Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | José A. Oteo | + 1426 word(s) | 1426 | 2021-05-27 11:13:39 | | | |
| 2 | Catherine Yang | Meta information modification | 1426 | 2021-06-10 03:36:55 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Oteo, J.A. Crimean-Congo Hemorrhagic Fever. Encyclopedia. Available online: https://encyclopedia.pub/entry/10673 (accessed on 08 February 2026).
Oteo JA. Crimean-Congo Hemorrhagic Fever. Encyclopedia. Available at: https://encyclopedia.pub/entry/10673. Accessed February 08, 2026.
Oteo, José A.. "Crimean-Congo Hemorrhagic Fever" Encyclopedia, https://encyclopedia.pub/entry/10673 (accessed February 08, 2026).
Oteo, J.A. (2021, June 09). Crimean-Congo Hemorrhagic Fever. In Encyclopedia. https://encyclopedia.pub/entry/10673
Oteo, José A.. "Crimean-Congo Hemorrhagic Fever." Encyclopedia. Web. 09 June, 2021.
Copy Citation
Crimean-Congo hemorrhagic fever virus (CCHFV) is an arthropod-borne virus (arbovirus), mainly transmitted by ticks, belonging to the genus Orthonairovirus (family Nairoviridae, order Bunyavirales). CCHFV causes a potentially severe, or even fatal, human disease, and it is widely distributed in Africa, Asia, eastern Europe and, more recently, in South-western Europe. Until a few years ago, no cases of Crimean-Congo hemorrhagic fever (CCHF) had been reported in western Europe, with the exception of several travel-associated cases.
Crimean-Congo hemorrhagic fever virus (CCHFV)
Crimean-Congo hemorrhagic fever (CCHF)
1. Introduction
Crimean Congo Hemorrhagic Fever (CCHF) is the most widely distributed tick-borne viral disease in the world (Africa, Asia, eastern and South-eastern Europe and recently, South-western Europe), and the second one (after dengue) among viral hemorrhagic fevers [1][2][3][4]. The clinical course of the infection varies from asymptomatic to severe and even fatal cases (3–40%) [5][6]. In fact, asymptomatic infections seem common [7], reaching up to 90% cases in some studies from hyperendemic areas [8]. CCHF is transmitted to people by the bites of hard ticks (or crushing of engorged specimens) and/or by direct contact with secretions, fluids or tissues of viraemic animals (slaughtering activity, animal abortion, farmers, animal husbandry, etc.) or with infected humans (blood, secretions and other biological fluids) without protective measures [9][10]. In the sanitary environment, nosocomial outbreaks are well reported [11], including those related to aerosol generation [12]. Nevertheless, the risk of suffering CCHF after exposition to infected biological fluids seems to be lower than the risk of healthcare-related exposure related to other hemorrhagic viruses such as Ebola [13]. There is an increased risk of nosocomial transmission when the disease is not suspected or the prevention measures are not used [14]. Nosocomial transmission of CCHF seems to be common in pregnancy [15]. Vertical transmission has been also reported [16][17], and sexual contact may represent an additional risk of CCHF transmission [18][19]. Laboratory-acquired accidental cases when handling viral material has been also described [9].
The etiological agent is an enveloped, segmented, negative-sense, single-stranded (ss) RNA arbovirus named Crimean-Congo hemorrhagic fever virus (CCHFV). It belongs to the genus Orthonairovirus (family Nairoviridae, order Bunyavirales), within the realm Riboviria (a new megataxonomic taxon rank), according to the most recent classification based on the advances in viral genomic and metagenomic comparison analysis [20][21]. The viral genome consists of three RNA segments: small (S), medium (M) and large (L), which encode the viral nucleoprotein (NP), the glycoprotein precursor (GPC) that yields the structural glycoproteins (GN and GC), and the RNA-dependent RNA polymerase, respectively [22]. CCHFV exhibits higher genetic diversity than other tick-borne viruses, which reveals a wide dispersion of the virus [23]. Since increasing complete genomic sequences of CCHFV are becoming available, new viral classifications are appearing in the last years. Previously classified into six main geographical clades [6], up to nine genetically different clades are currently proposed for CCHFV, according to the phylogenetic analysis of the complete genetic sequence of the S RNA segment of the genome, and based on the geographical origin [24]. Four of them are predominantly distributed in Africa, two in Asia and three in Europe, as follows: Africa-1 (genotype I), Africa-2 (genotype II), Africa-3, (genotype IIIa) and Africa 4 (genotype IIIb); Asia-1 (genotype IVa) and Asia-2 (genotype IVb); Europe-1 (genotype V), Europe-2 (genotype VI) and Europe-3 (genotype VII) [24].
