
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Guilhem ARRACHART | + 2096 word(s) | 2096 | 2021-07-22 04:27:41 | | | |
| 2 | Enzi Gong | Meta information modification | 2096 | 2021-08-03 02:34:53 | | | | |
| 3 | Enzi Gong | Meta information modification | 2096 | 2021-08-03 02:35:30 | | |
Video Upload Options
Rare earth elements (REEs) as defined by the International Union of Pure and Applied Chemistry (IUPAC) include metals characterized by similar properties, namely scandium (Sc), yttrium (Y) and all the lanthanides. The latter correspond to the chemical elements listed in the periodic table of Mendeleev that have an atomic number ranging from 57 for lanthanum (La) to 71 for lutetium (Lu). REEs are often subdivided into “light rare earths elements” (LREEs) and “heavy rare earths elements” (HREEs) according to their atomic numbers. Yttrium is oftentimes associated with HREEs due to chemical similarities, including ionic radii. In some cases, the elements from samarium to terbium are considered as the “middle rare earth elements” (MREEs).
1. Overview
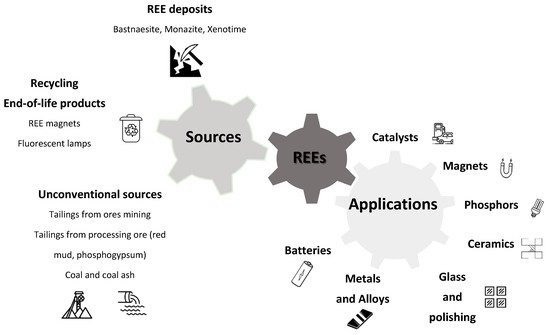
Rare earth elements (REEs) are becoming more and more significant as they play crucial roles in many advanced technologies. Therefore, the development of optimized processes for their recovery, whether from primary resources or from secondary sources, has become necessary, including recovery from mine tailings, recycling of end-of-life products and urban and industrial waste. Ionic solvents, including ionic liquids (ILs) and deep-eutectic solvents (DESs), have attracted much attention since they represent an alternative to conventional processes for metal recovery. These systems are used as reactive agents in leaching and extraction processes.
2. Rare Earth Elements
3. Description and Properties of Ionic Solvents
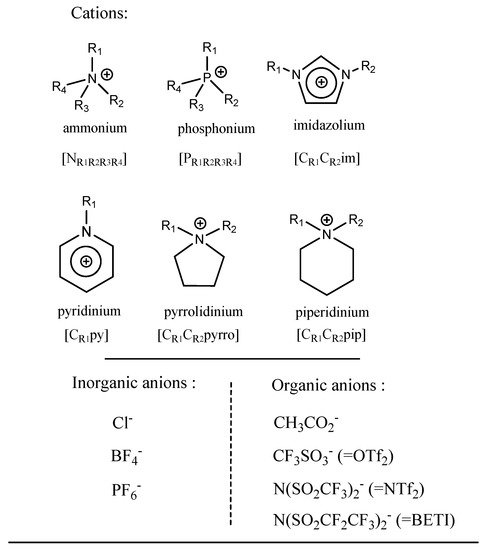
- -
-
Cat+ is usually a quaternary ammonium or phosphonium salt;
- -
-
X is the anionic moiety (generally a halide anion);
- -
-
Y is a metal chloride for type I, a metal chloride hydrate for type II and a hydrogen bond donor for type III.
