Sarcoidosis is a multisystem disorder of unknown origin and poorly understood pathogenesis that predominantly affects lungs and intrathoracic lymph nodes and is characterized by the presence of noncaseating granulomatous inflammation in involved organs. The disease is highly heterogeneous and can mimic a plethora of other disorders, making diagnosis a challenge even for experienced physicians. The evolution and severity of sarcoidosis are highly variable: many patients are asymptomatic and their disease course is generally benign with spontaneous resolution. However, up to one-third of patients develop chronic or progressive disease mainly due to pulmonary or cardiovascular complications that require long-term therapy. The diagnosis of sarcoidosis requires histopathological evidence of noncaseating granulomatous inflammation in one or more organs coupled with compatible clinical and radiological features and the exclusion of other causes of granulomatous inflammation; however, in the presence of typical disease manifestations such as Löfgren’s syndrome, Heerfordt’s syndrome, lupus pernio and asymptomatic bilateral and symmetrical hilar lymphadenopathy, the diagnosis can be established with high level of certainty on clinical grounds alone.
- sarcoidosis
- diagnosis
- histology
- imaging
- biomarkers
1. Clinical Features
2. Imaging
2.1. Chest Radiography
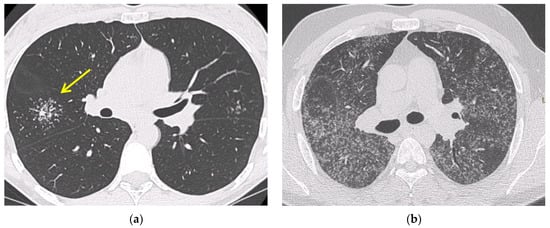
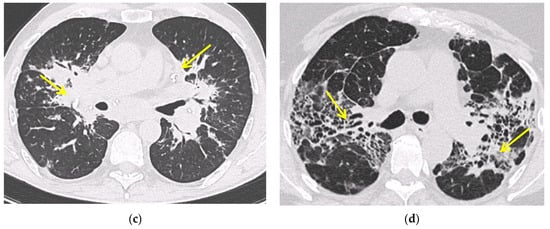
2.2. Computed Tomography (CT Scan)
2.3. Positron Emission Tomography (FDG PET/CT)
3. Confirmation of the Diagnosis
3.1. Fiberoptic Bronchoscopy
| Sensitivity | Specificity | Diagnostic Yield | References | |
|---|---|---|---|---|
| EBB | 46.2% | 85.7% | 30–70% | [39] |
| TBLB | 37% | 100% | 50–75% | [52,60] |
| EBUS/TBNA | 83–93% | 100% | 77–84% | [48,61] |
| Mediastinoscopy | 100% | 100% | 82–100% | [58] |
| BAL (CD4/CD8 ≥ 3.5) | 53–59% | 93–96% | 56% | [41,62] |
3.2. Bronchoalveolar Lavage
3.3. EBUS-TBNA
3.4. Transbronchial Lung Biopsy (TBLB)
3.5. Mediastinoscopy
3.6. Serological Biomarkers
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics11091558