The disease only affects humans, but CCHFV lives in a natural cycle affecting wild mammals, livestock, birds and ticks. Hard ticks are reservoirs of the virus in the nature through transovarial, transstadial and venereal transmission [25]. The cycle of CCHFV is shown in Figure 1.
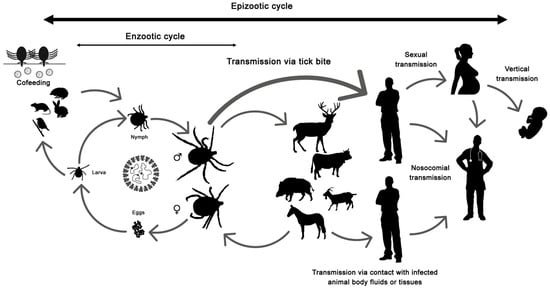
Figure 1. Crimean-Congo hemorrhagic fever virus (CCHFV) cycle and its routes of transmission.
2. What Is the Potential Future Impact of CCHF in Western Europe?
The recent emergence of CCHF in South-western Europe could lead to sporadic case outbreaks being endemic in this region (Spain) or become an epidemic (spreading to new areas). The Greek experience shows the most favorable situation. CCHFV was detected in 1975 in R. bursa ticks from Greek goats but no human cases were reported from this country until 2008 [26][27]. Previously to the confirmation of this autochthonous case, antibodies against the virus were detected in humans [28][29]. Nevertheless, up to date, only one more case has been reported from Greece and, it was imported from Bulgaria [30]. In contrast, the first CCHF human case was reported from Turkey in 2002, and 15 years later more than 11,000 cases had been notified, being endemic in many areas with a fatality rate close to 5% [31][32].
To predict the course of the disease in Spain, the sole western European country with autochthonous confirmed cases, or the incursion in neighboring countries is challenging. As it has been displayed in this review, there are several factors involved in the epidemiology of the CCHF, and presumably a combination of biological and environmental factors is responsible for its incidence [2][33]. The presence of the CCHFV virulent strains, competent vectors, reservoirs (the tick) and amplifiers (vertebrate hosts) of the virus are essential for the emergence of the disease, but a suitable environment is needed. The introduction of immature developmental stages of H. marginatum into western Europe through bird migrations has been reported [34][35][36][37] but local permanent Hyalomma have been only recognized in Portugal, Spain and France [38][35][39]. Tick-infested and/or infected (viraemic) wildlife or livestock to western European countries has been well documented, and even infected ticks [40]. Limited findings of adult H. marginatum and/or Hyalomma rufipes (formerly considered a subspecies of H. marginatum and also vector of CCHFV), most of them attached to horses and all showing negative results for CCHFV, have been recorded in Germany [41][42][43][44], UK [45][46], Austria [47] and The Netherlands [48]. In these cases, non-native immature Hyalomma ticks probably linked to the arrival of migrating birds were transmitted to the local fauna and molted into adults due to favorable conditions (warmer and dryer weather) outside the known distribution areas. Nevertheless, the potential introduction of non-autochthonous adult ticks associated to human travel cannot always be ruled out [42] and it has to also be considered that animals can travel with ticks and become the via of introduction of exotic tick species in new areas, as it occurred with one H. lusitanicum imported into the UK on a dog that had recently returned from Portugal [49]. In addition, other competent vectors, such as R. bursa, broadly distributed in the Mediterranean region [50][51], should be considered.