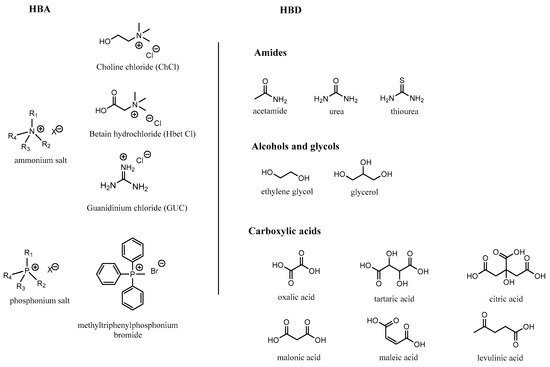
References
- Connelly, N.G.; Damhus, T.; Hartshorn, R.M.; Hutton, A.T. Nomenclature of Inorganic Chemistry—IUPAC Recommendations 2005. R. Soc. Chem. 2005, 27, 25–26.
- Rollinson, H.R. Using Geochemical Data: Evaluation, Presentation, Interpretation; Longman Scientific & Technica: Harlow, Essex, UK, 1993; ISBN 9780582067011.
- Balaram, V. Rare Earth Elements: A Review of Applications, Occurrence, Exploration, Analysis, Recycling, and Environmental Impact. Geosci. Front. 2019, 10, 1285–1303.
- Chakhmouradian, A.R.; Wall, F. Rare Earth Elements: Minerals, Mines, Magnets (and More). Elements 2012, 8, 333–340.
- Watari, T.; Nansai, K.; Nakajima, K. Review of Critical Metal Dynamics to 2050 for 48 Elements. Resour. Conserv. Recycl. 2020, 155, 104669.
- Jha, A.; Richards, B.; Jose, G.; Teddy-Fernandez, T.; Joshi, P.; Jiang, X.; Lousteau, J. Rare-Earth Ion Doped TeO2 and GeO2 Glasses as Laser Materials. Prog. Mater. Sci. 2012, 57, 1426–1491.
- Anashkina, E.A. Laser Sources Based on Rare-Earth Ion Doped Tellurite Glass Fibers and Microspheres. Fibers 2020, 8, 30.
- Coey, J.M.D. Perspective and Prospects for Rare Earth Permanent Magnets. Engineering 2020, 6, 119–131.
- Trench, A.; Sykes, J.P. Rare Earth Permanent Magnets and Their Place in the Future Economy. Engineering 2020, 6, 115–118.
- Lucas, J.; Lucas, P.; Le Mercier, T.; Rollat, A.; Davenport, W. Chapter 10—Rare Earths in Rechargeable Batteries. In Rare Earths; Lucas, J., Lucas, P., Le Mercier, T., Rollat, A., Davenport, W.B.T.-R.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 167–180. ISBN 978-0-444-62735-3.
- Zhao, H.; Xia, J.; Yin, D.; Luo, M.; Yan, C.; Du, Y. Rare Earth Incorporated Electrode Materials for Advanced Energy Storage. Coord. Chem. Rev. 2019, 390, 32–49.
- Alonso, E.; Sherman, A.M.; Wallington, T.J.; Everson, M.P.; Field, F.R.; Roth, R.; Kirchain, R.E. Evaluating Rare Earth Element Availability: A Case with Revolutionary Demand from Clean Technologies. Environ. Sci. Technol. 2012, 46, 3406–3414.
- Binnemans, K.; Jones, P.T. Rare Earths and the Balance Problem. J. Sustain. Metall. 2015, 1, 29–38.
- Kanazawa, Y.; Kamitani, M. Rare Earth Minerals and Resources in the World. J. Alloys Compd. 2006, 408–412, 1339–1343.
- Dutta, T.; Kim, K.-H.; Uchimiya, M.; Kwon, E.E.; Jeon, B.-H.; Deep, A.; Yun, S.-T. Global Demand for Rare Earth Resources and Strategies for Green Mining. Environ. Res. 2016, 150, 182–190.
- Khan, A.M.; Bakar, N.K.A.; Bakar, A.F.A.; Ashraf, M.A. Chemical Speciation and Bioavailability of Rare Earth Elements (REEs) in the Ecosystem: A Review. Environ. Sci. Pollut. Res. 2017, 24, 22764–22789.
