeneration from Seeds Responds to Phylogenetic Relatedness and Local Adapta-tion in Mediterranean Romulea (Iridaceae) Species. Ecol. Evol. 2016, 6, 4166–4178, doi:10.1002/ece3.2150.
- Escudero, A.; Pérez-García, F.; Luzuriaga, A.L. Effects of Light, Temperature and Population Variability on the Germination of Seven Spanish Pines. Seed Sci. Res. 2002, 12, 261–271, doi:10.1079/SSR2002116.
- Cochrane, A.; Yates, C.J.; Hoyle, G.L.; Nicotra, A.B. Will Among-Population Variation in Seed Traits Improve the Chance of Species Persistence under Climate Change? Glob. Ecol. Biogeogr. 2015, 24, 12–24, doi:10.1111/geb.12234.
- Chiang, G.C.K.; Bartsch, M.; Barua, D.; Nakabayashi, K.; Debieu, M.; Kronholm, I.; Koornneef, M.; Soppe, W.J.J.; Donohue, K.; De MEAUX, J. DOG1 Expression Is Predicted by the Seed-Maturation Environment and Contributes to Geographical Variation in Germination in Arabidopsis thaliana. Mol. Ecol. 2011, 20, 3336–3349, doi:10.1111/j.1365-294X.2011.05181.x.
- Baskin, J.; Baskin, C. A Classification System for Seed Dormancy. Seed Sci. Res. 2004, 14, 1–16, doi:10.1079/SSR2003150.
- Willis, C.G.; Baskin, C.C.; Baskin, J.M.; Auld, J.R.; Venable, D.L.; Cavender-Bares, J.; Donohue, K.; Rubio de Casas, R.; The NESCent Germination Working Group The Evolution of Seed Dormancy: Environmental Cues, Evolutionary Hubs, and Di-versification of the Seed Plants. New Phytol. 2014, 203, 300–309, doi:10.1111/nph.12782.
- Staszak, A.M.; Guzicka, M.; Pawłowski, T.A. Signalling Regulators of Abscisic and Gibberellic Acid Pathways Are Involved in Dormancy Breaking of Norway Maple (Acer platanoides L.) Seeds. Acta Physiol. Plant 2017, 39, 251, doi:10.1007/s11738-017-2544-0.
- Staszak, A.M.; Rewers, M.; Sliwinska, E.; Klupczyńska, E.A.; Pawłowski, T.A. DNA Synthesis Pattern, Proteome, and ABA and GA Signalling in Developing Seeds of Norway Maple (Acer platanoides). Funct. Plant Biol. 2019, 46, 152–164, doi:10.1071/FP18074.
- Donohue, K. Seeds and Seasons: Interpreting Germination Timing in the Field. Seed Sci. Res. 2005, 15, 175–187, doi:10.1079/SSR2005208.
- North, H.; Baud, S.; Debeaujon, I.; Dubos, C.; Dubreucq, B.; Grappin, P.; Jullien, M.; Lepiniec, L.; Marion-Poll, A.; Miquel, M.; et al. Arabidopsis Seed Secrets Unravelled after a Decade of Genetic and Omics-Driven Research. Plant J. 2010, 61, 971–981, doi:10.1111/j.1365-313X.2009.04095.x.
- Dekkers, B.J.W.; Bentsink, L. Regulation of Seed Dormancy by Abscisic Acid and Delay of Germination 1. Seed Sci. Res. 2015, 25, 82–98, doi:10.1017/S0960258514000415.
- Johnston, I.G.; Bassel, G.W. Identification of a Bet-Hedging Network Motif Generating Noise in Hormone Concentrations and Germination Propensity in Arabidopsis. J. R. Soc. Interface 2018, 15, 20180042, doi:10.1098/rsif.2018.0042.
- Nambara, E.; Okamoto, M.; Tatematsu, K.; Yano, R.; Seo, M.; Kamiya, Y. Abscisic Acid and the Control of Seed Dormancy and Germination. Seed Sci. Res. 2010, 20, 55–67, doi:10.1017/S0960258510000012.
- Bassel, G.W.; Lan, H.; Glaab, E.; Gibbs, D.J.; Gerjets, T.; Krasnogor, N.; Bonner, A.J.; Holdsworth, M.J.; Provart, N.J. Ge-nome-Wide Network Model Capturing Seed Germination Reveals Coordinated Regulation of Plant Cellular Phase Transi-tions. PNAS 2011, 108, 9709–9714, doi:10.1073/pnas.1100958108.
- Morris, K.; Linkies, A.; Müller, K.; Oracz, K.; Wang, X.; Lynn, J.R.; Leubner-Metzger, G.; Finch-Savage, W.E. Regulation of Seed Germination in the Close Arabidopsis Relative Lepidium sativum: A Global Tissue-Specific Transcript Analysis. Plant Physiol. 2011, 155, 1851–1870, doi:10.1104/pp.110.169706.
