| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Héctor González-Iglesias | + 6685 word(s) | 6685 | 2021-01-19 08:44:17 | | | |
| 2 | Rita Xu | -1926 word(s) | 4759 | 2021-01-26 04:17:27 | | |
Video Upload Options
This review describes the main antioxidant systems of the human eye, with particular emphasis in those expressed in the natural barriers of antioxidant protection, i.e., the ocular surface, the lens, the retina and its retinal pigment epithelium. In addition to superoxide dismutase, glutathione peroxidase, catalase, peroxiredoxins and selenoproteins, inter alia, metallothioneins (MTs) are considered antioxidant proteins of growing interest with further cell-mediated functions. The state of the art of MTs, including the isoforms classification, the main functions described to date and the zinc-MT redox cycle as antioxidant defense system are comprehensively described.
1. Introduction
The eye is subjected to a highly oxidative environment due to its intense exposure to light, its robust metabolic activity, and its high oxygen tension. Considering that solar ultraviolet (UV) radiation is the major environmental inducer of oxidant reactive species formation, the eye has developed a complex protection system against oxidative damage organized in three levels: (1) Prevention of free radical yield by pigments capable of absorbing and filtering light; (2) detoxification of free radicals by enzymatic or non-enzymatic antioxidants; and (3) repairment systems of oxidized biomolecules [1]. In the following sections, the antioxidant enzymes that are part of the defense system of the human eye will be discussed, with a particular focus on the redox system zinc-metallothioneins.
1.1. The Human Eye
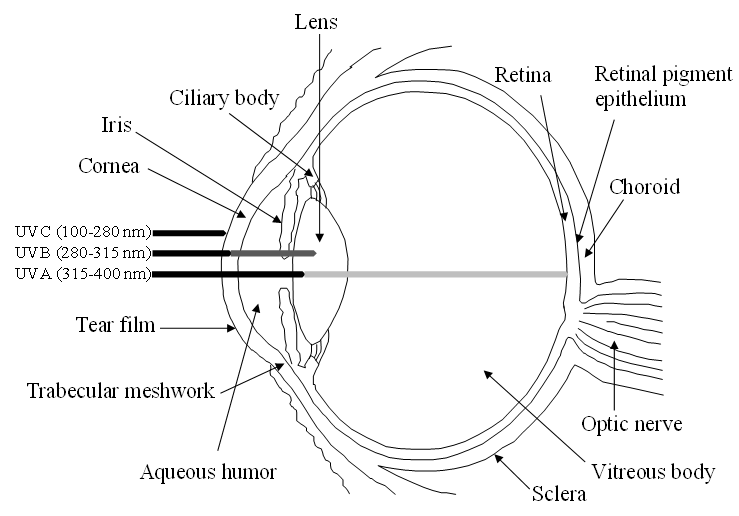
The human eye is a highly specialized organ of the visual system consisting of three primary layers surrounding the bulk of the ocular globe (Figure 1). The external part is the fibrous tunic, containing the sclera and cornea, the middle part is the uvea or vascular tunic composed of iris, ciliary body (CB), and choroid, and the inner part is the neural layer formed by the retina and its retinal pigment epithelium (RPE). These coats surround the lens and the transparent media, namely anterior chamber with aqueous humor and vitreous body [1]. Oxidative stress affects all the structures of the eye, specifically its antioxidant natural barriers formed by the ocular surface (i.e., cornea and the anterior part of the sclera), the lens and retina.
The cornea and sclera form a protective envelope that protects the ocular tissues and also provides structural support. The transparency of the cornea and its refractive power are among the most important properties, presenting a tough physical barrier to trauma and infection. The cornea has five different layers: The corneal epithelium, a stratified, squamous, non-keratinized epithelium; the anterior limiting membrane or Bowman’s layer, a modified acellular region of the stroma; the corneal stroma, a connective tissue composed of collagenous lamellae, keratocytes and modified fibroblasts; posterior limiting lamina or Descemet’s membrane; and corneal endothelium, a single layer of hexagonal cells. The lens, also responsible for light refraction and focus over the retina, possesses less refractive power than the cornea. This transparent body is composed of long-fibre like cells and epithelial cells highly organized and tightly packed, enclosed in an elastic collagenous capsule that confers its ability to change shape by the accommodation process. The retina is a light-sensitive tissue that lines the inner surface of the posterior segment of the eye, whose main function is to detect light, convert photochemical energy into neural signals, and transmit the signals to the visual cortex of the brain. The retina consists of an inner multilayer of neurosensory cells and an outer single neuroepithelium, the RPE. The neuroretina comprises the nerve fiber layer, the ganglion cell layer, the inner plexiform layer, the inner nuclear layer (INL), the outer plexiform layer and the outer nuclear layer (ONL), formed by different cell types including vascular endothelial, pericytes, glial, microglia, ganglion, horizontal, amacrine, bipolar, and photoreceptors. In addition, the RPE is a pigmented cell monolayer that nourishes and maintains the adhesion of the neurosensory retina and reduces light scattering within the eye [2].
Figure 1. Schematic view of the human eye anatomy and representation of the absorption of UV radiation by the main ocular barriers.
1.2. Reactive Oxygen Species within the Eye
Oxidative stress occurs from formation of multiple reactive oxygen species (ROS), including superoxide, hydrogen peroxide, and hydroxyl radicals, which promote free radical production. These oxygen-containing free radicals are produced as natural subproducts of the normal metabolism of oxygen, and have important roles in cell signaling and homeostasis. An imbalance of ROS production and the capability of the eye to counteract these free radicals causes oxidative stress that may be amplified by a continuing cycle of metabolic stress leading to increased free radical production. It compromises the antioxidant capacity of free radical scavenger systems and triggers cell damage and death. This intracellular disequilibrium results in the onset and/or progression of ocular diseases, which is exacerbated during long-term oxidative stress exposure and ageing [3].
