
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yanis Abdelhamid Gueche | + 2147 word(s) | 2147 | 2021-08-10 11:09:31 | | | |
| 2 | Lindsay Dong | Meta information modification | 2147 | 2021-08-27 10:30:43 | | |
Video Upload Options
3D printing is a new emerging technology in the pharmaceutical manufacturing landscape. Its potential advantages for personalized medicine have been widely explored and commented on in the literature over recent years. More recently, the selective laser sintering (SLS) technique has been investigated for oral drug-delivery applications.
1. Introduction
3D printing allows the production of objects of different sizes and shapes, according to a pre-established design. Therefore, it offers greater flexibility than conventional processes [1][2][3]. Three-dimensional printing could help improve individualized oral therapy, especially solid oral forms (SOFs), which currently present limited options for individually designed doses. Indeed, while liquid forms can be easily dosed using oral syringes, they also present main disadvantages such as poor stability, administration errors and unpleasant taste. In contrast, solid forms, especially tablets, present higher stability, but the possibilities of dosing them individually are limited [4].
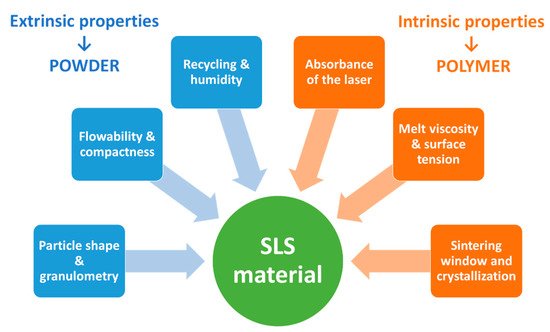
Selective laser sintering (SLS) is classified under the Powder Bed Fusion category according to the ASTM (American Society for Testing Material) [5]. It involves the building of objects by necking powder particles using the energy provided by a laser [6]. This additive manufacturing technique presents many benefits such as high resolution, the possibility of recycling the powder and the absence of pre-processing [7]. Moreover, pharmaceutical manufacturing requires a higher threshold of quality and safety. This justifies the relevance of using pharmaceutical grade powders, which are recognized to be safe for the human body, but these materials should also be printable and remain stable during the printing process. The literature on SLS provides knowledge of the requirements for printability and stability of the feedstock materials [8][9][10]. However, switching from conventional powders to pharmaceutical powders can be challenging [11][12][13].
2. Materials and Equipment
2.1. SLS Printer and Process Parameters
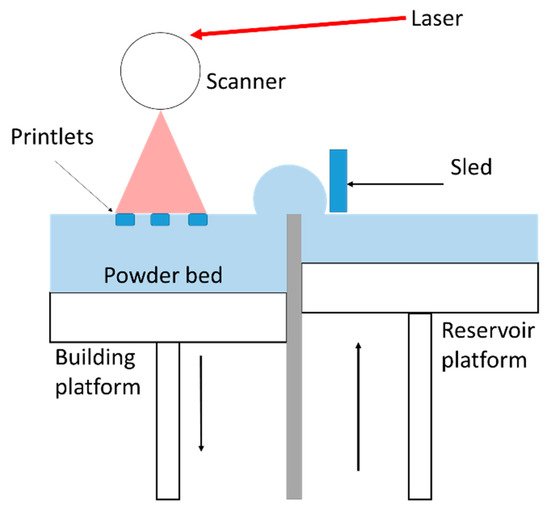
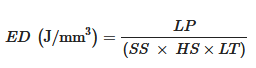
2.2. Raw Materials
2.2.1. Polymers
Like FDM, SLS requires the use of thermoplastic polymers as matrices to carry drugs. This type of polymers present the ability to be processed and remolded upon thermal variations (heating and cooling) [17]. Before sintering, the heating temperature is set below the melting temperature for semi-crystalline polymers or below the glass transition temperature for amorphous polymers [10]. Then, the laser, depending on its scanning speed, will act as a final push to more or less fuse the powder particles. Several pharmaceutical thermoplastic polymers widely used for HME and FDM have also been explored for SLS such as copovidone (Kollidon VA64) [18][19][20][21][22][23], PEG-PVA (Kollicoat IR) [24][25][26][27], hydroxypropyl methylcellulose (HPMC) [18][28][29], ethylcellulose [30][25][29], acrylic polymers (Eudragit) [24][30][28][29] and polyethylene oxide (PEO) [30][31].
