1000/1000
Hot
Most Recent

Sarcoidosis is a multisystem disorder of unknown origin and poorly understood pathogenesis that predominantly affects lungs and intrathoracic lymph nodes and is characterized by the presence of noncaseating granulomatous inflammation in involved organs. The disease is highly heterogeneous and can mimic a plethora of other disorders, making diagnosis a challenge even for experienced physicians. The evolution and severity of sarcoidosis are highly variable: many patients are asymptomatic and their disease course is generally benign with spontaneous resolution. However, up to one-third of patients develop chronic or progressive disease mainly due to pulmonary or cardiovascular complications that require long-term therapy. The diagnosis of sarcoidosis requires histopathological evidence of noncaseating granulomatous inflammation in one or more organs coupled with compatible clinical and radiological features and the exclusion of other causes of granulomatous inflammation; however, in the presence of typical disease manifestations such as Löfgren’s syndrome, Heerfordt’s syndrome, lupus pernio and asymptomatic bilateral and symmetrical hilar lymphadenopathy, the diagnosis can be established with high level of certainty on clinical grounds alone.
Sarcoidosis has numerous clinical manifestations, but respiratory tract involvement occurs at some point in nearly all patients. Dry cough, dyspnea and chest discomfort are the most common symptoms and are generally more prominent in patients with significant endobronchial or parenchymal involvement [1]. The skin, eyes, liver, spleen and peripheral lymph nodes are the next most common disease sites, with the frequency of involvement ranging from 10% to 30% [2]. Cardiac and neurological disease are uncommon but potentially lethal complications [3][4]. Manifestations such as small fiber neuropathy and fatigue are not organ-specific and have been reported in as many as 86% and more than 90% of patients, respectively [5][6].
Some forms of sarcoidosis are characterized by specific constellation of manifestations: Löfgren’s syndrome, which is generally acute, benign and self-limiting, is characterized by fever, BHL, erythema nodosum, and periarthritis [7], whereas Heerfordt’s syndrome is characterized by parotid or salivary glands enlargement, fever, facial nerve paralysis and anterior uveitis [8][9].
Pulmonary imaging has a key role in the diagnosis of sarcoidosis, and all patients evaluated for suspected sarcoidosis should have a chest X-ray. This is abnormal in more than 90% of cases, with bilateral hilar lymphadenopathy and parenchymal changes being observed in 50 to 85% and 25 to 60% of cases, respectively [10]. In the 1960s, Guy Scadding developed a “staging system” for pulmonary sarcoidosis using chest radiography: stage I is defined by the presence of BHL; stage II consists of BHL and parenchymal infiltrates; stage III is characterized by parenchymal abnormalities without BHL; stage IV consists of upper-lobe predominant fibrotic changes with volume loss [11]. The frequencies of the different stages at presentation are 50% for stage I, 25–30% for stage II, 10–12% for stage III and 5% for stage IV [12].
Radiographic resolution occurs in three-quarters of patients with stage I and two-thirds of patients with stage II. Patients with higher radiographic stages tend to have more pulmonary symptoms, greater functional impairment and lower likelihood of remission. However, due to the significant overlap between stages, the Scadding system alone cannot be used to predict outcome in individual patients [13]. In addition, there is not necessarily a progression to a higher stage, and, with the exception of stage IV, radiographic resolution may occur with any stage.
Chest high resolution CT (HRCT) is more sensitive than chest X-ray and provides a more precise assessment of hilar, mediastinal and parenchymal abnormalities (Figure 1). A typical HRCT feature in sarcoidosis is the presence of well-defined micronodules scattered along the broncho-vascular bundle, veins, fissures and pleura in a characteristic lymphatic distribution [14]. The micronodules can be confluent, leading to mass-like conglomerations and distortion of the lung parenchyma [15]. Occasionally, conglomerate masses are surrounded by a multitude of micronodules, hence the term “galaxy sign”, which is highly suggestive of pulmonary sarcoidosis. All these abnormalities typically display a mid-to-upper zone predominance.
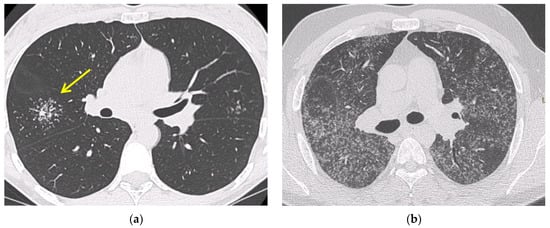
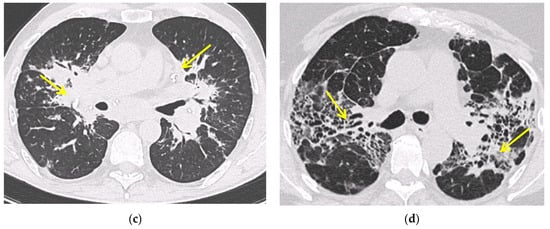
Figure 1. Irregularly marginated nodule surrounded by multiple small nodules (“Galaxy sign”, yellow narrow), this is typical of sarcoidosis (a); ground-glass-like increased attenuation resulting from diffuse micronodules randomly distributed (“Miliary sarcoidosis”) (b); enlarged and partially calcified (yellow narrows) bilateral hilar lymph nodes (c); fibrotic sarcoidosis with cystic changes and traction bronchiectases (yellow narrows) predominantly in the perihilar region and upper lobes. Nodular abnormalities are minimal/absent, but the appearance and the location of the fibrosis are very suggestive of the diagnosis of sarcoidosis (d).