While exogenous ROS generators include UV light exposure, viral infection, chemical insults and drug intake, among others, endogenous ROS are mainly produced in the mitochondria due to cellular respiration [4]. UV light, one of the main factors of ROS production triggering oxidative damage, consists of radiation in different wavelengths: UVA (315–400 nm), UVB (280–315 nm) and UVC (100–280 nm). Whilst the cornea and lens absorb UVC radiation and most UVB, a small part of UVA radiation reaches the retina-RPE system (see Figure 1). This continuous oxidative damage plays a key role in the onset of age-related eye diseases affecting the cornea, lens and retina-RPE [5] In the cornea, UV light producing ROS interferes with epithelial cells proliferation and reduces epithelial thickness, which may promote Fuchs’ endothelial dystrophy. This corneal disorder associated to oxidative stress leads to degeneration of endothelium causing stromal and epithelial edema resulting in visual acuity reduction. This corneal dystrophy is characterized by the loss of hexagonal shape and cell density of endothelial cells and formation of excrescences of Descemet’s membrane called guttae [6]. Besides, the lack of blood vessels in the lens exacerbates oxidative stress, increasing the risk of its opacification and resulting in cataract formation, which is one the most common causes of blindness worldwide [7][8][9]. Furthermore, the high oxygen consumption rate of retinal cells due to cellular respiration contributes synergistically with UV light and vascular flux defects to the pathogenesis of retinal diseases, including glaucoma, age-related macular degeneration (AMD), diabetic retinopathy and retinitis pigmentosa, among others [10]. During glaucoma, a neuropathy characterized by the abnormal increase of intraocular pressure that brings to degeneration of retinal ganglion cells (RGCs) causing irreversible blindness, a prolonged imbalance of oxidative species and antioxidants is considered a significant risk factor where high intraocular pressure stimulates in a feedback process the production of ROS, inducing RGCs to autophagy and apoptosis [11]. Moreover, the continuous exposure of the RPE to light energy, the oxygen-rich environment and the high metabolic activity of this neuroepithelial monolayer provide an ideal framework for the formation of ROS with the potential to damage proteins, DNA and lipids, resulting in AMD, a neurodegenerative disease, characterized by the irreversible damage in the macula and the formation of extracellular deposits between RPE and Bruch’s membrane (a modified connective tissue layer), called drusen [12]. Finally, the microvascular disease diabetic retinopathy is a complication of diabetes mellitus, caused by high mitochondrial ROS production, due to hyperglycemia. This results in capillaries cell damage and the release of plasma and erythrocytes in the retina causing edema and stimulating retinal angiogenesis [13].
2. Antioxidant Defense Systems in the Eye
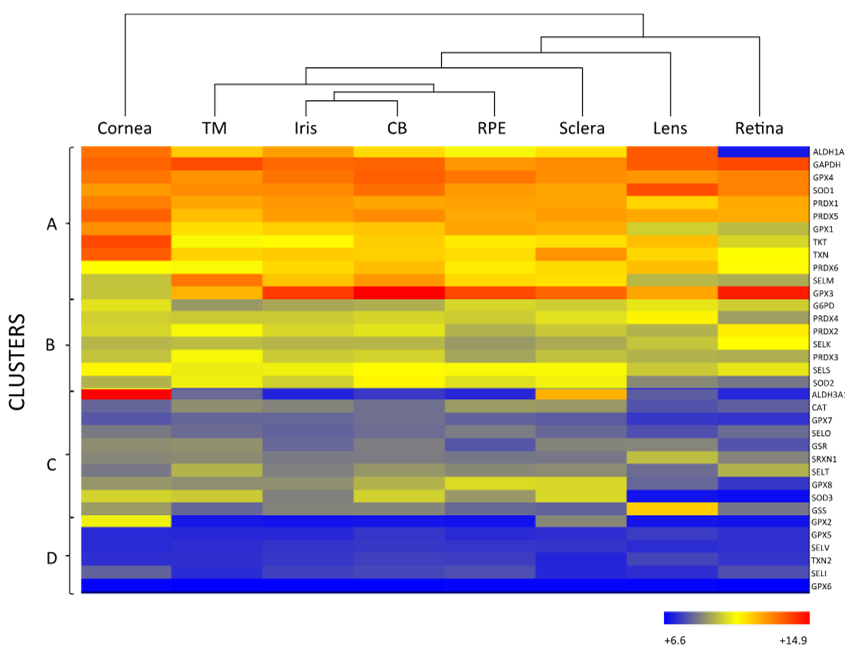
As mentioned above, the eye is constantly exposed to both exogenous and endogenous sources of oxidants, which put the cells under continuous pressure from oxidative stress. The eye relies on a complex antioxidant defense system to keep free radicals under control and maintain correct physiological functions [5]. Defense against oxidative stress is slightly different across the eye, matching with its structural complexity and accounting for the different sources of ROS threatening each region. For the purpose of this review, we have divided the eye into three natural barriers of defense: (1) The ocular surface, composed by the tear film, cornea, anterior sclera and aqueous humor; (2) the lens; and (3) the retina (neurosensory retina and RPE). Although it is out of the scope of this review, it should be noted the potential antioxidant role of the vitreous body, a body fluid of gel nature filling the posterior pole between the lens and the retina, filtering infrared light, has been recently reviewed [14]. Figure 2 shows the hierarchical ranking and clustering of enzymatic antioxidant genes present in the human eye, based on whole-genome expression microarray analysis by Alvarez et al., 2012 [15]. Four clusters could be distinguished (A, B, C and D), each containing genes expressed at levels ranging from highly abundant (i.e., cluster A) to moderate (i.e., cluster B and C), and almost absent (cluster D), which will be further discussed with respect to their ocular tissue antioxidant barrier function.