2.2.2. Active Pharmaceutical Ingredients (API)
2.2.3. Fillers and Other Components
3. Variability of the Structure
3.1. Variability of the Macrostructure with the Design
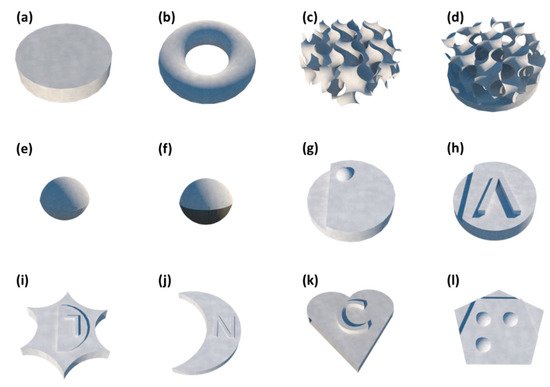
The ability to fabricate printlets presenting various geometries is not specific to the SLS technique but to 3D printing in general. However, precision varies from a printer to another and SLS shows good potential to produce highly complex structures with high resolution. The design of the reported SOFs varies from one study to another (Figure 3), depending on the intended application.
3.2. Variability of the Microstructure with the Process Parameters and the Material Attributes
3.2.1. Porosity of Printlets Produced by SLS
It is also interesting to note that the internal structure is not only influenced by the pre-established design but also by the printing parameters. As in the case of FDM, where slicing parameters such as infill rate and infill shape could modify the geometry of printlets and hence influence their properties [36]; SLS printing parameters such as laser scanning speed can also affect the size, the form and the distribution of pores by modulating the degree of sintering [10].
3.2.2. Critical Process Parameters and Material Attributes for Porosity in SLS
3.3. Relationship between Porosity–Mechanical Properties and Drug Release
3.3.1. Mechanical Properties
In general, hardness tended to decrease when the laser scanning speed was accelerated [18], or when the heating temperature was reduced [19]. Hence, the mechanical strength is directly related to the porosity, as porous printlets present weak interparticular bonding and break easily. This is consistent with previous results on the influence of high laser energy density on improving stiffness of the sintered DDDs [45][38][46][47][48][49][39]. It is, therefore, necessary to determine the optimal energy interval that balances between the mechanical integrity and stability of the drug [50].
3.3.2. Drug Release
4. Amorphous Solid Dispersions (ASDs)
Difference scanning calorimetry (DSC) and X-Ray powder diffraction (XRPD) were used to determine the effect of sintering on the solid state of the material components by comparing the sintered printlets with their corresponding physical mixtures. DSC analysis showed that most of the printlets obtained by SLS presented a reduction or even a disappearance of the characteristic API melting peak. XRPD analysis generally corroborated the precedent results by demonstrating a more or less pronounced flattening of the specific crystalline peaks of the API. This suggests that the drug dissolves partially or totally into an amorphous form within the molten polymer during the sintering process [24][18][30][28][25][19][31][20][21][22]. These results highlight the potential of SLS for the fabrication of solid amorphous dispersions.