For the emergence of outbreaks, the tick–vertebrate–tick enzootic cycle of the virus should be established (and data in Spain points in that direction), and the exposition of susceptible people to the infected competent vector and/or to the virus (virulent strains) would be also necessary (the risk is higher in people from rural areas and/or with professions at risk). To date, the ‘hot spot’ of CCHFV infection seems to be more related to a certain area (Central-West and South-western Spain) and, hopefully, more closely associated to wild animals, but the ‘spill-over’ into livestock is a fact, and the demonstrated capacity of the virus for adaptation, among other factors, makes it a dangerous threat in our environment. CCHF, as other vector-borne viral diseases, is affected by dynamic factors such as globalization, climate change, social and cultural changes, alterations of land uses, habitats fragmentation, loss of biodiversity, introduction of exotic species, etc. [52][53][54][55][56][57]. Whether the vector in Spain is H. marginatum or H. lusitanicum is still not clear. Hyalomma ticks are abundant in southern Europe and a trend of their increasing human tick-bites has been observed for more than a decade. Thus, according to our data, the number of Hyalomma spp. detached from humans at San Pedro University Hospital (La Rioja, Spain) has almost doubled if we compare the years 2009–2014 with 2015–2020 (data not shown). All these facts, among others, combined with the different genotypes detected in ticks and humans in different areas from Spain suggest the potential establishment of a CCHFV transmission cycle. Therefore, a greater awareness and surveillance of this threat is needed. Strategies based on a One Health approach are essential for the prevention, contention, and control of the CCHF in western Europe [58]. To improve detection, diagnosis, treatment and prevention approaches under a global and coordinated perspective is essential.
Addendum: After submission of this review, an early release article (not the final version) about the CCHFV genetic sequence corresponding to the fatal case of patient 3 (Badajoz, 2018) from Spain was published online [https://wwwnc.cdc.gov/eid/article/27/4/20-3462_article (released on: 9 March 2021)]. Based on these data, the reassortant S segment was similar to genotype IV and related to a Nigeria strain that differed from the Asia strains of this genotype. Other authors [24] had already included this same S sequence from Spain within a new genotype designated IIIb (Africa-4). To reach a consensus about designation of genotypes, genetic linages and groups is a pending challenge.
References
- Watts, D.M.; Ksiazek, T.G.; Linthicum, K.J.; Hoogstraal, H. Crimean-Congo Hemorrhagic Fever. In The Arboviruses: Epidemiology and Ecology; CRC Press: Boca Raton, FL, USA, 1988; Volume 2, pp. 177–260.
- Ergönül, O. Crimean-Congo haemorrhagic fever. Lancet Infect. Dis. 2006, 6, 203–214.
- World Health Organization (WHO). Roadmap for Research and Product Development against Crimean-Congo Haemorrhagic Fever (CCHF). 2018. Available online: (accessed on 27 February 2021).
- ECDC (European Centre for Disease Prevention and Control. Crimean-Congo Haemorrhagic Fever. In Annual Epidemiological Report for 2018; ECDC: Stockholm, Sweden, 2019; Available online: (accessed on 27 February 2021).
- Sidira, P.; Maltezou, H.C.; Haidich, A.B.; Papa, A. Seroepidemiological study of Crimean-Congo haemorrhagic fever in Greece, 2009–2010. Clin. Microbiol. Infect. 2012, 18, E16–E19.
- Bente, D.A.; Forrester, N.L.; Watts, D.M.; McAuley, A.J.; Whitehouse, C.A.; Bray, M. Crimean-Congo hemorrhagic fever: History, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antivir. Res. 2013, 100, 159–189.
- Ergönül, O. Clinical and pathological features of Crimean-Congo hemorrhagic fever. In Crimean-Congo Hemorrhagic Fever—A Global Perspective; Ergönül, O., Whitehouse, C.A., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 207–220.