The antioxidant defense system of any cell is composed of a vast variety of compounds, including both enzymatic and non-enzymatic molecules. Non-enzymatic antioxidants are water-soluble (e.g., ascorbic acid and glutathione) or fat-soluble (e.g., carotenoids and vitamins A and E) molecules with relevant roles, not only in antioxidant protection, but also in UV-light absorption [16][17], by which other scientific papers are referenced [18][19][20]. In relation to enzymatic antioxidants, primary and secondary enzymatic defenses can be distinguished. Primary enzymatic antioxidants consist of enzymes that act at the root of the oxidative propagation chain, preventing the formation of new free radicals by removing precursors or inhibiting catalysts. These primary antioxidants include enzymes like superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT) and peroxiredoxins (PRDXs). By comparison, secondary enzymatic antioxidants have preventive and supportive roles in antioxidant protection and may tackle different processes like metal deactivation, regeneration of primary antioxidants and maintenance of the cellular reducing power. Secondary antioxidants include metabolic enzymes like transketolase (TKT), glucose-6-phosphate dehydrogenase (G6PD) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH); glutathione-redox cycle enzymes like glutathione reductase (GSR) and glutathione synthetase (GSS); selenoproteins (SELM, SELO, SELT, SELK, SELS, SELV and SELI) and thioredoxin-related enzymes like thioredoxin (TXN) and sulfiredoxin (SRXN) [21][22]. Both primary and secondary antioxidants are important constituents of the ocular surface, the lens, and the retina of the human eye.
Figure 2. Hierarchical cluster and heat map (whole-genome expression microarrays) of the main antioxidant enzymes in human ocular tissues. The row at the top shows the clustering information in the form of a dendogram and the similarity relationships among the genes and tissues: cornea (n = 11), trabecular meshwork (TM; n = 9), CB (n = 12), sclera (n = 7), iris (11), RPE (n = 8), retina (n = 12) and lens (n = 10). The column at the left of the heat map shows four clusters (A to D), each with antioxidant enzymes expressed at different abundance. Mean values of 6.6–14.9 from biological replicas per tissue are indicated according to the log2 scale, in arbitrary units, depicted at the bottom. Hierarchical cluster and heat maps were created using Illumina BeadChip array platform (HumanHT-12 v4.0 Expression BeadChip Kit, Illumina, San Diego, CA, USA) and ArrayStar software, version 4 (DNASTAR, Inc, Madison, WI, USA) according to Alvarez et al., 2012 [15]. n: number of samples per tissue.
2.1. Enzymatic Antioxidants of the Ocular Surface
As the outermost region of the eye, the ocular surface comprised of the cornea, sclera, tear film and aqueous humor, is directly exposed to environmental damaging agents. Considering that UV-light radiation is the major source of ROS among these environmental stressors, the ocular surface has a crucial function in UV-light filtering and absorption, protecting the inner ocular tissues from oxidative damage and constituting the first barrier of defense against oxidants [19][20]. However, UV-light absorption makes the cells of the cornea and anterior sclera themselves vulnerable to the effects of ROS, thus, requiring a robust antioxidant defense system optimized for both their function and protection [23].
2.1.1. Primary Antioxidant Enzymes
SOD catalyzes the dismutation of superoxide anion (O2·) to the less toxic free radical hydrogen peroxide (H2O2), being the only antioxidant, capable of reacting with O2·, and thus, often regarded as the most important primary antioxidant of the corneal antioxidant defense system [5]. Humans have three isoforms of SOD, namely SOD1, SOD2 and SOD3, that differ in their location (SOD1 is located in the cytosol, SOD2 in the mitochondria and SOD3 in the extracellular space) and cofactors (SOD1 and SOD3 use Cu and Zn, while SOD2 uses Mn) [16]. SOD isoforms also have different sensitivity to UV-light and oxidative stress, given that SOD1 is easily down-regulated by UVB, while SOD2 can be moderately induced under light-induced oxidative stress [24]. Due to their differences, some authors suggest that SOD isoforms perform different functions in the eye physiology. This hypothesis is supported by the fact that knockout animals show significantly different phenotypes depending on the isoform inactivated [19][25]. According to Figure 2, all three SOD isoforms are present in the ocular surface, being SOD1 the most expressed isoform in all the considered eye tissues, including the cornea. While SOD2 is a minor isoform of SOD in the human cornea, it is abundant in other mammalian ocular tissues and has gained interest because of its antioxidant function within the mitochondria, and its role in the prevention of apoptosis initiated by mitochondrial damage [26][27].
Although H2O2 is more stable than O2·, it can diffuse across hydrophobic membranes and generate other ROS such as OH- by reacting with metal ions. Therefore, H2O2 poses an important threat to cell function and numerous enzymes are committed to its detoxification, i.e., CAT, GPX and PRDX [28][29]. CAT is a heme-containing enzyme that catalyzes the dismutation of H2O2 into O2 and H2O using Mn as a cofactor. It also protects SOD from inactivation and may be directly involved in UVB-light filtering. CAT is ubiquitously expressed in mammalian cells, predominantly in peroxisomes [19][25], and the microarray analysis data depicted in Figure 2 suggest that CAT is expressed at low levels both in the cornea and sclera.
GPX catalyzes the same reaction as CAT, reducing H2O2 to H2O, but using reduced glutathione (GSH) as an electron donor. GPX activity depends on the regeneration of GSH, which is carried out by enzymes like GSR and GSS that produce GSH by reducing oxidized glutathione (glutathione disulfide, GSSG) or by de novo synthesizing it, respectively [5][19]. Eight isoforms of GPX are known in humans and five of them (GPX1–4 and GPX6) require Se as cofactor [25]. Like other selenoproteins, GPX family follows a hierarchical order for Se supply, being GPX1 and GPX4 the most preferentially isoforms [30]. Six isoforms of GPX are expressed in the ocular surface, with preferential presence of GPX1 and GPX4, in addition to GPX3 in the sclera (Figure 2). In comparing GPX and CAT expression within the eye, it seems that GPX rather than CAT may predominantly perform H2O2 decomposition in the cornea and sclera. Conversely, CAT may gain importance over the GSH redox system when H2O2 concentration increases [20][31].
PRDX is the third type of enzyme able to reduce and detoxify H2O2 using its redox active structural cysteines without the need of specific cofactors [14]. Six PRDX isoforms are expressed in humans, namely PRDX1-6, all localized both in the cornea and the sclera. PRDX may also have chaperone-like and redox-signaling properties, which link them to inflammation and aging processes [28][32].