5. Applications of SLS in Personalized Medicine
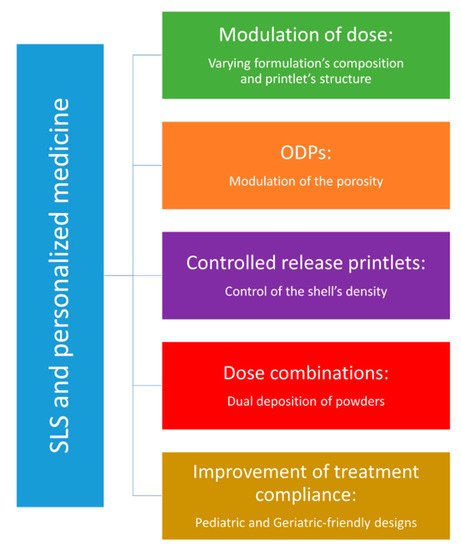
6. Conclusions
Porosity stands out as the main contribution of SLS technology, as both sintering parameters and material properties show the ability to modulate the internal structure of printlets. Therefore, orally disintegrating printlets appear as the most promising application for SLS of solid oral forms. Moreover, previous studies conducted on SLS of drug-delivery devices help to predict potential pharmaceutical applications such controlled-release printlets. In the long term, SLS could be an interesting asset for precision medicine, but in the meantime, there are still some technical and regulatory aspects to be addressed.
References
- Trenfield, S.J.; Awad, A.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing Pharmaceuticals: Drug Development to Frontline Care. Trends Pharmacol. Sci. 2018, 39, 440–451.
- El Aita, I.; Ponsar, H.; Quodbach, J. A Critical Review on 3D-Printed Dosage Forms. CPD 2019, 24, 4957–4978.
- Afsana; Jain, V.; Haider, N.; Jain, K. 3D Printing in Personalized Drug Delivery. CPD 2019, 24, 5062–5071.
- Wening, K.; Breitkreutz, J. Oral Drug Delivery in Personalized Medicine: Unmet Needs and Novel Approaches. Int. J. Pharm. 2011, 404, 1–9.
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Advances in Powder Bed Fusion 3D Printing in Drug Delivery and Healthcare. Adv. Drug Deliv. Rev. 2021, 174, 406–424.
- Mazzoli, A. Selective Laser Sintering in Biomedical Engineering. Med. Biol. Eng. Comput. 2013, 51, 245–256.
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printing: Principles and Pharmaceutical Applications of Selective Laser Sintering. Int. J. Pharm. 2020, 586, 119594.
- Tan, J.H.; Wong, W.L.E.; Dalgarno, K.W. An Overview of Powder Granulometry on Feedstock and Part Performance in the Selective Laser Melting Process. Addit. Manuf. 2017, 18, 228–255.
- Chatham, C.A.; Long, T.E.; Williams, C.B. A Review of the Process Physics and Material Screening Methods for Polymer Powder Bed Fusion Additive Manufacturing. Prog. Polym. Sci. 2019, 93, 68–95.
- Goodridge, R.D.; Tuck, C.J.; Hague, R.J.M. Laser Sintering of Polyamides and Other Polymers. Prog. Mater. Sci. 2012, 57, 229–267.
- Ilyés, K.; Kovács, N.K.; Balogh, A.; Borbás, E.; Farkas, B.; Casian, T.; Marosi, G.; Tomuță, I.; Nagy, Z.K. The Applicability of Pharmaceutical Polymeric Blends for the Fused Deposition Modelling (FDM) 3D Technique: Material Considerations–Printability–Process Modulation, with Consecutive Effects on in Vitro Release, Stability and Degradation. Eur. J. Pharm. Sci. 2019, 129, 110–123.
- Alhijjaj, M.; Belton, P.; Qi, S. An Investigation into the Use of Polymer Blends to Improve the Printability of and Regulate Drug Release from Pharmaceutical Solid Dispersions Prepared via Fused Deposition Modeling (FDM) 3D Printing. Eur. J. Pharm. Biopharm. 2016, 108, 111–125.
- Nasereddin, J.M.; Wellner, N.; Alhijjaj, M.; Belton, P.; Qi, S. Development of a Simple Mechanical Screening Method for Predicting the Feedability of a Pharmaceutical FDM 3D Printing Filament. Pharm Res. 2018, 35, 151.