- Dekkers, B.J.W.; Pearce, S.; Bolderen-Veldkamp, R.P. van; Marshall, A.; Widera, P.; Gilbert, J.; Drost, H.-G.; Bassel, G.W.; Müller, K.; King, J.R.; et al. Transcriptional Dynamics of Two Seed Compartments with Opposing Roles in Arabidopsis Seed Germination. Plant Physiol. 2013, 163, 205–215, doi:10.1104/pp.113.223511.
- Finch-Savage, W.E.; Bassel, G.W. Seed Vigour and Crop Establishment: Extending Performance beyond Adaptation. J. Exp. Bot. 2016, 67, 567–591, doi:10.1093/jxb/erv490.
- Bassel, G.W. To Grow or Not to Grow? Trends Plant Sci. 2016, 21, 498–505, doi:10.1016/j.tplants.2016.02.001.
- Bentsink, L.; Jowett, J.; Hanhart, C.J.; Koornneef, M. Cloning of DOG1, a Quantitative Trait Locus Controlling Seed Dor-mancy in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 17042–17047, doi:10.1073/pnas.0607877103.
- Graeber, K.; Linkies, A.; Müller, K.; Wunchova, A.; Rott, A.; Leubner-Metzger, G. Cross-Species Approaches to Seed Dor-mancy and Germination: Conservation and Biodiversity of ABA-Regulated Mechanisms and the Brassicaceae DOG1 Genes. Plant Mol. Biol. 2010, 73, 67–87, doi:10.1007/s11103-009-9583-x.
- Nonogaki, H. Seed Dormancy and Germination—Emerging Mechanisms and New Hypotheses. Front. Plant Sci. 2014, 5, doi:10.3389/fpls.2014.00233.
- Alonso-Blanco, C.; Bentsink, L.; Hanhart, C.J.; Vries, H.B.; Koornneef, M. Analysis of Natural Allelic Variation at Seed Dor-mancy Loci of Arabidopsis Thaliana. Genetics 2003, 164, 711–729.
- Bentsink, L.; Hanson, J.; Hanhart, C.J.; Vries, H.B.; Coltrane, C.; Keizer, P.; El-Lithy, M.; Alonso-Blanco, C.; Andrés, M.T. de; Reymond, M.; et al. Natural Variation for Seed Dormancy in Arabidopsis Is Regulated by Additive Genetic and Molecular Pathways. PNAS 2010, 107, 4264–4269, doi:10.1073/pnas.1000410107.
- Chiang, G.C.K.; Barua, D.; Dittmar, E.; Kramer, E.M.; de Casas, R.R.; Donohue, K. Pleiotropy in the Wild: The Dormancy Gene Dog1 Exerts Cascading Control on Life Cycles. Evolution 2013, 67, 883–893, doi:10.1111/j.1558-5646.2012.01828.x.
- Kronholm, I.; Picó, F.X.; Alonso-Blanco, C.; Goudet, J.; de Meaux, J. Genetic Basis of Adaptation in Arabidopsis Thaliana: Local Adaptation at the Seed Dormancy QTL DOG1. Evolution 2012, 66, 2287–2302, doi:10.1111/j.1558-5646.2012.01590.x.
- Footitt, S.; Clay, H.A.; Dent, K.; Finch-Savage, W.E. Environment Sensing in Spring-Dispersed Seeds of a Winter Annual Arabidopsis Influences the Regulation of Dormancy to Align Germination Potential with Seasonal Changes. New Phytol. 2014, 202, 929–939, doi:10.1111/nph.12694.
- Nakabayashi, K.; Bartsch, M.; Xiang, Y.; Miatton, E.; Pellengahr, S.; Yano, R.; Seo, M.; Soppe, W.J.J. The Time Required for Dormancy Release in Arabidopsis Is Determined by DELAY OF GERMINATION1 Protein Levels in Freshly Harvested Seeds[OA]. Plant Cell 2012, 24, 2826–2838, doi:10.1105/tpc.112.100214.
- Graeber, K.; Linkies, A.; Müller, K.; Wunchova, A.; Rott, A.; Leubner-Metzger, G. Cross-Species Approaches to Seed Dor-mancy and Germination: Conservation and Biodiversity of ABA-Regulated Mechanisms and the Brassicaceae DOG1 Genes. Plant Mol. Biol. 2010, 73, 67–87, doi:10.1007/s11103-009-9583-x.
- Zhao, M.; Yang, S.; Liu, X.; Wu, K. Arabidopsis Histone Demethylases LDL1 and LDL2 Control Primary Seed Dormancy by Regulating DELAY OF GERMINATION 1 and ABA Signaling-Related Genes. Front. Plant Sci. 2015, 6, 159, doi:10.3389/fpls.2015.00159.
- Graeber, K.; Linkies, A.; Steinbrecher, T.; Mummenhoff, K.; Tarkowská, D.; Turečková, V.; Ignatz, M.; Sperber, K.; Voegele, A.; Jong, H. de; et al. DELAY OF GERMINATION 1 Mediates a Conserved Coat-Dormancy Mechanism for the Temperature- and Gibberellin-Dependent Control of Seed Germination. PNAS 2014, 111, E3571–E3580, doi:10.1073/pnas.1403851111.