2.1.2. Secondary Antioxidant Enzymes
A big part of the secondary antioxidant enzymes of the cornea belongs to a group of water-soluble proteins formerly called crystallins, which include very diverse proteins, like chaperones, metabolic enzymes, and members of the heat-shock family. Berzelius originally coined the name in 1830 to describe highly abundant proteins present in the bovine crystal-clear lens [33]. Later, it was found that crystallins are also present in the cornea and, less abundantly, in some outer-eye tissues [34]. In the eye, their main function is to provide the lens and cornea with the required transparency and refractive index to filter and focus the light adequately. These proteins are carefully packed to minimize variations in concentration and to create an appropriate concentration gradient across the eye [33]. Developmental modulators and environmental factors tightly regulate crystallins genes in order to create such concentration gradient [35]. During aging and/or post-translational modifications, crystallins are prone to aggregate, which disturbs their concentration pattern and the light focusing capacity of the eye, even possibly leading to cataracts exacerbated by oxidative stress [33][36][37]. Interestingly, crystallins in the eye also have additional functions involved in UV-light filtering, protein folding and ROS detoxification [24]. The multifunctionality of crystallins is caused by a phenomenon called “gene sharing”, which occurs when a gene codifies a protein that has remarkably different functions depending on its concentration and expression pattern [33]. This finding expanded the knowledge about crystallins by including enzymes that were long thought to only be involved in cellular processes, such as metabolism and stress response [35].
One of such called enzymatic crystallins is the aldehyde dehydrogenase (ALDH) family, which regulates the metabolism of aldehydes and has a function as crystallins, albeit only ALDH1A1 and ALDH3A1 are expressed in some tissues of the eye [38]. In the cornea, ALDH family is the most abundant secondary antioxidant, especially the isoform 3A1 (ALDH3A1), which is the most expressed corneal antioxidant enzyme (Figure 2) accounting for 5 to 50% of the total water-soluble protein fraction of the corneal epithelium in mammalian species [39]. ALDH superfamily catalyzes the NAD(P)+-dependent oxidation of a wide range of endogenous and exogenous toxic aldehydes and participates in the antioxidant defense by directly scavenging ROS and indirectly producing NADPH. Although, ALDH3A1 is considered a cytosolic protein, it has been also found in the cellular nucleus, suggesting that it may also protect DNA from oxidative damage [40]. While ALDH3A1 is almost exclusively expressed in the cornea and sclera, ALDH1A1 is present in all the eye tissues with the exception of the retina, being highly expressed in the cornea ahead of other abundant enzymes like SOD (Figure 2). These findings support the idea that the cornea requires a higher number of antioxidants than other parts of the eye to maintain its UV-light filtering function.
The enzymes TKT and GAPDH are found in great abundance in the cornea—hence, they are recognized as being crystallins—highlighting that TKT is the second most abundant secondary antioxidant enzyme just behind ALDH3A1. TKT metabolizes glycolytic products as part of the pentose phosphate pathway (PPP) and GAPDH is an enzyme involved in the glycolysis [41]. PPP and glycolysis are closely associated routes that generate ribose-5-phosphate, the essential building block for RNA synthesis, and ATP via glucose oxidation, respectively. Both pathways also generate NADPH, which is the main reducing agent of the cell, so enzymes like TKT and GAPDH serve an additional antioxidant role by promoting NADPH production [24].
It should be noted that the cornea counts with other crystallins that are non-enzymatic, such as chaperones, whose activity is fundamental to prevent protein aggregation and to maintain the transparency of the cornea [34]. In some instances, chaperones also prevent oxidative stress by conserving the structure of antioxidant enzymes, e.g., αB-crystallin reduces aggregation of mutant SOD-1 in familial amyotrophic lateral sclerosis and secures its antioxidant activity [42].
2.2. Enzymatic Antioxidants of the Lens
Together with the cornea, the lens is responsible for focusing the incident light into the retina, as well as filtering harmful UV radiation to protect the inner tissues, constituting a second barrier of antioxidant defense. The UV radiation that reaches the lens is greatly composed of UVA light, since the cornea absorbs UVC and most UVB but only a small fraction of UVA (see Figure 1). UVA radiation has been shown to be more effective at causing oxidative damage than any other UV radiation, putting the lens at a higher risk of oxidative stress induction since its absorption may cause damage to the cells [43][44]. Also, lens metabolism occurs mainly in the epithelium, while the synthesis of new proteins ceases with lens fiber cell formation, making the lens especially susceptible to the accumulation of oxidative damage, and accounts for its different antioxidant needs in comparison with the aforementioned cornea [28][45][46].
The lens contains high levels of GPX and SOD [1]. Superoxide dismutases are one of the main antioxidant enzymes of the ocular lens, where they show a clear distribution consisting of high levels of protein in the anterior layers of the lens (i.e., lens capsule and epithelial cells) and low levels in the posterior layers (i.e., cortex and nucleus composed of lens fibers) [47]. Although SOD2 and SOD3 may not be present at RNA level (Figure 2). The isoform SOD1 contributes about a 90% to the total SOD activity in the lens [48], whilst the overall SOD activity significantly decreases with age and is probably caused by the life-long accumulation of inhibitory modifications, as protein turnover is almost non-existent in the cortex and the nucleus. In contrast, the activity of SOD in the cornea does not decline to such extent during aging, reflecting the differences in protein synthesis and turnover between these eye tissues [47][49].
Low CAT RNA-expression levels are observed in the whole lens (Figure 2). Some studies show that CAT is more concentrated in the periphery of the lens epithelium, where cells may require a stronger defense system [50]. Recent studies have been centered on the signaling function of H2O2 in the cell, which has been shown to occur by transient bursts of oxidants that inactivate both CAT and PRDXs [51][52]. However, it is most likely that daily H2O2 decomposition in the lens is carried out by PRDXs and GPX, according to their raised abundance in the tissue, and that CAT is more active against chronic exposure to H2O2 similarly to its function in the cornea [18][28]. All PRDX isoforms are, to a greater or lesser extent, expressed in the lens, as well as in the rest of the ocular tissues, suggesting a distinct and specific role in eye physiology for each of them (Figure 2) [53].
The glutathione redox system is especially important in the lens, since its high protein content calls for a strict redox control on thiol groups to avoid protein aggregation and this system can fulfill such function [54]. According to Figure 2, among the eight isoforms of human GPX, the lens mostly expresses GPX1, GPX3 and GPX4. GPX1 is the main intracellular isoform of GPX and its deficiency has been linked with membrane damage in the lenticular nucleus. Neither the epithelium nor the cortex of the lens is significantly affected by GPX1 loss, probably due to the abundance of other compensating antioxidants [55]. In another study, GPX3 is secreted and appears in the extracellular space, while GPX4, a mediator in the non-apoptotic and iron-dependent programmed cell death pathway that produces a rapid release of ROS (i.e., ferroptosis) is coupled with cell membranes [56].