- Ho, H.C.H.; Gibson, I.; Cheung, W.L. Effects of Energy Density on Morphology and Properties of Selective Laser Sintered Polycarbonate. J. Mater. Process. Technol. 1999, 89–90, 204–210.
- Southon, N.; Stavroulakis, P.; Goodridge, R.; Leach, R. In-Process Measurement and Monitoring of a Polymer Laser Sintering Powder Bed with Fringe Projection. Mater. Des. 2018, 157, 227–234.
- Araújo, M.; Sa-Barreto, L.; Gratieri, T.; Gelfuso, G.; Cunha-Filho, M. The Digital Pharmacies Era: How 3D Printing Technology Using Fused Deposition Modeling Can Become a Reality. Pharmaceutics 2019, 11, 128.
- Yuan, S.; Shen, F.; Chua, C.K.; Zhou, K. Polymeric Composites for Powder-Based Additive Manufacturing: Materials and Applications. Prog. Polym. Sci. 2019, 91, 141–168.
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D Printed Orally Disintegrating Printlets Using Selective Laser Sintering. Int. J. Pharm. 2018, 541, 101–107.
- Barakh Ali, S.F.; Mohamed, E.M.; Ozkan, T.; Kuttolamadom, M.A.; Khan, M.A.; Asadi, A.; Rahman, Z. Understanding the Effects of Formulation and Process Variables on the Printlets Quality Manufactured by Selective Laser Sintering 3D Printing. Int. J. Pharm. 2019, 570, 118651.
- Allahham, N.; Fina, F.; Marcuta, C.; Kraschew, L.; Mohr, W.; Gaisford, S.; Basit, A.W.; Goyanes, A. Selective Laser Sintering 3D Printing of Orally Disintegrating Printlets Containing Ondansetron. Pharmaceutics 2020, 12, 110.
- Awad, A.; Yao, A.; Trenfield, S.J.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Tablets (Printlets) with Braille and Moon Patterns for Visually Impaired Patients. Pharmaceutics 2020, 12, 172.
- Mohamed, E.M.; Barakh Ali, S.F.; Rahman, Z.; Dharani, S.; Ozkan, T.; Kuttolamadom, M.A.; Khan, M.A. Formulation Optimization of Selective Laser Sintering 3D-Printed Tablets of Clindamycin Palmitate Hydrochloride by Response Surface Methodology. AAPS PharmSciTech 2020, 21, 232.
- Davis, D.A.; Thakkar, R.; Su, Y.; Williams, R.O.; Maniruzzaman, M. Selective Laser Sintering 3-Dimensional Printing as a Single Step Process to Prepare Amorphous Solid Dispersion Dosage Forms for Improved Solubility and Dissolution Rate. J. Pharm. Sci. 2021, 110, 1432–1443.
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective Laser Sintering (SLS) 3D Printing of Medicines. Int. J. Pharm. 2017, 529, 285–293.
- Awad, A.; Fina, F.; Trenfield, S.; Patel, P.; Goyanes, A.; Gaisford, S.; Basit, A. 3D Printed Pellets (Miniprintlets): A Novel, Multi-Drug, Controlled Release Platform Technology. Pharmaceutics 2019, 11, 148.
- Hamed, R.; Mohamed, E.M.; Rahman, Z.; Khan, M.A. 3D-Printing of Lopinavir Printlets by Selective Laser Sintering and Quantification of Crystalline Fraction by XRPD-Chemometric Models. Int. J. Pharm. 2020, 120059.
- Januskaite, P.; Xu, X.; Ranmal, S.R.; Gaisford, S.; Basit, A.W.; Tuleu, C.; Goyanes, A. I Spy with My Little Eye: A Paediatric Visual Preferences Survey of 3D Printed Tablets. Pharmaceutics 2020, 12, 1100.