- Bodur, H.; Akinci, E.; Ascioglu, S.; Öngürü, P.; Uyar, Y. Subclinical Infections with Crimean-Congo Hemorrhagic Fever Virus, Turkey. Emerg. Infect. Dis. 2012, 18, 640–642.
- Whitehouse, C.A. Crimean-Congo hemorrhagic fever. Antiviral Res. 2004, 64, 145–160.
- Gunes, T.; Engin, A.; Poyraz, O.; Elaldi, N.; Kaya, S.; Dokmetas, I.; Bakir, M.; Cinar, Z. Crimean-Congo hemorrhagic fever virus in high-risk population, Turkey. Emerg. Infect. Dis. 2009, 15, 461–464.
- Tsergouli, K.; Karampatakis, T.; Haidich, A.B.; Metallidis, S.; Papa, A. Nosocomial infections caused by Crimean-Congo haemorrhagic fever virus. J. Hosp. Infect. 2020, 105, 43–52.
- Pshenichnaya, N.Y.; Nenadskaya, S.A. Probable Crimean-Congo hemorrhagic fever virus transmission occurred after aerosol-generating medical procedures in Russia: Nosocomial cluster. Intern. J. Infect. Dis. 2015, 33, 120–122.
- Leblebicioglu, H.; Sunbul, M.; Guner, R.; Bodur, H.; Bulut, C.; Duygu, F.; Elaldi, N.; Cicek Senturk, G.; Ozkurt, Z.; Yilmaz, G.; et al. Healthcare-associated Crimean-Congo haemorrhagic fever in Turkey, 2002–2014: A multicentre retrospective cross-sectional study. Clin. Microbiol. Infect. 2016, 22, 387.
- Nabeth, P.; Cheikh, D.O.; Lo, B.; Faye, O.; Vall, I.O.; Niang, M.; Wague, B.; Diop, D.; Diallo, M.; Diallo, B.; et al. Crimean-Congo hemorrhagic fever, Mauritania. Emerg. Infect. Dis. 2004, 10, 2143–2149.
- Pshenichnaya, N.Y.; Leblebicioglu, H.; Bozkurt, I.; Sannikova, I.V.; Abuova, G.N.; Zhuravlev, A.S.; Barut, S.; Shermetova, M.B.; Fletcher, T.E. Crimean-Congo hemorrhagic fever in pregnancy: A systematic review and case series from Russia, Kazakhstan and Turkey. Int. J. Infect. Dis. 2017, 58, 58–64.
- Ergonul, O.; Celikbas, A.; Yildirim, U.; Zenciroglu, A.; Erdogan, D.; Ziraman, I.; Saracoglu, F.; Demirel, N.; Cakmak, O.; Dokuzoguz, B. Pregnancy and Crimean-Congo haemorrhagic fever. Clin. Microbiol. Infect. 2010, 16, 647–650.
- Ahmeti, S.; Berisha, L.; Halili, B.; Ahmeti, F.; von Possel, R.; Thomé-Bolduan, C.; Michel, A.; Priesnitz, S.; Reisinger, E.C.; Günther, S.; et al. Crimean-Congo Hemorrhagic Fever, Kosovo, 2013–2016. Emerg. Infect. Dis. 2019, 25, 321–324.
- Ergönül, O.; Battal, I. Potential sexual transmission of Crimean-Congo hemorrhagic fever infection. Jpn. J. Infect. Dis. 2014, 67, 137–138.
- Pshenichnaya, N.Y.; Sydenko, I.S.; Klinovaya, E.P.; Romanova, E.B.; Zhuravlev, A.S. Possible sexual transmission of Crimean-Congo hemorrhagic fever. Int. J. Infect. Dis. 2016, 45, 109–111.
- International Committee on Taxonomy of Viruses (ICTV), Taxonomy History: Crimean-Congo Hemorrhagic Fever Orthonairovirus. Available online: (accessed on 24 February 2021).