As discussed in the previous section, crystallins are extremely important to the lens due to their dual role in antioxidant protection and light refraction. Among the enzymatic crystallins, members of the ALDH superfamily are the most abundant in the lens, specifically the isoform ALDH1A1, just behind SOD1 levels [38]. In contrast to the cornea, ALDH activity in the lens is carried out entirely by ALDH1A1 and not ALDH3A1. Although, in some species, ALDH1A1 has been described in the cornea at even greater levels than ALDH3A1, the later has never been reported to have significant abundance in the lens [44][57]. This suggests that ALDH1A1 has an important function both in the cornea and the lens, which could be related to the detoxification of reactive aldehydes. Meanwhile, ALDH3A1 may have a bigger role in UV radiation filtering than aldehyde detoxification, since by filtering light in the cornea it also indirectly protects the underlying lens [16]. ALDH1A1 has a heterogeneous distribution throughout the lens, being more concentrated in the epithelial cells and the cortex than in the nucleus, presumably because of its bigger exposure to free radicals coming from the aqueous humor [57].
Finally, metabolic enzymes, involved in NADPH regeneration, like GAPDH and TKT, are also an important part of the lens antioxidant defense system. Differential expression of TKT in the lens and cornea (Figure 2) may be the result of different gene regulatory mechanisms. It is suggested that crystallin gene expression in the lens is mostly regulated by tissue- and developmental-specific transcription factors, while environmental agents may play a more determining role in the expression profile of the cornea [58].
2.3. Enzymatic Antioxidants of the Retina
The retina is the innermost part of the eye and has a crucial role in vision, being the structure that transforms light energy into electrical outputs that are transmitted to the brain. Due to its function in vision, the retina has the highest oxygen consumption rate per kg of the body, and thus, is particularly exposed to ROS of endogenous origin [20]. In addition, photoreceptors contain high concentrations of polyunsaturated lipids that are easily oxidized and further increase the susceptibility of the retina to oxidative stress. Apart from endogenous sources, ROS in the retina can also arise from short-wavelength light that is not filtered by the first and second barriers of defense, specifically UVA and blue visible light. The RPE decreases light scatter and protects against oxidative stress generated by photooxidation. However, light-induced oxidative stress risk increases with age due to antioxidant deficiency and the accumulation of oxidative agents [59], contributing to the onset of age-related eye diseases affecting the retina. The macular region, located near the center of the retina-RPE, is particularly susceptible to light damage, and contains yellow pigments (carotenoids) that reduce glare from short wavelength blue light. These carotenoids, referred to xanthophyll macular pigments, also have an antioxidant role particularly at low levels of oxygenation. In addition, the pigmented RPE contains high amounts of melanin, which in the reduced form is a very effective free radical scavenger [1].
Primary enzymes like SOD, GPX, CAT and PRDXs are the main components of the enzymatic antioxidant defense system of the retina-RPE. The CAT concentration in the retina increases upon light exposure, but its levels do not change during prolonged exposure times. In such cases, SOD and GPX may take over CAT for antioxidant protection. In fact, CAT activity has been found to decrease with age and during age-related pathologies like AMD, while SOD does not show a clear correlation with aging [20]. However, the SOD distribution across the retina seems to change during aging [60], and recently, it was reported that SOD2 isoform experiences an age-related increase in the neural retina and more prominently in the macular region. Although, it should be noted that SOD2 levels in the retina are low in comparison to SOD1 and other antioxidant enzymes [59].
Interestingly, GPX isoform composition in the retina differs from that of the cornea and lens, being GPX3 the most abundant isoform followed by GPX4. Moreover, the GPX3 is the enzymatic antioxidant with the highest expression in the retina (Figure 2). GPX3 is present in the extracellular space, where it may protect the cell surface and basal membranes [61]. It has been reported that GPX3 is present in high levels in the aqueous humor and ciliary body of human and bovine eyes, but its presence and role in the retina has not been deeply studied [62][63]. It should be noted that GPX1 is abundantly expressed in the retina of the immature eye, likely acting as an early defense mechanism [64]. During aging, a similar pattern of expression to SOD has been described for GPX, with up-regulated levels in the photoreceptor outer segments, down-regulated levels on the peripheral retina and increased concentration upon light exposure [65]. In a recent work, the enzyme glyoxalase 1 prevented induced oxidative stress damage through the detoxification of methylglyoxalm, whose excess inactivates GPx and SOD, which may be involved in the etiopathogenesis of retinitis pigmentosa [66].
The PRDX family also contributes to the detoxification of H2O2 in the retina, although it does not show the same relevance as SOD and GPX enzymes. PRDX may complement the SOD/GPX H2O2-detoxification system by acting as redox sensors in the retina, since under oxidative stress they can become hyperoxidized and prompt redox signaling cascades, as well as function as protein chaperones. PRDX show a distinct distribution in the neural retina depending on the cellular type, where PRDX1 is mainly associated with cells in the inner nuclear layer and cone photoreceptors and PRDX5 is ubiquitously expressed in the mitochondria and sometimes found in peroxisomes, the cytoplasm and the nucleus [67].
Other antioxidants like GAPDH and some selenoproteins work in a supportive role to SOD, GPX, PRDX and CAT enzymes. Specifically, GAPDH is the secondary antioxidant most expressed in the retina due to its role in glycolysis and NADPH generation and contributes to the maintenance of the cellular reducing power under oxidative stress. Interestingly up to twenty crystallins have been described in the retina, most of them sparsely expressed and carrying out different functions than those they have in the cornea and lens. It is plausible that some crystallins interact with the cytoskeleton of retinal cells to maintain its structural integrity [68]. The overexpression of crystallins has been found to protect the RPE from oxidative stress, since some of them act as heat-shock proteins and metabolic enzymes involved in NADPH generation [69].
