
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francesco Gentile | + 2380 word(s) | 2380 | 2020-10-16 09:44:09 | | | |
| 2 | Conner Chen | -21 word(s) | 2359 | 2020-11-17 02:55:05 | | | | |
| 3 | Conner Chen | Meta information modification | 2359 | 2021-01-12 03:45:21 | | | | |
| 4 | Conner Chen | Meta information modification | 2359 | 2021-01-12 03:45:53 | | |
Video Upload Options
The central nervous system (CNS) is a highly energy demanding organ, as it uses about 20% of the total oxygen and glucose consumed by the body, despite representing only 2% of the total body mass. Neurons heavily rely on glucose as the main energy substrate, but in stressful conditions, other resources, such as ketone bodies and lactate, provided by glial cells, may be used. Fatty acids (FA) are poorly used by the CNS as a fuel due to a low expression of the β-oxidation enzyme machinery, an evolutionarily acquired feature necessary to limit excessive oxygen consumption and consequent reactive oxygen species generation in mitochondria generally associated with FA catabolism . Furthermore, the CNS has a limited ability to build internal energy stores, as only astrocytes have been shown to synthesize glycogen in small amounts. Cholesterol is essential for brain function. It is involved in cell maintenance, neuronal transmission, and synaptic formation. Its metabolism in the CNS relies on local de novo synthesis and catabolism, as the blood–brain barrier (BBB) blocks the passage of diet-derived cholesterol into the CNS.
Thus, to maintain a constant delivery of energy substrates for neuronal activity, the CNS engages in intensive crosstalk with organs involved in metabolism, such as the gut, adipose tissue and liver, regulating several functions such as food behavior, hormonal status and commence of adaptive responses to dietary changes. Due to its metabolic setting, the maintenance of glucose homeostasis is essential for proper neuronal functioning. Receptors for insulin and insulin-like growth factor-1 (IGF-1) are present throughout the CNS, mostly concentrated on the hypothalamus and hippocampus, where local production of these hormones has also been demonstrated, especially during growth. Insulin and IGF-1 exert an important role in neuronal development and survival by stimulating synaptic plasticity and long-term potentiation, which aid in learning and memory. Interestingly, insulin modulates phosphorylation of tau protein, supporting a potential involvement of insulin metabolism in AD. Furthermore, fibroblast growth factor 21 (FGF21), a hepatocyte-derived hormone, signals protein and glucose status to the brain, allowing the refinement of food choice and metabolism according to dietary changes. On the other hand, CNS insulin sensitivity modulates adiposity and body fat accumulation. Along the brain-periphery signaling network, diet and microbiota deeply influence these communication pathways through several mechanisms.
1. Diet-CNS Interactions
Cognition and behavior are tightly connected to the nutritional state of the organism. Regulation in many neural- and nutrition-related genes underwent strong positive selection during human evolution, representing an important step forward in the separation from primates along the evolutionary timeline and contributing to the high complexity of human communication and habits [1].
Besides its basic function as an energy substrate, the type and composition of diet during early life has important long-lasting effects on brain function. Breastfeeding is associated with better neural development in childhood [2] and its effects persist during adulthood, as the better cognitive performance of breastfed progeny associates with higher educational status and income [3]. Nonetheless, these effects may be viable only within precise time windows during growth. Indeed, consumption of 2′-fucosyllactose, a component of human milk, from 1 month after birth predicted better cognition at 24 months, but this effect was lost if supplied from 6 months of age [4].
Long-term effects may be explained by the ability of nutrients to exert stable epigenetic changes on neurons, critical for proper development of the nervous system. Omega-3 polyunsaturated FAs (ω-3 PUFA), including docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), are essential components of neuronal cells’ membrane, affecting its composition and fluidity. Examples of food enriched in ω-3 PUFAs are fish, evo oil, walnuts and soybeans. High concentrations of DHA and EPA are present in the nervous tissue, representing 40% of the lipid content in neuronal membranes, and their accumulation in the brain during gestational and perinatal age is important for vision, memory, and behavior. Besides their structural role, perinatal ω-3 PUFA-enriched diet induces wide ranging changes in the brains of rats at the transcriptional level, affecting the expression of genes such as transthyretin, α-synuclein, and calmodulins, important for synaptic plasticity, hippocampal neurogenesis and learning [5]. Furthermore, ω-3 PUFAs exhibit anti-inflammatory effects. For these reasons, ω-3 PUFA supplementation, mainly through breastfeeding, is recommended in pregnant women and newborns [6].