- Kuhn, J.H.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Amarasinghe, G.K.; Anthony, S.J.; Avšič-Županc, T.; Ayllón, M.A.; Bahl, J.; Balkema-Buschmann, A.; et al. 2020 taxonomic update for phylum Negarnaviricota (Riboviria: Orthornavirae), including the large orders Bunyavirales and Mononegavirales. Arch. Virol. 2020, 165, 3023–3072.
- Garrison, A.R.; Alkhovsky, S.V.; Avšič-Županc, T.; Bente, D.A.; Bergeron, É.; Burt, F.; Di Paola, N.; Ergünay, K.; Hewson, R.; Kuhn, J.H.; et al. ICTV Virus Taxonomy Profile: Nairoviridae. J. Gen. Virol. 2020, 101, 798–799.
- Mild, M.; Simon, M.; Albert, J.; Mirazimi, A. Towards an under- standing of the migration of Crimean-Congo hemorrhagic fever virus. J. Gen. Virol. 2010, 91, 199–207.
- Gruber, C.E.M.; Bartolini, B.; Castilletti, C.; Mirazimi, A.; Hewson, R.; Christova, I.; Avšič, T.; Grunow, R.; Papa, A.; Sánchez-Seco, M.P.; et al. Geographical Variability Affects CCHFV Detection by RT-PCR: A Tool for In-Silico Evaluation of Molecular Assays. Viruses 2019, 11, 953.
- Turell, M. Role of ticks in the transmission of Crimean-Congo hemorrhagic fever virus. In Crimean-Congo Hemorrhagic Fever: A Global Perspective; Ergönül, O., Whitehouse, C.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 143–154.
- Papadopoulos, O.; Koptopoulos, G. Crimean-Congo hemorrhagic fever (CCHF) in Greece: Isolation of the virus from Rhipicephalus bursa ticks and a preliminary serological survey. Zentbl. Bakteriol. Hyg. 1980, 1, 189–193.
- Papa, A.; Maltezou, H.C.; Tsiodras, S.; Dalla, V.G.; Papadimitriou, T.; Pierroutsakos, I.; Kartalis, G.N.; Antoniadis, A. A case of Crimean-Congo haemorrhagic fever in Greece, June 2008. Eur. Surveill. 2008, 13, 18952.
- Antoniadis, A.; Casals, J. Serological evidence of human infection with Congo-Crimean hemorrhagic fever virus in Greece. Am. J. Trop. Med. Hyg. 1982, 31, 1066–1067.
- Antoniadis, A.; Alexiou-Daniel, S.; Malissiovas, N.; Doutsos, J.; Polyzoni, T.; LeDuc, J.W.; Peters, C.J.; Saviolakis, G. Seroepidemiological survey for antibodies to arboviruses in Greece. Arch. Virol. 1990, 1, 277–285.
- Papa, A.; Markatou, F.; Maltezou, H.C.; Papadopoulou, E.; Terzi, E.; Ventouri, S.; Pervanidou, D.; Tsiodras, S.; Maltezos, E. Crimean-Congo haemorrhagic fever in a Greek worker returning from Bulgaria, June 2018. Euro Surveill. 2018, 23, 1800432.
- Kar, S.; Rodriguez, S.E.; Akyildiz, G.; Cajimat, M.N.B.; Bircan, R.; Mears, M.C.; Bente, D.A.; Keles, A.G. Crimean-Congo hemorrhagic fever virus in tortoises and Hyalomma aegyptium ticks in East Thrace, Turkey: Potential of a cryptic transmission cycle. Parasit Vectors 2020, 13, 201.
- Karakecili, F.; Cikman, A.; Aydin, M.; Binay, U.D.; Kesik, O.A.; Ozcicek, F. Evaluation of epidemiological, clinical, and laboratory characteristics and mortality rate of patients with Crimean-Congo hemorrhagic fever in the northeast region of Turkey. J. Vector Borne Dis. 2018, 55, 215–221.
- Burt, F.J.; Swanepoel, R. Molecular epidemiology of African and Asian Crimean-Congo haemorrhagic fever isolates. Epidemiol. Infect. 2005, 133, 659–666.