References
- Forrester, J.V.; Dick, A.D.; McMenamin, P.G.; Roberts, F. The Eye, Basic Sciences in Practice, 3rd ed.; Saunders Elsevier: Edinburgh, UK, 2008; Volume 540, ISBN 978-0702028410.
- Levin, L.; Nilsson, S.; Ver Hoeve, J.; Wu, S.; Kaufman, P.; Alm, A. Adler’s Physiology of the Eye, 11th ed.; Saunders Elsevier: St. Louis/Mosby, MO, USA, 2011, ISBN 9780323057141.
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183, doi:10.1016/j.redox.2015.01.002.
- Ung, L.; Pattamatta, U.; Carnt, N.; Wilkinson-Berka, J.L.; Liew, G.; White, A.J.R. Oxidative stress and reactive oxygen species: A review of their role in ocular disease. Sci. 2017, 131, 2865–2883, doi:10.1042/CS20171246.
- Cabrera, M.P.; Chihuailaf, R.H. Antioxidants and the integrity of ocular tissues. Med. Int. 2011, 905153, doi:10.4061/2011/905153.
- Wojcik, K.A.; Kaminska, A.; Blasiak, J.; Szaflik, J.; Szaflik, J.P. Oxidative stress in the pathogenesis of keratoconus and Fuchs endothelial corneal dystrophy. J. Mol. Sci. 2013, 14, 19294–19308, doi:10.3390/ijms140919294.
- Vinson, J.A. Oxidative stress in cataracts. Pathophysiology 2006, 13, 151–162, doi:10.1016/j.pathophys.2006.05.006.
- Beebe, D.C.; Holekamp, N.M.; Shui, Y.B. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res. 2010, 44, 155–165, doi:10.1159/000316481.
- Varma, S.D.; Chand, D.; Sharma, Y.R.; Kuck, J.F. Jr.; Richards, R.D. Oxidative stress on lens and cataract formation: Role of light and oxygen. Eye Res. 1984, 3, 35–57, doi:10.3109/02713688408997186.
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Med. Cell. Longev. 2016, 16, 3164734, doi:10.1155/2016/3164734.
- Tanito, M.; Kaidzu, S.; Takai, Y.; Ohira, A. Association between systemic oxidative stress and visual field damage in open-angle glaucoma. Rep. 2016, 6, 25792, doi:10.1038/srep25792.
- Beatty, S.; Koh, H.H.; Phil, M.; Henson, D.; Boulton, M. The Role of Oxidative Stress in the Pathogenesis of Age-Related Macular Degeneration. Ophthalmol. 2000, 45, 115–134, doi:0.1016/S0039-6257(00)00140-5.
- Kowluru, R.A.; Kowluru, A.; Mishra, M.; Kumar, B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Retin. Eye Res. 2015, 48, 40–61, doi:10.1016/j.preteyeres.2015.05.001.
- Ankamah, E.; Sebag, J.; Ng, E.; Nolan, J.M. Vitreous Antioxidants, Degeneration, and Vitreo-Retinopathy: Exploring the Links. Antioxidants 2020, 9, 7, doi:10.3390/antiox9010007.
- Alvarez, L.; Gonzalez-Iglesias, H.; Garcia, M.; Ghosh, S.; Sanz-Medel, A.; Coca-Prados, M. The stoichiometric transition from Zn6Cu1-metallothionein to Zn7-metallothionein underlies the up-regulation of metallothionein (MT) expression: Quantitative analysis of MT-metal load in eye cells. Biol. Chem. 2012, 287, 28456-28469, doi:10.1074/jbc.M112.365015.
- Marchitti, S.A.; Chen, Y.; Thompson, D.C.; Vasiliou, V. Ultraviolet radiation: Cellular antioxidant response and the role of ocular aldehyde dehydrogenase enzymes. Eye Contact Lens 2011, 37, 206–213, doi:10.1097/ICL.0b013e3182212642.
- Zoidis, E.; Seremelis, I.; Kontopoulos, N.; Danezis, G.P. Selenium-Dependent Antioxidant Enzymes: Actions and Properties of Selenoproteins. Antioxidants 2018, 7, doi:10.3390/antiox7050066.
- Brennan, L.A.; McGreal, R.S.; Kantorow, M. Oxidative stress defense and repair systems of the ocular lens. Biosci. 2012, 4, 141–155, doi:10.2741/365.
- Chen, Y.; Mehta, G.; Vasiliou, V. Antioxidant defenses in the ocular surface. Surf. 2009, 7, 176–185, doi:10.1016/s1542-0124(12)70185-4.
- Saccà, S.C.; Roszkowska, A.M.; Izzotti, A. Environmental light and endogenous antioxidants as the main determinants of non-cancer ocular diseases. Res. 2013, 752, 153–171, doi:10.1016/j.mrrev.2013.01.001.
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25, doi:10.1016/j.fct.2012.09.021.
- Laher, I. Systems Biology of Free Radicals and Antioxidants; Springer: Heidelberg/Berlin, Germany, 2014; Chapter LXI, p. 4178, ISBN:978-3-642-30017-2.
- Hammond, B.R.; Johnson, B.A.; George, E.R. Oxidative photodegradation of ocular tissues: Beneficial effects of filtering and exogenous antioxidants. Eye Res. 2014, 129, 135–150, doi:10.1016/j.exer.2014.09.005.
- Lassen, N.; Black, W.J.; Estey, T.; Vasiliou, V. The role of corneal crystallins in the cellular defense mechanisms against oxidative stress. Cell. Dev. Biol. 2008, 19, 100–112, doi:10.1016/j.semcdb.2007.10.004.
- Lei, X.G.; Zhu, J.H.; Cheng, W.H.; Bao, Y.; Ho, Y.S.; Reddi, A.R.; Holmgren, A.; Arnér, E.S. Paradoxical Roles of Antioxidant Enzymes: Basic Mechanisms and Health Implications. Rev. 2016, 96, 307–364, doi:10.1152/physrev.00010.2014.
- Siu, A.W.; Maldonado, M.; Sanchez-Hidalgo, M.; Tan, D.X.; Reiter, R.J. Protective effects of melatonin in experimental free radical-related ocular diseases. Pineal Res. 2006, 40, 101–109, doi:10.1111/j.1600-079X.2005.00304.x.