Other micronutrients contribute to brain development and function. Flavonoids are plant-extracted polyphenols, which sustain spatial working memory by increasing hippocampal levels of brain-derived neurotrophic factor (BDNF), important for hippocampal neurogenesis [7][8]. Dietary choline supports fetal development during pregnancy by increasing neuronal proliferation and brain angiogenesis [9]. It also serves as a substrate for the production of the neurotransmitter acetylcholine and as a methyl-group donor, contributing to epigenetic modifications such as DNA and histone methylation. The 1958G>A polymorphism in the methylenetetrahydrofolate dehydrogenase gene (MTHFD1), which encodes for an enzyme involved in folate metabolism, decreases the availability of methyltetrahydrofolate, creating an extra demand of alternative methyl-group donors, such as choline, for the formation of methionine [10]. In a scenario of low choline diet, individuals carrying this SNP show a higher chance of developing fatty liver and muscle injury [10]. Finally, minerals and vitamins also exert numerous effects on neuronal signaling and communication. B vitamins are important for fiber myelination and neuronal survival, while vitamin E is a powerful antioxidant, supporting mitochondrial function in cells [11].
Despite several studies that analyzed isolated nutrients, investigating the effects of dietary patterns yields more reliable results as it closely reproduces natural human practices. The Mediterranean Diet (MeD) roots from southern Europe eating habits and is based on high consumption of vegetables, fruits, nuts and whole grains, moderate consumption of dairy products and limited amounts of meat and saturated fat. Besides its known effects in cardiovascular and cancer prevention, adherence to MeD demonstrated protection against a wide range of CNS diseases, including stroke, mild cognitive impairment and AD [12]. The results of functional tests are coherent with longitudinal neuroimaging studies, which showed that a healthy diet is associated with larger hippocampal volumes compared to individuals consuming a Western diet [13][14]. Caloric restriction has been also demonstrated to prolong lifespan and protects against neurodegeneration [15]. Reduction in calories stimulates a mild chronic stress response in neurons, which favors an increased production of neurotrophic factors, such as BDNF, and chaperones, protective against neuronal death and protein aggregation [15].
2. Gut Microbiota-CNS Interactions
The symbiotic relationship between gut microbiota and the brain, also known as the “microbiota-gut-brain axis”, exerts an important role in several aspects of brain health, such as development, function, and senescence. It is based on a continuous bi-directional flux of molecular signals using two main different routes: neural pathways and the systemic circulation (Figure 1). Viscero-fugal fibers make up 80% of the total vagus nerve, allowing the brain to constantly sense the gut environment. Indeed, neurotransmitters such as serotonin, dopamine and γ-aminobutyric acid (GABA) are produced by microbiota and bind the sensory neurons of the enteric nervous system (ENS), sending afferent impulses through the vagus nerve and sympathetic/parasympathetic pathways [16]. Gut inflammation induced by bacterial pathogens elicits activation of the vagal sensory ganglia and nucleus tractus solitarius in the brainstem, providing a method of early warning to the brain during infections [17]. Interestingly, some studies highlighted the existence of lateralized functions of the vagal pathway, such that the right vagus nerve is involved in stimulation of neural circuits responsible for diet-induced reward behavior [18], while the left vagus nerve takes part to a liver–brain–gut neural arc responsible for proper differentiation and maintenance of T regulatory (Treg) cells in the gut [19].
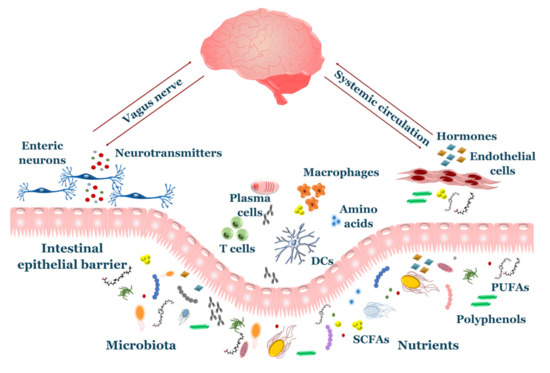
Figure 1. Overview of gut–brain axis. The gut and brain communicate through an intensive crosstalk involving neuroendocrine axis, circulating metabolites and immune system. Nutrients and microbial products pass through the intestinal epithelial barrier where they participate on enterocytes’ physiology and drive behavior of immune cells. The enteric nervous system uses numerous signals to sense the gut environment, including neurotransmitters produced by microbiota. Afferent fibers (vagus nerve/sympathetic nerves) transmit these signals to the central nervous system (CNS). Gut-derived hormones and metabolites are released to the systemic circulation and reach the brain.