- Jameson, L.J.; Morgan, P.J.; Medlock, J.M.; Watola, G.; Vaux, A.G. Importation of Hyalomma marginatum, vector of Crimean-Congo haemorrhagic fever virus, into the United Kingdom by migratory birds. Ticks Tick Borne Dis. 2012, 3, 95–99.
- Vial, L.; Stachurski, F.; Leblond, A.; Huber, K.; Vourc’h, G.; René-Martellet, M.; Desjardins, I.; Balança, G.; Grosbois, V.; Pradier, S.; et al. Strong evidence for the presence of the tick Hyalomma marginatum Koch, 1844 in southern continental France. Ticks Tick Borne Dis. 2016, 7, 1162–1167.
- De Liberato, C.; Frontoso, R.; Magliano, A.; Montemaggiori, A.; Autorino, G.L.; Sala, M.; Bosworth, A.; Scicluna, M.T. Monitoring for the possible introduction of Crimean-Congo haemorrhagic fever virus in Italy based on tick sampling on migratory birds and serological survey of sheep flocks. Prev. Vet. Med. 2018, 149, 47–52.
- Mancuso, E.; Toma, L.; Polci, A.; d’Alessio, S.G.; Di Luca, M.; Orsini, M.; Di Domenico, M.; Marcacci, M.; Mancini, G.; Spina, F.; et al. Crimean-Congo Hemorrhagic Fever Virus Genome in Tick from Migratory Bird, Italy. Emerg. Infect. Dis. 2019, 25, 1418–1420.
- Palomar, A.M.; Portillo, A.; Mazuelas, D.; Roncero, L.; Arizaga, J.; Crespo, A.; Gutiérrez, Ó.; Márquez, F.J.; Cuadrado, J.F.; Eiros, J.M.; et al. Molecular analysis of Crimean-Congo hemorrhagic fever virus and Rickettsia in Hyalomma marginatum ticks removed from patients (Spain) and birds (Spain and Morocco), 2009–2015. Ticks Tick Borne Dis. 2016, 7, 983–987.
- Santos-Silva, M.M.; Beati, L.; Santos, A.S.; De Sousa, R.; Núncio, M.S.; Melo, P.; Santos-Reis, M.; Fonseca, C.; Formosinho, P.; Vilela, C.; et al. The hard-tick fauna of mainland Portugal (Acari: Ixodidae): An update on geographical distribution and known associations with hosts and pathogens. Exp. Appl. Acarol. 2011, 55, 85–121.
- Spengler, J.R.; Bergeron, É.; Spiropoulou, C.F. Crimean-Congo hemorrhagic fever and expansion from endemic regions. Curr. Opin. Virol. 2019, 34, 70–78.
- Chitimia-Dobler, L.; Nava, S.; Bestehorn, M.; Dobler, G.; Wölfel, S. First detection of Hyalomma rufipes in Germany. Ticks Tick Borne Dis. 2016, 7, 1135–1138.
- Kampen, H.; Poltz, W.; Hartelt, K.; Wölfel, R.; Faulde, M. Detection of a questing Hyalomma marginatum marginatum adult female (Acari, Ixodidae) in southern Germany. Exp. Appl. Acarol. 2007, 43, 227–231.
- Oehme, R.; Bestehorn, M.; Wölfel, S.; Chitimia-Dobler, L. Hyalomma marginatum in Tübingen, Germany. Syst. Appl. Acarol. 2017, 22, 1–6.
- Chitimia-Dobler, L.; Schaper, S.; Rieß, R.; Bitterwolf, K.; Frangoulidis, D.; Bestehorn, M.; Springer, A.; Oehme, R.; Drehmann, M.; Lindau, A.; et al. Imported Hyalomma ticks in Germany in 2018. Parasit Vectors 2019, 12, 134.