- Trenfield, S.J.; Goyanes, A.; Telford, R.; Wilsdon, D.; Rowland, M.; Gaisford, S.; Basit, A.W. 3D Printed Drug Products: Non-Destructive Dose Verification Using a Rapid Point-and-Shoot Approach. Int. J. Pharm. 2018, 549, 283–292.
- Yang, Y.; Xu, Y.; Wei, S.; Shan, W. Oral Preparations with Tunable Dissolution Behavior Based on Selective Laser Sintering Technique. Int. J. Pharm. 2020, 120127.
- Fina, F.; Goyanes, A.; Madla, C.M.; Awad, A.; Trenfield, S.J.; Kuek, J.M.; Patel, P.; Gaisford, S.; Basit, A.W. 3D Printing of Drug-Loaded Gyroid Lattices Using Selective Laser Sintering. Int. J. Pharm. 2018, 547, 44–52.
- Trenfield, S.J.; Tan, H.X.; Goyanes, A.; Wilsdon, D.; Rowland, M.; Gaisford, S.; Basit, A.W. Non-Destructive Dose Verification of Two Drugs within 3D Printed Polyprintlets. Int. J. Pharm. 2020, 577, 119066.
- Schmid, M.; Amado, A.; Wegener, K. Polymer Powders for Selective Laser Sintering (SLS). AIP Conf. Proc. 2015, 1664, 160009.
- Tan, L.J.; Zhu, W.; Zhou, K. Recent Progress on Polymer Materials for Additive Manufacturing. Adv. Funct. Mater. 2020, 30, 2003062.
- Aho, J.; Bøtker, J.P.; Genina, N.; Edinger, M.; Arnfast, L.; Rantanen, J. Roadmap to 3D-Printed Oral Pharmaceutical Dosage Forms: Feedstock Filament Properties and Characterization for Fused Deposition Modeling. J. Pharm. Sci. 2019, 108, 26–35.
- Charoo, N.A.; Barakh Ali, S.F.; Mohamed, E.M.; Kuttolamadom, M.A.; Ozkan, T.; Khan, M.A.; Rahman, Z. Selective Laser Sintering 3D Printing—An Overview of the Technology and Pharmaceutical Applications. Drug Dev. Ind. Pharm. 2020, 46, 869–877.
- Korte, C.; Quodbach, J. 3D-Printed Network Structures as Controlled-Release Drug Delivery Systems: Dose Adjustment, API Release Analysis and Prediction. AAPS PharmSciTech 2018, 19, 3333–3342.
- Leong, K.F.; Phua, K.K.S.; Chua, C.K.; Du, Z.H.; Teo, K.O.M. Fabrication of Porous Polymeric Matrix Drug Delivery Devices Using the Selective Laser Sintering Technique. Proc. Inst. Mech Eng. H 2001, 215, 191–192.
- Salmoria, G.V.; Klauss, P.; Zepon, K.M.; Kanis, L.A. The Effects of Laser Energy Density and Particle Size in the Selective Laser Sintering of Polycaprolactone/Progesterone Specimens: Morphology and Drug Release. Int. J. Adv. Manuf. Technol. 2013, 66, 1113–1118.
- Salmoria, G.V.; Vieira, F.E.; Muenz, E.A.; Gindri, I.M.; Marques, M.S.; Kanis, L.A. Additive Manufacturing of PE/Fluorouracil/Progesterone Intrauterine Device for Endometrial and Ovarian Cancer Treatments. Polym. Test. 2018, 71, 312–317.
- Low, K.H.; Leong, K.F.; Chua, C.K.; Du, Z.H.; Cheah, C.M. Characterization of SLS Parts for Drug Delivery Devices. Rapid Prototyp. J. 2001, 7, 262–268.