Neurotransmitters produced by GM also exert other functions outside of the nervous system with indirect effects of neuronal function. For instance, serotonin and dopamine affect immune system response [20][21], while GABA plays a role in the local defense against bacterial translocation [22]. The gut releases significant amounts of hormones, peptides and microbial metabolites, such as short chain fatty acids (SCFAs), secondary bile acids, tryptophan- and polyphenol-derived products, which exerts important effects on neuronal function and survival. Most of these compounds cross the BBB, including SCFAs, which exploit active membrane transporters on the endothelium to reach the CNS [23]. Conversely, the CNS also sends efferent responses to the gut, regulating motility, mucus secretion, barrier integrity and visceral sensitivity [24].
The GM exerts a broad role in the regulation of behavior and cognition. Germ-free (GF) animals show reduced anxiety [25][26], but impaired social development, providing links with neurodevelopmental and neuropsychiatric illnesses, such as autism spectrum disorders and schizophrenia [27]. Furthermore, gut bacteria participate in the proper development of non-spatial memory and fear extinction learning in mice, revealing targeted actions also towards the hippocampus [28] and prefrontal cortex [29], respectively. Food choice is also affected by GM, as O’Donnell et al. showed the ability of microbes to shape host’s dietary preferences towards food enriched in those specific bacteria, thus ensuring their survival and maintenance in the gut [30]. Microbial-derived metabolites stimulate the secretion of anorexigenic and incretin hormones, thus affecting appetite sensation and glucose metabolism after a meal [31]. Recent evidence revealed that GM might influence behavior in humans as well. Indeed, GM composition is associated with emotionality and fear reactivity in infants [32], important traits that have been shown to predict the risk of anxiety and depression. Furthermore, gut bacteria may model human personality traits, with sociability being associated with higher GM diversity, while anxiety and stress is related to reduced diversity [33].
GF mice develop gross and ultrastructural alterations in the amygdala and hippocampus, with the latter displaying shorter and less branched dendritic trees [34]. Microbiota also affects neuronal proliferation, as post-natal administration of antibiotics reduces hippocampal neurogenesis [35]. Among candidate mediators for this effect, sodium butyrate, a SCFA, restores antibiotic-induced impairment in neuronal proliferation [35] and its administration boosts widespread neurogenesis after an ischemic insult [36]. The GM may also exert region-specific effects in the brain. GF mice upregulate genes involved in myelination of the prefrontal cortex, but not in other regions, which can be reversed by colonization with commensal bacteria [37]. The microbiota also affects glial development and function. Microglia participate to several events during growth, such as synaptic pruning, promotion of network wiring and release of cytokines necessary for neuronal differentiation. Defects in microglia occur in GF conditions, as they become dysfunctional due to disruption of developmental genes and immune response pathways [38]. SCFA-producing bacteria may mediate such an effect, as a supply of butyrate restores normal microglial morphology [39]. Astrocytes may also be regulated by gut microbes, as some bacterial species produce tryptophan-derived metabolites which bind to the aryl hydrocarbon receptor (AHR), present on astrocytes’ membrane, stimulating type I interferon signaling and reducing inflammation and disease scores in experimental autoimmune encephalomyelitis (EAE) mice, a model of multiple sclerosis (MS) [40].
Finally, the BBB integrity is influenced by the microbiota. Depletion of microbial community leads to a 75% loss of tight junction proteins occludin and claudin-5 in the BBB of mice, increasing the CNS susceptibility to exogenous stimuli such as lipopolysaccharide (LPS) and oxidative stress, both potent inducers of systemic and neuroinflammation [41]. One study demonstrated that this defect may be restored by the supplementation of propionate or butyrate, highlighting a potential role of SCFAs also in BBB homeostasis [42].