- Hansford, K.M.; Carter, D.; Gillingham, E.L.; Hernandez-Triana, L.M.; Chamberlain, J.; Cull, B.; McGinley, L.; Paul Phipps, L.; Medlock, J.M. Hyalomma rufipes on an untraveled horse: Is this the first evidence of Hyalomma nymphs successfully moulting in the United Kingdom? Ticks Tick Borne Dis. 2019, 10, 704–708.
- McGinley, L.; Hansford, K.M.; Cull, B.; Gillingham, E.L.; Carter, D.P.; Chamberlain, J.F.; Hernandez-Triana, L.M.; Phipps, L.P.; Medlock, J.M. First report of human exposure to Hyalomma marginatum in England: Further evidence of a Hyalomma moulting event in north-western Europe? Ticks Tick Borne Dis. 2021, 12, 101541.
- Duscher, G.G.; Hodžić, A.; Hufnagl, P.; Wille-Piazzai, W.; Schötta, A.M.; Markowicz, M.A.; Estrada-Peña, A.; Stanek, G.; Allerberger, F. Adult Hyalomma marginatum tick positive for Rickettsia aeschlimannii in Austria, October 2018. Euro. Surveill. 2018, 23, 1800595.
- RIVM, National Institute for Public Health and the Environment. Tick Found in Drenthe is a Hyalomma Tick|RIVM [WWW Document]. Natl. Inst. Public Health Environ. 2019. Available online: (accessed on 30 January 2021).
- Hansford, K.M.; Medlock, J.M.; Atkinson, B.; Santos-Silva, M.M. Importation of a Hyalomma lusitanicum tick into the UK on a dog. Vet. Rec. 2016, 179, 415.
- Gargili, A.; Estrada-Peña, A.; Spengler, J.R.; Lukashev, A.; Nuttall, P.A.; Bente, D.A. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: A review of published field and laboratory studies. Antivir. Res. 2017, 144, 93–119.
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T. Ticks of Europe and North Africa. A Guide to Species Identification. Switzerland; Springer International Publishing AG: Cham, Switzerland, 2017; p. 403.
- Estrada-Peña, A.; Vatansever, Z.; Gargili, A.; Ergönul, O. The trend towards habitat fragmentation is the key factor driving the spread of Crimean-Congo haemorrhagic fever. Epidemiol. Infect. 2010, 138, 1194–1203.
- Estrada-Peña, A.; Jameson, L.; Medlock, J.; Vatansever, Z.; Tishkova, F. Unraveling the ecological complexities of tick-associated Crimean-Congo hemorrhagic fever virus transmission: A gap analysis for the western Palearctic. Vector Borne Zoonotic Dis. 2012, 12, 743–752.
- Kilpatrick, A.M.; Randolph, S.E. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 2012, 380, 1946–1955.
- Esser, H.J.; Mögling, R.; Cleton, N.B.; van der Jeugd, H.; Sprong, H.; Stroo, A.; Koopmans, M.P.G.; de Boer, W.F.; Reusken, C.B.E.M. Risk factors associated with sustained circulation of six zoonotic arboviruses: A systematic review for selection of surveillance sites in non-endemic areas. Parasit. Vectors 2019, 12, 265.
- Estrada-Peña, A.; D’Amico, G.; Fernández-Ruiz, N. Modelling the potential spread of Hyalomma marginatum ticks in Europe by migratory birds. Int. J. Parasitol. 2021, 51, 1–11.
- Fernández-Ruiz, N.; Estrada-Peña, A. Towards New Horizons: Climate Trends in Europe Increase the Environmental Suitability for Permanent Populations of Hyalomma marginatum (Ixodidae). Pathogens 2021, 10, 95.
- Sorvillo, T.E.; Rodriguez, S.E.; Hudson, P.; Carey, M.; Rodriguez, L.L.; Spiropoulou, C.F.; Bird, B.H.; Spengler, J.R.; Bente, D.A. Towards a Sustainable One Health Approach to Crimean-Congo Hemorrhagic Fever Prevention: Focus Areas and Gaps in Knowledge. Trop. Med. Infect. Dis. 2020, 5, 113.
More
Information
Subjects:
Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
10 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