- Brennan, L.A.; Kantorow, M. Mitochondrial function and redox control in the aging eye: Role of MsrA and other repair systems in cataract and macular degenerations. Eye. Res. 2009, 88, 195–203, doi:10.1016/j.exer.2008.05.018.
- Babizhayev, M.A.; Yegorov, Y.E. Reactive Oxygen Species and the Aging Eye: Specific Role of Metabolically Active Mitochondria in Maintaining Lens Function and in the Initiation of the Oxidation-Induced Maturity Onset Cataract—A Novel Platform of Mitochondria-Targeted Antioxidants With Broad Therapeutic Potential for Redox Regulation and Detoxification of Oxidants in Eye Diseases. J. Ther. 2016, 23, e98–117, doi:10.1097/MJT.0b013e3181ea31ff.
- Ohia, S.E.; Opere, C.A.; Leday, A.M. Pharmacological consequences of oxidative stress in ocular tissues. Res. 2005, 579, 22–36, doi:10.1016/j.mrfmmm.2005.03.025.
- Brigelius-Flohé, R. Glutathione peroxidases and redox-regulated transcription factors. Chem. 2006, 387, 1329–1335, doi:10.1515/bc.2006.166.
- Ma, W.; Kleiman, N.J.; Sun, F.; Li, D.; Spector, A. Peroxide toxicity in conditioned lens epithelial cells—Evaluation of multi-defense systems. Eye Res. 2003, 77, 711–720, doi:10.1016/j.exer.2003.08.004.
- Detienne, G.; De Haes, W.; Mergan, L.; Edwards, S.L.; Temmerman, L.; Van Bael, S. beyond ROS clearance: Peroxiredoxins in stress signaling and aging. Ageing Res. Rev. 2018, 44, 33–48, doi:10.1016/j.arr.2018.03.005.
- Piatigorsky, J. Gene Sharing and Evolution the Diversity of Protein Functions; Harvard University Press: Cambridge, UK, 2007; Chapter XV, p. 320, ISBN 978-0674023413.
- Cooper, D.L.; Isola, N.R.; Stevenson, K.; Baptist, E.W. Members of the ALDH gene family are lens and corneal crystallins. Exp. Med. Biol. 1993, 328, 169–179, doi:10.1007/978-1-4615-2904-0_19.
- Sax, C.M.; Kays, W.T.; Salamon, C.; Chervenak, M.M.; Xu, Y.S.; Piatigorsky, J. Transketolase gene expression in the cornea is influenced by environmental factors and developmentally controlled events. Cornea 2000, 19, 833–841, doi:10.1097/00003226-200011000-00014.
- Lampi, K.J.; Wilmarth, P.A.; Murray, M.R.; David, L.L. Lens β-crystallins: The role of deamidation and related modifications in aging and cataract. Biophys. Mol. Biol. 2014, 115, 21–31, doi:10.1016/j.pbiomolbio.2014.02.004.
- Anbarasu, K.; Sivakumar, J. Multidimensional significance of crystallin protein-protein interactions and their implications in various human diseases. Biophys. Acta 2016, 1860, 222–233, doi:10.1016/j.bbagen.2015.09.005.
- Chen, Y.; Thompson, D.C.; Koppaka, V.; Jester, J.V.; Vasiliou, V. Ocular aldehyde dehydrogenases: Protection against ultraviolet damage and maintenance of transparency for vision. Retin. Eye Res. 2013, 33, 28–39, doi:10.1016/j.preteyeres.2012.10.001.
- Estey, T.; Piatigorsky, J.; Lassen, N.; Vasiliou, V. ALDH3A1: A corneal crystallin with diverse functions. Eye Res. 2007, 84, 3–12, doi:10.1016/j.exer.2006.04.010.
- Voulgaridou, G.P.; Tsochantaridis, I.; Tolkas, C.; Franco, R.; Giatromanolaki, A.; Panayiotidis, M.I.; Pappa, A. Aldehyde dehydrogenase 3A1 confers oxidative stress resistance accompanied by altered DNA damage response in human corneal epithelial cells. Free Radic. Biol. Med. 2020, 150, 66–74, doi:10.1016/j.freeradbiomed.2020.01.183.
- Xu, I.M.; Lai, R.K.; Lin, S.H.; Tse, A.P.; Chiu, D.K.; Koh, H.Y.; Law, C.T.; Wong, C.M.; Cai, Z.; Wong, C.C.; et al. Transketolase counteracts oxidative stress to drive cancer development. Natl. Acad. Sci. USA 2016, 113, E725-734, doi:10.1073/pnas.1508779113.
- Romi, F.; Helgeland, G.; Gilhus, N.E. Heat-shock proteins in clinical neurology. Neurol. 2011, 66, 65–69, doi:10.1159/000329373.
- Ivanov, I.V.; Mappes, T.; Schaupp, P.; Lappe, C.; Wahl, S. Ultraviolet radiation oxidative stress affects eye health. Biophotonics 2018, 11, e201700377, doi:10.1002/jbio.201700377.
- Stagos, D.; Chen, Y.; Cantore, M.; Jester, J.V.; Vasiliou, V. Corneal aldehyde dehydrogenases: Multiple functions and novel nuclear localization. Brain Res. Bull. 2010, 81, 211–218, doi:10.1016/j.brainresbull.2009.08.017.
- Piatigorsky, J. Lens and cornea: The “refracton hypothesis”. Cell. Dev. Biol. 2008, 19, 69–70, doi:10.1016/j.semcdb.2007.10.010.
- Berthoud, V.M.; Beyer, E.C. Oxidative stress, lens gap junctions, and cataracts. Redox Signal. 2009, 11, 339–353, doi:10.1089/ars.2008.2119.
- Crouch, R.K.; Patrick, J.; Goosey, J.; Coles, W.H. The effect of age on corneal and lens superoxide dismutase. Eye Res. 1984, 3, 1119–1123, doi:10.3109/02713688409000811.