3. Diet and Microbiota on the Immune System: An Indirect Link to the CNS
Nutrition and microbiota also impact the immune system, as priming of the adaptive immune response towards an antigen may depend on metabolic factors. The heavy supply of FAs observed in mice fed on high-fat diets elicits a profound transcriptional change in CD4 T lymphocytes due to the activation of acetyl-CoA carboxylase 1, an important enzyme regulating FA metabolism in cells. This protein in turn switches on transcription factors involved in the differentiation towards Th17 cells [43]. Indeed, saturated FAs derived from diet directly enhance the differentiation and proliferation of Th1 and/or Th17 cells and their escape from the intestinal environment, which favors the rise in autoimmune events in the CNS [44]. Factors from protein catabolism also modify the immune response in the gut. Dietary serine [45] and methionine [46] affect the activation of naïve T cells and differentiation to a Th17 effector cell, respectively, by altering the epigenomic pattern of the cell. Indeed, dietary methionine restriction dampened neuroinflammation in a murine model of MS by decreasing Th17 cell activation, delaying disease onset and progression. In this context, it is not surprising that metabolic disorders are associated with a chronic low-grade Th1- and Th17-mediated inflammation [43], which has a strong pull towards the development of autoimmune diseases, including MS and chronic inflammatory demyelinating polyneuropathy (CIDP) [47][48]. Recently, Doneddu et al. reported that higher fish consumption may decrease the risk of CIDP [49], an association possibly mediated by the anti-inflammatory effects of ω-3 PUFAs, highly contained in seafood.
Commensal bacteria are essential to maintaining gut barrier integrity and participate in proper development of both B and T lymphocytes. The microbiota is involved in B cell maturation and response, shaping its function towards a tolerant state during mucosal exposure while rapidly switching to attack mode in case of entry in the systemic circulation [50]. The colonization of microbiota is essential in triggering an immune response, as the recruitment of autoreactive B and T cells in CNS demyelinating lesions relies on the availability of target autoantigen and commensal bacteria [51]. Indeed, GF mice showed a reduced inflammation in the EAE model, as the immune system failed to mount a Th17-mediated response [52]. Nonetheless, gut bacteria may also modulate immune response by shaping the fate of T cell differentiation in the gut. Bacteroides fragilis stimulates differentiation towards Treg cells [53]. Additionally, butyrate increases the expression of Treg cells and reduces EAE symptoms and axonal damage, suppressing at the same time the activation of Th17 cells [54]. An important therapeutic mechanism of dimethyl fumarate, a first-line approved drug for MS, is the activation of hydroxycarboxylic acid receptor 2 (HCAR2), a transmembrane receptor binding β-hydroxybutyrate and butyrate, which induces phenotype switching in microglia from pro-inflammatory to a neuroprotective state [55].
References
- Johnson, K.V.A. Gut microbiome composition and diversity are related to human personality traits. Hum. Microbiome J. 2020, 15, 100069, doi:10.1016/j.humic.2019.100069.
- Luczynski, P.; Whelan, S.O.; O’Sullivan, C.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 2016, 44, 2654–2666, doi:10.1111/ejn.13291.
- Mohle, L.; Mattei, D.; Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Alutis, M.; French, T.; Hambardzumyan, D.; Matzinger, P.; Dunay, I.R.; et al. Ly6C(hi) Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cell Rep. 2016, 15, 1945–1956, doi:10.1016/j.celrep.2016.04.074.
- Kim, H.J.; Leeds, P.; Chuang, D.M. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J. Neurochem. 2009, 110, 1226–1240, doi:10.1111/j.1471-4159.2009.06212.x.
- Hoban, A.E.; Stilling, R.M.; Ryan, F.J.; Shanahan, F.; Dinan, T.G.; Claesson, M.J.; Clarke, G.; Cryan, J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 2016, 6, e774, doi:10.1038/tp.2016.42.
- Matcovitch-Natan, O.; Winter, D.R.; Giladi, A.; Vargas Aguilar, S.; Spinrad, A.; Sarrazin, S.; Ben-Yehuda, H.; David, E.; Zelada Gonzalez, F.; Perrin, P.; et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016, 353, aad8670, doi:10.1126/science.aad8670.
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977, doi:10.1038/nn.4030.
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597, doi:10.1038/nm.4106.