- Hennebel, T.; Boon, N.; Maes, S.; Lenz, M. Biotechnologies for Critical Raw Material Recovery from Primary and Secondary Sources: R&D Priorities and Future Perspectives. New Biotechnol. 2015, 32, 121–127.
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of Rare Earths: A Critical Review. J. Clean. Prod. 2013, 51, 1–22.
- Gaustad, G.; Williams, E.; Leader, A. Rare Earth Metals from Secondary Sources: Review of Potential Supply from Waste and Byproducts. Resour. Conserv. Recycl. 2021, 167, 105213.
- Rivera, R.M.; Ounoughene, G.; Malfliet, A.; Vind, J.; Panias, D.; Vassiliadou, V.; Binnemans, K.; Van Gerven, T. A Study of the Occurrence of Selected Rare-Earth Elements in Neutralized–Leached Bauxite Residue and Comparison with Untreated Bauxite Residue. J. Sustain. Metall. 2019, 5, 57–68.
- Akcil, A.; Akhmadiyeva, N.; Abdulvaliyev, R.; Abhilash; Meshram, P. Overview On Extraction and Separation of Rare Earth Elements from Red Mud: Focus on Scandium. Miner. Process. Extr. Metall. Rev. 2018, 39, 145–151.
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards Zero-Waste Valorisation of Rare-Earth-Containing Industrial Process Residues: A Critical Review. J. Clean. Prod. 2015, 99, 17–38.
- Evans, K. The History, Challenges, and New Developments in the Management and Use of Bauxite Residue. J. Sustain. Metall. 2016, 2, 316–331.
- Cánovas, C.R.; Pérez-López, R.; Macías, F.; Chapron, S.; Nieto, J.M.; Pellet-Rostaing, S. Exploration of Fertilizer Industry Wastes as Potential Source of Critical Raw Materials. J. Clean. Prod. 2017, 143, 497–505.
- Cánovas, C.R.; Chapron, S.; Arrachart, G.; Pellet-Rostaing, S. Leaching of Rare Earth Elements (REEs) and Impurities from Phosphogypsum: A Preliminary Insight for Further Recovery of Critical Raw Materials. J. Clean. Prod. 2019.
- León, R.; Macías, F.; Cánovas, C.R.; Pérez-López, R.; Ayora, C.; Nieto, J.M.; Olías, M. Mine Waters as a Secondary Source of Rare Earth Elements Worldwide: The Case of the Iberian Pyrite Belt. J. Geochem. Explor. 2021, 224, 106742.
- Kawasaki, A.; Kimura, R.; Arai, S. Rare Earth Elements and Other Trace Elements in Wastewater Treatment Sludges. Soil Sci. Plant Nutr. 1998, 44, 433–441.
- Royer-Lavallée, A.; Neculita, C.M.; Coudert, L. Removal and Potential Recovery of Rare Earth Elements from Mine Water. J. Ind. Eng. Chem. 2020, 89, 47–57.
- Zhang, W.; Rezaee, M.; Bhagavatula, A.; Li, Y.; Groppo, J.; Honaker, R. A Review of the Occurrence and Promising Recovery Methods of Rare Earth Elements from Coal and Coal By-Products. Int. J. Coal Prep. Util. 2015, 35, 295–330.
- Rybak, A.; Rybak, A. Characteristics of Some Selected Methods of Rare Earth Elements Recovery from Coal Fly Ashes. Metals 2021, 11, 142.
- Tunsu, C.; Petranikova, M.; Gergorić, M.; Ekberg, C.; Retegan, T. Reclaiming Rare Earth Elements from End-of-Life Products: A Review of the Perspectives for Urban Mining Using Hydrometallurgical Unit Operations. Hydrometallurgy 2015, 156, 239–258.