Additional HRCT findings include ground glass opacities and septal and nonseptal lines [16]. Up to 20% of cases develop pulmonary fibrosis, which manifests on HRCT as bronchial distortion (40% of cases), honeycombing (26% of cases) and linear scarring (14% of cases) [17][18]. Complications of fibrotic pulmonary sarcoidosis include pulmonary hypertension and mycetoma, with hemoptysis representing a rare but potentially life-threatening manifestation [19].
Fluorine 18 fluorodeoxyglucose (FDG) PET/CT is a useful tool for assessing sarcoidosis activity, with a sensitivity of 89 to 100% [20]. In addition, FDG PET/CT may be useful for identifying occult disease (i.e., cardiac or osseous sarcoidosis), or the most suitable site to biopsy and for assessing disease extent and response to treatment [20][21].
In a small study by Mostard et al., more than 90% of patients with fibrotic lesions (Scadding stage IV) still had active inflammatory disease as measured by FDG-PET [22]. Additional studies are needed to further confirm the utility of PET imaging in the diagnosis and assessment of treatment response in sarcoidosis [23].
Because of the high prevalence of pulmonary involvement, bronchoscopy, with its ancillary sampling techniques, has the highest diagnostic yield in sarcoidosis, unless more easily accessible biopsy sites, such as skin or superficial lymph nodes, are available [24]. Bronchoscopy may reveal a cobblestone appearance of the mucosa—the typical feature of endobronchial granulomatous inflammation. In such cases, the diagnostic yield of endobronchial biopsy is >70%, but it is only about 30% if the airway mucosa appears normal (Table 1) [25].
Table 1. Sensitivity and specificity of the main diagnostic procedures in suspicion of pulmonary sarcoidosis.
| Sensitivity | Specificity | Diagnostic Yield | References | |
|---|---|---|---|---|
| EBB | 46.2% | 85.7% | 30–70% | [25] |
| TBLB | 37% | 100% | 50–75% | [26][27] |
| EBUS/TBNA | 83–93% | 100% | 77–84% | [28][29] |
| Mediastinoscopy | 100% | 100% | 82–100% | [30] |
| BAL (CD4/CD8 ≥ 3.5) | 53–59% | 93–96% | 56% | [31][32] |
EBUS/TBNA: endobronchial ultrasound with real-time guided transbronchial needle aspiration; EBB: endobronchial biopsy; TBLB: transbronchial lung biopsy; BAL: bronchoalveolar lavage.
Although the main role of BAL in sarcoidosis is to exclude alternative diagnoses, such as, among others, infection and malignancy, it may also provide supportive evidence for a diagnosis of sarcoidosis, provided BAL data are interpreted in the context of clinical and radiological features. An increased cellular count with a lymphocytosis > 25% and an elevated CD4/CD8 ratio are common findings, though they are neither sensitive nor specific for a diagnosis of sarcoidosis. Indeed, the BAL cell count may be normal in approximately 15% of newly diagnosed cases [33]. However, a CD4/CD8 ratio > 3.5 has a specificity of 93–96%, although with a sensitivity of 53 to 59% and a CD4/CD8 ratio > 10 has a >99% specificity for a diagnosis of sarcoidosis [31]. Additional features suggestive of a diagnosis of sarcoidosis include a lymphocytosis > 15%, CD4/CD8 ratio > 3.5, CD103 ratio of <0.2 and a transbronchial biopsy demonstrating noncaseating granulomas, whereas a CD4/CD8 ratio < 1 and elevated neutrophil and eosinophil counts make the diagnosis of sarcoidosis unlikely [31][34][35]. Patients with severe and progressive disease may show increased BAL neutrophils, which portend a poor response to immunosuppressive therapy [36].