- Johnson, K.V.A. Gut microbiome composition and diversity are related to human personality traits. Hum. Microbiome J. 2020, 15, 100069, doi:10.1016/j.humic.2019.100069.
- Luczynski, P.; Whelan, S.O.; O’Sullivan, C.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 2016, 44, 2654–2666, doi:10.1111/ejn.13291.
- Mohle, L.; Mattei, D.; Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Alutis, M.; French, T.; Hambardzumyan, D.; Matzinger, P.; Dunay, I.R.; et al. Ly6C(hi) Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cell Rep. 2016, 15, 1945–1956, doi:10.1016/j.celrep.2016.04.074.
- Kim, H.J.; Leeds, P.; Chuang, D.M. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J. Neurochem. 2009, 110, 1226–1240, doi:10.1111/j.1471-4159.2009.06212.x.
- Hoban, A.E.; Stilling, R.M.; Ryan, F.J.; Shanahan, F.; Dinan, T.G.; Claesson, M.J.; Clarke, G.; Cryan, J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 2016, 6, e774, doi:10.1038/tp.2016.42.
- Matcovitch-Natan, O.; Winter, D.R.; Giladi, A.; Vargas Aguilar, S.; Spinrad, A.; Sarrazin, S.; Ben-Yehuda, H.; David, E.; Zelada Gonzalez, F.; Perrin, P.; et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016, 353, aad8670, doi:10.1126/science.aad8670.
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977, doi:10.1038/nn.4030.
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597, doi:10.1038/nm.4106.
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158, doi:10.1126/scitranslmed.3009759.
- Hoyles, L.; Snelling, T.; Umlai, U.-K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome—Host systems interactions: Protective effects of propionate upon the blood–brain barrier. Microbiome 2018, 6, 55, doi:10.1186/s40168-018-0439-y.
- Mohle, L.; Mattei, D.; Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Alutis, M.; French, T.; Hambardzumyan, D.; Matzinger, P.; Dunay, I.R.; et al. Ly6C(hi) Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cell Rep. 2016, 15, 1945–1956, doi:10.1016/j.celrep.2016.04.074.
- Kim, H.J.; Leeds, P.; Chuang, D.M. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J. Neurochem. 2009, 110, 1226–1240, doi:10.1111/j.1471-4159.2009.06212.x.
- Hoban, A.E.; Stilling, R.M.; Ryan, F.J.; Shanahan, F.; Dinan, T.G.; Claesson, M.J.; Clarke, G.; Cryan, J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 2016, 6, e774, doi:10.1038/tp.2016.42.
- Matcovitch-Natan, O.; Winter, D.R.; Giladi, A.; Vargas Aguilar, S.; Spinrad, A.; Sarrazin, S.; Ben-Yehuda, H.; David, E.; Zelada Gonzalez, F.; Perrin, P.; et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016, 353, aad8670, doi:10.1126/science.aad8670.
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977, doi:10.1038/nn.4030.
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597, doi:10.1038/nm.4106.
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158, doi:10.1126/scitranslmed.3009759.
- Hoyles, L.; Snelling, T.; Umlai, U.-K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome—Host systems interactions: Protective effects of propionate upon the blood–brain barrier. Microbiome 2018, 6, 55, doi:10.1186/s40168-018-0439-y.
- Endo, Y.; Asou, H.K.; Matsugae, N.; Hirahara, K.; Shinoda, K.; Tumes, D.J.; Tokuyama, H.; Yokote, K.; Nakayama, T. Obesity Drives Th17 Cell Differentiation by Inducing the Lipid Metabolic Kinase, ACC1. Cell Rep. 2015, 12, 1042–1055, doi:10.1016/j.celrep.2015.07.014.
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829, doi:10.1016/j.immuni.2015.09.007.
- Ma, E.H.; Bantug, G.; Griss, T.; Condotta, S.; Johnson, R.M.; Samborska, B.; Mainolfi, N.; Suri, V.; Guak, H.; Balmer, M.L.; et al. Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab. 2017, 25, 345–357, doi:10.1016/j.cmet.2016.12.011.