- Rajkumar, S.; Vasavada, A.R.; Praveen, M.R.; Ananthan, R.; Reddy, G.B.; Tripathi, H.; Ganatra, D.A.; Arora, A.I.; Patel, A.R. Exploration of molecular factors impairing superoxide dismutase isoforms activity in human senile cataractous lenses. Ophthalmol. Vis. Sci. 2013, 54, 6224–6233, doi:10.1167/iovs.13-11935.
- Joyce, N.C.; Harris, D.L.; Zhu, C.C. Age-related gene response of human corneal endothelium to oxidative stress and DNA damage. Ophthalmol. Vis. Sci. 2011, 52, 1641–1649, doi:10.1167/iovs.10-6492.
- Reddan, J.R.; Steiger, C.A.; Dziedzic, D.C.; Gordon, S.R. Regional differences in the distribution of catalase in the epithelium of the ocular lens. Mol. Biol. 1996, 42, 209–219.
- Sepasi Tehrani, H.; Moosavi-Movahedi, A.A. Catalase and its mysteries. Biophys. Mol. Biol. 2018, 140, 5–12, doi:10.1016/j.pbiomolbio.2018.03.001.
- Wahlig, S.; Lovatt, M.; Mehta, J.S. Functional role of peroxiredoxin 6 in the eye. Free Radic. Biol. Med. 2018, 126, 210–220, doi:10.1016/j.freeradbiomed.2018.08.017.
- Shibata, S.; Shibata, N.; Shibata, T.; Sasaki, H.; Singh, D.P.; Kubo, E. The role of Prdx6 in the protection of cells of the crystalline lens from oxidative stress induced by UV exposure. J. Ophthalmol. 2016, 60, 408–418, doi:10.1007/s10384-016-0461-1.
- Ganea, E.; Harding, J.J. Glutathione-related enzymes and the eye. Eye Res. 2006, 31, 1–11, doi:10.1080/02713680500477347.
- Reddy, V.N.; Giblin, F.J.; Lin, L.R.; Dang, L.; Unakar, N.J.; Musch, D.C.; Boyle, D.L.; Takemoto, L.J.; Ho, Y.S.; Knoernschild, T.; et al. Glutathione peroxidase-1 deficiency leads to increased nuclear light scattering, membrane damage, and cataract formation in gene-knockout mice. Ophthalmol. Vis. Sci. 2001, 42, 3247–3255.
- Cardoso, B.R.; Hare, D.J.; Bush, A.I.; Roberts, B.R. Glutathione peroxidase 4: A new player in neurodegeneration? Psychiatry 2017, 22, 328–335, doi:10.1038/mp.2016.196.
- Choudhary, S.; Xiao, T.; Vergara, L.A.; Srivastava, S.; Nees, D.; Piatigorsky, J.; Ansari, N.H. Role of aldehyde dehydrogenase isozymes in the defense of rat lens and human lens epithelial cells against oxidative stress. Ophthalmol. Vis. Sci. 2005, 46, 259–267, doi:10.1167/iovs.04-0120.
- Sax, C.M.; Salamon, C.; Kays, W.T.; Guo, J.; Yu, F.X.; Cuthbertson, R.A.; Piatigorsky, J. Transketolase is a major protein in the mouse cornea. Biol. Chem. 1996, 271, 33568–33574, doi:10.1074/jbc.271.52.33568.
- Rath, P.C. Models, Molecules and Mechanisms in Biogerontology: Physiological Abnormalities, Diseases and Interventions; Springer: Singapore, Singapore, 2019; Chapter XL, p. 436, doi:10.1007/978-981-13-3585-3.
- Cejková, J.; Vejrazka, M.; Pláteník, J.; Stípek, S. Age-related changes in superoxide dismutase, glutathione peroxidase, catalase and xanthine oxidoreductase/xanthine oxidase activities in the rabbit cornea. Gerontol. 2004, 39, 1537–1543, doi:10.1016/j.exger.2004.08.006.
- Chang, C.; Worley, B.L.; Phaëton, R.; Hempel, N. Extracellular Glutathione Peroxidase GPx3 and Its Role in Cancer. Cancers 2020, 12, doi:10.3390/cancers12082197.
- Martín-Alonso, J.M.; Ghosh, S.; Coca-Prados, M. Cloning of the bovine plasma selenium-dependent glutathione peroxidase (GP) cDNA from the ocular ciliary epithelium: Expression of the plasma and cellular forms within the mammalian eye. Biochem. 1993, 114, 284–291, doi:10.1093/oxfordjournals.jbchem.a124168.
- Yildirim, Z.; Ucgun, N.I.; Yildirim, F. The role of oxidative stress and antioxidants in the pathogenesis of age-related macular degeneration. Clinics 2011, 66, 743–746, doi:10.1590/s1807-59322011000500006.
- Tan, S.M.; Stefanovic, N.; Tan, G.; Wilkinson-Berka, J.L.; de Haan, J.B. Lack of the antioxidant glutathione peroxidase-1 (GPx1) exacerbates retinopathy of prematurity in mice. Ophthalmol. Vis. Sci. 2013, 54, 555–562, doi:10.1167/iovs.12-10685.
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461–482, doi:10.1007/s10522-013-9463-2.
- Donato, L.; Scimone, C.; Alibrandi, S.; Nicocia, G.; Rinaldi, C.; Sidoti, A.; D’Angelo, R. Discovery of GLO1 New Related Genes and Pathways by RNA-Seq on A2E-Stressed Retinal Epithelial Cells Could Improve Knowledge on Retinitis Pigmentosa. Antioxidants 2020, 9, 416.
- Chidlow, G.; Wood, J.P.; Knoops, B.; Casson, R.J. Expression and distribution of peroxiredoxins in the retina and optic nerve. Struct. Funct. 2016, 221, 3903–3925, doi:10.1007/s00429-015-1135-3.
- Andley, U.P. Crystallins in the eye: Function and pathology. Retin. Eye Res. 2007, 26, 78–98, doi:10.1016/j.preteyeres.2006.10.003.
- Kannan, R.; Sreekumar, P.G.; Hinton, D.R. Alpha crystallins in the retinal pigment epithelium and implications for the pathogenesis and treatment of age-related macular degeneration. Biophys. Acta. 2016, 1860, 258–268, doi:10.1016/j.bbagen.2015.05.016.