- Salmoria, G.V.; Leite, J.L.; Lopes, C.N.; Machado, R.A.F.; Lago, A. The Manufacturing of PMMA/PS Blends by Selective Laser Sintering. In Virtual and Rapid Manufacturing: Advanced Research in Virtual and Rapid Prototyping; Taylor & Francis: New York, NY, USA, 2007; pp. 305–311.
- Salmoria, G.V.; Ahrens, C.H.; Klauss, P.; Paggi, R.A.; Oliveira, R.G.; Lago, A. Rapid Manufacturing of Polyethylene Parts with Controlled Pore Size Gradients Using Selective Laser Sintering. Mat. Res. 2007, 10, 211–214.
- Salmoria, G.V.; Klauss, P.; Paggi, R.A.; Kanis, L.A.; Lago, A. Structure and Mechanical Properties of Cellulose Based Scaffolds Fabricated by Selective Laser Sintering. Polym. Test. 2009, 28, 648–652.
- Salmoria, G.V.; Fancello, E.A.; Roesler, C.R.M.; Dabbas, F. Functional Graded Scaffold of HDPE/HA Prepared by Selective Laser Sintering: Microstructure and Mechanical Properties. Int. J. Adv. Manuf. Technol. 2013, 65, 1529–1534.
- Salmoria, G.V.; Klauss, P.; Roesler, C.R.M.; Kanis, L.A. Structure and Mechanical Properties of PCL/PG Devices Prepared by Selective Laser Sintering for Drug Delivery Applications. In Proceedings of the ASME 2013 Summer Bioengineering Conference, Sunriver, OR, USA, 26–29 June 2013.
- Salmoria, G.V.; Cardenuto, M.R.; Roesler, C.R.M.; Zepon, K.M.; Kanis, L.A. PCL/Ibuprofen Implants Fabricated by Selective Laser Sintering for Orbital Repair. Procedia CIRP 2016, 49, 188–192.
- Salmoria, G.V.; Klauss, P.; Kanis, L.A. Laser Printing of PCL/Progesterone Tablets for Drug Delivery Applications in Hormone Cancer Therapy. Lasers Manuf. Mater. Process. 2017, 4, 108–120.
- Salmoria, G.; Vieira, F.; Ghizoni, G.; Marques, M.; Kanis, L. 3D Printing of PCL/Fluorouracil Tablets by Selective Laser Sintering: Properties of Implantable Drug Delivery for Cartilage Cancer Treatment. Rheumatol. Orthop. Med. 2017, 2, 1–7.
- Salmoria, G.V.; Vieira, F.E.; Ghizoni, G.B.; Gindri, I.M.; Kanis, L.A. Additive Manufacturing of PE/Fluorouracil Waffles for Implantable Drug Delivery in Bone Cancer Treatment. Eng. J. 2017, 3, 62–70.
- Antonov, E.N.; Bagratashvili, V.N.; Whitaker, M.J.; Barry, J.J.A.; Shakesheff, K.M.; Konovalov, A.N.; Popov, V.K.; Howdle, S.M. Three-Dimensional Bioactive and Biodegradable Scaffolds Fabricated by Surface-Selective Laser Sintering. Adv. Mater. 2005, 17, 327–330.
- Cailleaux, S.; Sanchez-Ballester, N.M.; Gueche, Y.A.; Bataille, B.; Soulairol, I. Fused Deposition Modeling (FDM), the new asset for the production of tailored medicines. J. Control. Release 2021, 330, 821–841.
- Pietrzak, K.; Isreb, A.; Alhnan, M.A. A Flexible-Dose Dispenser for Immediate and Extended Release 3D Printed Tablets. Eur. J. Pharm. Biopharm. 2015, 96, 380–387.
- Yang, Y.; Wang, H.; Li, H.; Ou, Z.; Yang, G. 3D Printed Tablets with Internal Scaffold Structure Using Ethyl Cellulose to Achieve Sustained Ibuprofen Release. Eur. J. Pharm. Sci. 2018, 115, 11–18.