- Roy, D.G.; Chen, J.; Mamane, V.; Ma, E.H.; Muhire, B.M.; Sheldon, R.D.; Shorstova, T.; Koning, R.; Johnson, R.M.; Esaulova, E.; et al. Methionine Metabolism Shapes T Helper Cell Responses through Regulation of Epigenetic Reprogramming. Cell Metab. 2020, 31, 250–266 e259, doi:10.1016/j.cmet.2020.01.006.
- Hedstrom, A.K.; Lima Bomfim, I.; Barcellos, L.; Gianfrancesco, M.; Schaefer, C.; Kockum, I.; Olsson, T.; Alfredsson, L. Interaction between adolescent obesity and HLA risk genes in the etiology of multiple sclerosis. Neurology 2014, 82, 865–872, doi:10.1212/WNL.0000000000000203.
- Bril, V.; Blanchette, C.M.; Noone, J.M.; Runken, M.C.; Gelinas, D.; Russell, J.W. The dilemma of diabetes in chronic inflammatory demyelinating polyneuropathy. J. Diabetes Its Complicat. 2016, 30, 1401–1407, doi:10.1016/j.jdiacomp.2016.05.007.
- Doneddu, P.E.; Bianchi, E.; Cocito, D.; Manganelli, F.; Fazio, R.; Filosto, M.; Mazzeo, A.; Cosentino, G.; Cortese, A.; Jann, S.; et al. Risk factors for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): Antecedent events, lifestyle and dietary habits. Data from the Italian CIDP Database. Eur. J. Neurol. 2020, 27, 136–143, doi:10.1111/ene.14044.
- Hoyles, L.; Snelling, T.; Umlai, U.-K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome—Host systems interactions: Protective effects of propionate upon the blood–brain barrier. Microbiome 2018, 6, 55, doi:10.1186/s40168-018-0439-y.
- Endo, Y.; Asou, H.K.; Matsugae, N.; Hirahara, K.; Shinoda, K.; Tumes, D.J.; Tokuyama, H.; Yokote, K.; Nakayama, T. Obesity Drives Th17 Cell Differentiation by Inducing the Lipid Metabolic Kinase, ACC1. Cell Rep. 2015, 12, 1042–1055, doi:10.1016/j.celrep.2015.07.014.
- Haghikia, A.; Jörg, S.; Duscha, A.; Berg, J.; Manzel, A.; Waschbisch, A.; Hammer, A.; Lee, D.H.; May, C.; Wilck, N.; et al. Dietary Fatty Acids Directly Impact Central Nervous System Autoimmunity via the Small Intestine. Immunity 2015, 43, 817–829, doi:10.1016/j.immuni.2015.09.007.
- Ma, E.H.; Bantug, G.; Griss, T.; Condotta, S.; Johnson, R.M.; Samborska, B.; Mainolfi, N.; Suri, V.; Guak, H.; Balmer, M.L.; et al. Serine Is an Essential Metabolite for Effector T Cell Expansion. Cell Metab. 2017, 25, 345–357, doi:10.1016/j.cmet.2016.12.011.
- Roy, D.G.; Chen, J.; Mamane, V.; Ma, E.H.; Muhire, B.M.; Sheldon, R.D.; Shorstova, T.; Koning, R.; Johnson, R.M.; Esaulova, E.; et al. Methionine Metabolism Shapes T Helper Cell Responses through Regulation of Epigenetic Reprogramming. Cell Metab. 2020, 31, 250–266 e259, doi:10.1016/j.cmet.2020.01.006.
- Hedstrom, A.K.; Lima Bomfim, I.; Barcellos, L.; Gianfrancesco, M.; Schaefer, C.; Kockum, I.; Olsson, T.; Alfredsson, L. Interaction between adolescent obesity and HLA risk genes in the etiology of multiple sclerosis. Neurology 2014, 82, 865–872, doi:10.1212/WNL.0000000000000203.
- Bril, V.; Blanchette, C.M.; Noone, J.M.; Runken, M.C.; Gelinas, D.; Russell, J.W. The dilemma of diabetes in chronic inflammatory demyelinating polyneuropathy. J. Diabetes Its Complicat. 2016, 30, 1401–1407, doi:10.1016/j.jdiacomp.2016.05.007.
- Doneddu, P.E.; Bianchi, E.; Cocito, D.; Manganelli, F.; Fazio, R.; Filosto, M.; Mazzeo, A.; Cosentino, G.; Cortese, A.; Jann, S.; et al. Risk factors for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): Antecedent events, lifestyle and dietary habits. Data from the Italian CIDP Database. Eur. J. Neurol. 2020, 27, 136–143, doi:10.1111/ene.14044.