- Sethurajan, M.; van Hullebusch, E.D.; Fontana, D.; Akcil, A.; Deveci, H.; Batinic, B.; Leal, J.P.; Gasche, T.A.; Ali Kucuker, M.; Kuchta, K.; et al. Recent Advances on Hydrometallurgical Recovery of Critical and Precious Elements from End of Life Electronic Wastes—A Review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 212–275.
- Rademaker, J.H.; Kleijn, R.; Yang, Y. Recycling as a Strategy against Rare Earth Element Criticality: A Systemic Evaluation of the Potential Yield of NdFeB Magnet Recycling. Environ. Sci. Technol. 2013, 47, 10129–10136.
- Bandara, H.M.D.; Darcy, J.W.; Apelian, D.; Emmert, M.H. Value Analysis of Neodymium Content in Shredder Feed: Toward Enabling the Feasibility of Rare Earth Magnet Recycling. Environ. Sci. Technol. 2014, 48, 6553–6560.
- Binnemans, K.; Jones, P.T. Perspectives for the Recovery of Rare Earths from End-of-Life Fluorescent Lamps. J. Rare Earths 2014, 32, 195–200.
- Tan, Q.; Li, J.; Zeng, X. Rare Earth Elements Recovery from Waste Fluorescent Lamps: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 749–776.
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Rare Earths. Int. Mater. Rev. 1992, 37, 197–248.
- Joshi, D.; Adhikari, N. An Overview on Common Organic Solvents and Their Toxicity. J. Pharm. Res. Int. 2019, 28, 1–18.
- Smiglak, M.; Reichert, W.M.; Holbrey, J.D.; Wilkes, J.S.; Sun, L.; Thrasher, J.S.; Kirichenko, K.; Singh, S.; Katritzky, A.R.; Rogers, R.D. Combustible Ionic Liquids by Design: Is Laboratory Safety Another Ionic Liquid Myth? Chem. Commun. 2006, 24, 2554–2556.
- Earle, M.J.; Esperança, J.M.S.S.; Gilea, M.A.; Lopes, J.N.C.; Rebelo, L.P.N.; Magee, J.W.; Seddon, K.R.; Widegren, J.A. The Distillation and Volatility of Ionic Liquids. Nature 2006, 439, 831–834.
- Esperança, J.M.S.S.; Canongia Lopes, J.N.; Tariq, M.; Santos, L.M.N.B.F.; Magee, J.W.; Rebelo, L.P.N. Volatility of Aprotic Ionic Liquids—A Review. J. Chem. Eng. Data 2010, 55, 3–12.
- Domínguez de María, P. Chapter 6—Ionic Liquids, Switchable Solvents, and Eutectic Mixtures. In The Application of Green Solvents in Separation Processes; Pena-Pereira, F., Tobiszewski, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 139–154. ISBN 978-0-12-805297-6.
- Singh, S.K.; Savoy, A.W. Ionic Liquids Synthesis and Applications: An Overview. J. Mol. Liq. 2020, 297, 112038.
- Abbott, A.P.; Frisch, G.; Gurman, S.J.; Hillman, A.R.; Hartley, J.; Holyoak, F.; Ryder, K.S. Ionometallurgy: Designer Redox Properties for Metal Processing. Chem. Commun. 2011, 47, 10031–10033.
- Leclerc, N.; Legeai, S.; Balva, M.; Hazotte, C.; Comel, J.; Lapicque, F.; Billy, E.; Meux, E. Recovery of Metals from Secondary Raw Materials by Coupled Electroleaching and Electrodeposition in Aqueous or Ionic Liquid Media. Metals 2018, 8, 556.
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576.
- Plechkova, N.V.; Seddon, K.R. Applications of Ionic Liquids in the Chemical Industry. Chem. Soc. Rev. 2008, 37, 123–150.
- Abbott, A.P.; Barron, J.C.; Ryder, K.S.; Wilson, D. Eutectic-Based Ionic Liquids with Metal-Containing Anions and Cations. Chem. Eur. J. 2007, 13, 6495–6501.