Mediastinal lymphadenopathy is the most common manifestation of sarcoidosis across all ethnic groups [15], and different techniques can be used to sample the mediastinum, including endobronchial ultrasound with real-time guided transbronchial needle aspiration [EBUS-TBNA], transesophageal endoscopic ultrasound-guided fine-needle aspiration [EUS-TBNA] and endoscopic ultrasound bronchoscope guided fine-needle aspiration [EUS-b-FNA]. In particular, EBUS-TBNA can safely sample virtually any nodes in contact with the large airways and, when coupled with cytologic evaluation, has a consistently high success rate in diagnosing sarcoidosis [37][38], particularly in stage I and stage II disease [39]. Indeed, its diagnostic yield in stage I disease is 84% (95% CI, 74–92%) compared with 77% (95% CI, 64–86%) in stage II. In patients with hilar and/or mediastinal adenopathy, EUS-TBNA performs better than EBUS-TBNA, with a diagnostic yield of 88% (95% CI, 80–93%) vs. 66% (95% CI, 53–77%) [28]. However, EBUS-TBNA gives better access to the lymph nodes commonly involved in sarcoidosis than EUS–FNA [23][40]. Importantly, endosonography may help differentiating sarcoidosis from an adenopathy of a different nature, such as tuberculosis, based on sonographic features (i.e., the presence of heterogeneous echotexture in B-mode or necrosis in intrathoracic lymph nodes in a patient with positive tuberculin skin test (TST), especially in countries with high tuberculosis burden, may support a diagnosis of tuberculosis rather than sarcoidosis) [41]. In patients with suspected sarcoidosis, it is recommended to sample more than one nodal station. Indeed, the sampling of two lymph node stations versus one was significantly associated with the likelihood of a positive needle aspirate or biopsy sample [42]; the best success rates were obtained with aspirates or biopsies performed in the right paratracheal and subcarinal areas.
Transbronchial Lung Biopsy (TBLB) has a relatively high diagnostic yield (50 to 75%) in patients with suspected sarcoidosis based on bilateral hilar adenopathy or compatible lung parenchymal abnormalities on HRCT. Conversely, in patients with stage I disease, the diagnostic accuracy of TBLB is suboptimal (12–66%), at least when four to five biopsies per patient are obtained [26]. When coupled with EBUS-TBNA, the diagnostic yield of TBLB (versus TBLB alone) increases from 82% to about 93% [43][44]. The parenchymal areas to sample should be chosen based on CT findings, avoiding areas with significant architectural distortion and, in cases with mild radiological abnormalities, the right lower lobe segments [44]. TBLB is generally a safe procedure, although bleeding and pneumothorax are uncommon but well-established complications [45]. The diagnostic yield of TBLB positively correlates with the number and size of samples obtained; the highest success rates are achieved when 8–10 biopsies are obtained [46], but such a high number of biopsies is seldom obtained in clinical practice. EBUS-TBNA is the preferred technique in the diagnostic work-up of patients with suspected stage I sarcoidosis. However, in order to obtain the highest success rate in the detection of granulomas, EBUS-TBNA needs to be combined with TBLB [43]. Recent data suggest that transbronchial lung cryobiopsy (TBLC) may be useful in cases without significant lymphadenopathy and parenchymal abnormalities and in atypical cases when other sampling techniques are inconclusive, thus avoiding surgical lung biopsy [47]. However, more data are needed to clarify the role of TBLC in the diagnostic work-up of patients with suspected sarcoidosis.
If less invasive tests are not diagnostic or inconclusive, the next steps to sample the mediastinal lymph nodes are mediastinoscopy [30], and, in highly selected cases, thoracotomy (open or video-assisted thoracoscopy). However, surgical procedures are invasive, expensive and associated with longer hospital stays and significant morbidity, with prolonged air leakage being the most common postoperative complication. The benefits of lung biopsy to secure a histological diagnosis of sarcoidosis remain controversial, and many clinicians are reluctant to suggest surgical procedures to their patients due the non-negligible operative risks. In cases that remain undiagnosed following less invasive procedures and in the presence of mediastinal lymphadenopathy, mediastinoscopy should be the preferred sampling modality [48].
As with other interstitial lung diseases, diagnostic, prognostic and theragnostic biomarkers are also lacking in sarcoidosis. Angiotensin converting enzyme (ACE) and the soluble interleukin-2 receptor (sIL-2R) are the most widely used biomarkers in sarcoidosis, though with suboptimal sensitivity and specificity. ACE is produced by the epithelioid cells within the sarcoid granuloma, and high levels of serum ACE are believed to reflect the burden of granulomatous inflammation [49][50]. However, elevated ACE levels may also be found in other granulomatous disorders such as chronic beryllium disease (CBD) or leprosy, as well as in liver disease, lymphoma, diabetes and hyperthyroidism [51]. In addition, ACE inhibitors, which are widely used antihypertensive drugs, reduce serum ACE level, thus leading to false negative values [52]. Furthermore, an insertion/deletion polymorphism within the ACE gene is known to affect plasma ACE levels [53]. Therefore, the value of ACE levels as diagnostic or prognostic tool remains a matter of debate.
sIL-2R is released by activated mononuclear cells and sIL2R levels correlate with disease activity, particularly in patients with extrapulmonary disease. In addition, sIL2R is more sensitive than serum ACE and lysozyme levels for a diagnosis of sarcoidosis [54], although sIL-2R levels can also be elevated in hematologic malignancy, autoimmune disorders and idiopathic pulmonary fibrosis [55]. A more recent study in patients with suspected sarcoidosis demonstrated a sensitivity of 88% and a specificity of 85% [56]. This indicates that sIL-2R can be a useful tool in the diagnosis of sarcoidosis when combined with imaging and clinical features.
To date, neither ACE nor sIL2R measurement are recommended as routine tests in the diagnostic work-up, initial assessment or follow up of patients with sarcoidosis [57].