- Li, H.; Limenitakis, J.P.; Greiff, V.; Yilmaz, B.; Scharen, O.; Urbaniak, C.; Zund, M.; Lawson, M.A.E.; Young, I.D.; Rupp, S.; et al. Mucosal or systemic microbiota exposures shape the B cell repertoire. Nature 2020, 10.1038/s41586-020-2564-6, doi:10.1038/s41586-020-2564-6.
- Berer, K.; Mues, M.; Koutrolos, M.; Rasbi, Z.A.; Boziki, M.; Johner, C.; Wekerle, H.; Krishnamoorthy, G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479, 538–541, doi:10.1038/nature10554.
- Lee, Y.K.; Menezes, J.S.; Umesaki, Y.; Mazmanian, S.K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2011, 108 S1, 4615-4622, doi:10.1073/pnas.1000082107.
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209, doi:10.1073/pnas.0909122107.
- Chen, H.; Assmann, J.C.; Krenz, A.; Rahman, M.; Grimm, M.; Karsten, C.M.; Kohl, J.; Offermanns, S.; Wettschureck, N.; Schwaninger, M. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J. Clin. Investig. 2014, 124, 2188–2192, doi:10.1172/JCI72151.
- Parodi, B.; Rossi, S.; Morando, S.; Cordano, C.; Bragoni, A.; Motta, C.; Usai, C.; Wipke, B.T.; Scannevin, R.H.; Mancardi, G.L.; et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol. 2015, 130, 279–295, doi:10.1007/s00401-015-1422-3.
- Bril, V.; Blanchette, C.M.; Noone, J.M.; Runken, M.C.; Gelinas, D.; Russell, J.W. The dilemma of diabetes in chronic inflammatory demyelinating polyneuropathy. J. Diabetes Its Complicat. 2016, 30, 1401–1407, doi:10.1016/j.jdiacomp.2016.05.007.
- Doneddu, P.E.; Bianchi, E.; Cocito, D.; Manganelli, F.; Fazio, R.; Filosto, M.; Mazzeo, A.; Cosentino, G.; Cortese, A.; Jann, S.; et al. Risk factors for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): Antecedent events, lifestyle and dietary habits. Data from the Italian CIDP Database. Eur. J. Neurol. 2020, 27, 136–143, doi:10.1111/ene.14044.
- Li, H.; Limenitakis, J.P.; Greiff, V.; Yilmaz, B.; Scharen, O.; Urbaniak, C.; Zund, M.; Lawson, M.A.E.; Young, I.D.; Rupp, S.; et al. Mucosal or systemic microbiota exposures shape the B cell repertoire. Nature 2020, 10.1038/s41586-020-2564-6, doi:10.1038/s41586-020-2564-6.
- Berer, K.; Mues, M.; Koutrolos, M.; Rasbi, Z.A.; Boziki, M.; Johner, C.; Wekerle, H.; Krishnamoorthy, G. Commensal microbiota and myelin autoantigen cooperate to trigger autoimmune demyelination. Nature 2011, 479, 538–541, doi:10.1038/nature10554.
- Lee, Y.K.; Menezes, J.S.; Umesaki, Y.; Mazmanian, S.K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2011, 108 S1, 4615-4622, doi:10.1073/pnas.1000082107.
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209, doi:10.1073/pnas.0909122107.
- Chen, H.; Assmann, J.C.; Krenz, A.; Rahman, M.; Grimm, M.; Karsten, C.M.; Kohl, J.; Offermanns, S.; Wettschureck, N.; Schwaninger, M. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J. Clin. Investig. 2014, 124, 2188–2192, doi:10.1172/JCI72151.
- Parodi, B.; Rossi, S.; Morando, S.; Cordano, C.; Bragoni, A.; Motta, C.; Usai, C.; Wipke, B.T.; Scannevin, R.H.; Mancardi, G.L.; et al. Fumarates modulate microglia activation through a novel HCAR2 signaling pathway and rescue synaptic dysregulation in inflamed CNS. Acta Neuropathol. 2015, 130, 279–295, doi:10.1007/s00401-015-1422-3.