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082.
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982.
- Plechkova, N.V.; Seddon, K.R. Ionic Liquids: “Designer” Solvents for Green Chemistry. In Methods and Reagents for Green Chemistry: An Introduction; John Wiley & Sons: Hoboken, NJ, USA, 2007; pp. 103–130. ISBN 9780471754008.
- Micheau, C.; Arrachart, G.; Turgis, R.; Lejeune, M.; Draye, M.; Michel, S.; Legeai, S.; Pellet-Rostaing, S. Ionic Liquids as Extraction Media in a Two-Step Eco-Friendly Process for Selective Tantalum Recovery. ACS Sustain. Chem. Eng. 2020, 8, 1954–1963.
- Jacquemin, J.; Husson, P.; Padua, A.A.H.; Majer, V. Density and Viscosity of Several Pure and Water-Saturated Ionic Liquids. Green Chem. 2006, 8, 172–180.
- Bonhôte, P.; Dias, A.-P.; Papageorgiou, N.; Kalyanasundaram, K.; Grätzel, M. Hydrophobic, Highly Conductive Ambient-Temperature Molten Salts. Inorg. Chem. 1996, 35, 1168–1178.
- Paiva, A.; Matias, A.A.; Duarte, A.R.C. How Do We Drive Deep Eutectic Systems towards an Industrial Reality? Curr. Opin. Green Sustain. Chem. 2018, 11, 81–85.
- Florindo, C.; Lima, F.; Ribeiro, B.D.; Marrucho, I.M. Deep Eutectic Solvents: Overcoming 21st Century Challenges. Curr. Opin. Green Sustain. Chem. 2019, 18, 31–36.
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690.
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071.
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146.
- Qin, H.; Hu, X.; Wang, J.; Cheng, H.; Chen, L.; Qi, Z. Overview of Acidic Deep Eutectic Solvents on Synthesis, Properties and Applications. Green Energy Environ. 2020, 5, 8–21.
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 1, 70–71.
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285.
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147.
- van Osch, D.J.G.P.; Zubeir, L.F.; van den Bruinhorst, A.; Rocha, M.A.A.; Kroon, M.C. Hydrophobic Deep Eutectic Solvents as Water-Immiscible Extractants. Green Chem. 2015, 17, 4518–4521.
- Dwamena, A.K. Recent Advances in Hydrophobic Deep Eutectic Solvents for Extraction. Separations 2019, 6, 9.
- Makos, P.; Slupek, E.; Gebicki, J. Hydrophobic Deep Eutectic Solvents in Microextraction Techniques—A Review. Microchem. J. 2020, 152.
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for Green-Solvent Design: From Hydrophilic to Hydrophobic (Deep) Eutectic Solvents. Chem. Sus. Chem. 2019, 12, 1549–1559.
- Ribeiro, B.D.; Florindo, C.; Iff, L.C.; Coelho, M.A.Z.; Marrucho, I.M. Menthol-Based Eutectic Mixtures: Hydrophobic Low Viscosity Solvents. ACS Sustain. Chem. Eng. 2015, 3, 2469–2477.
- Martins, M.A.R.; Crespo, E.A.; Pontes, P.V.A.; Silva, L.P.; Bülow, M.; Maximo, G.J.; Batista, E.A.C.; Held, C.; Pinho, S.P.; Coutinho, J.A.P. Tunable Hydrophobic Eutectic Solvents Based on Terpenes and Monocarboxylic Acids. ACS Sustain. Chem. Eng. 2018, 6, 8836–8846.
- Abranches, D.O.; Martins, M.A.R.; Silva, L.P.; Schaeffer, N.; Pinho, S.P.; Coutinho, J.A.P. Phenolic Hydrogen Bond Donors in the Formation of Non-Ionic Deep Eutectic Solvents: The Quest for Type V DES. Chem. Commun. 2019, 55, 10253–10256.




