Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kinga Humińska-Lisowska | + 1818 word(s) | 1818 | 2021-08-20 09:55:20 | | | |
| 2 | Amina Yu | Meta information modification | 1818 | 2021-08-26 05:25:07 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Humińska-Lisowska, K. CfDNA, Sport Adaptation Predictor. Encyclopedia. Available online: https://encyclopedia.pub/entry/13565 (accessed on 08 February 2026).
Humińska-Lisowska K. CfDNA, Sport Adaptation Predictor. Encyclopedia. Available at: https://encyclopedia.pub/entry/13565. Accessed February 08, 2026.
Humińska-Lisowska, Kinga. "CfDNA, Sport Adaptation Predictor" Encyclopedia, https://encyclopedia.pub/entry/13565 (accessed February 08, 2026).
Humińska-Lisowska, K. (2021, August 25). CfDNA, Sport Adaptation Predictor. In Encyclopedia. https://encyclopedia.pub/entry/13565
Humińska-Lisowska, Kinga. "CfDNA, Sport Adaptation Predictor." Encyclopedia. Web. 25 August, 2021.
Copy Citation
Changes of circulating free plasma DNA (cfDNA) are associated with different types of tissue injury, including those induced by intensive aerobic and anaerobic exercises. Observed changes are dependent from induced inflammation, and thus it may be a potential marker for athletic overtraining.
cfDNA
aerobic exercise
exercise load
anaerobic exercise
1. Introduction
High intensity exercises lead to transient muscle fibre damage, performance deterioration, increased inflammation processes [1] and oxidative stress induced by the generation of free radicals [2]. All those factors are associated with muscle injury, and occur during normal competition, especially when the duration or intensity of sport competition is very high. In a situation in which the accumulation of exercise load is very high, and decreased athletic performance is induced, the presence of muscle injury and increased inflammation processes are also observed. It is called ‘overtraining’ and may lead to increased immune system activity with elevated secretion of inflammatory factors [3][4][5][6].
There is rising evidence suggesting that, in addition to its health benefits, intensive exercise may have potential adverse effects on the immune system [3][7]. Therefore, clarification on this matter is necessary for training recommendations or personalized training load adjustment. Preventing injuries during training is critical to optimum performance. While exercise has both acute and chronic effects on the immune system, most biomarkers are insufficient and show only modest association with susceptibility to tissue injury. Adaptation mechanisms against soft tissue injuries as well as accelerating recovery time after different types of exercise are still poorly understood and these biomarkers still need to be established. In recent years cell-free DNA (cfDNA) has emerged as a potential biomarker of injury level, inflammation, cellular stress and cell death, generating more and more interest across various biomedical disciplines. Its changes are correlated with the presence of tissue injury, immune system activation, and even cancer [8][9][10][11][12]
Such pathological conditions have already been reported in patients with sepsis, myocardial infarction, cardiovascular diseases, autoimmune disorders, cancer, or metabolic disorders, but also in patients after trauma [13].
Circulating cfDNA is defined as double-stranded DNA molecules found in body liquids such as plasma, serum or urine, resulting from biological fragmentation upon stress induction. cfDNA circulating in the body has several fragment sizes. Most cfDNA molecules display short fragments (~150–180 bp), which are mainly derived from apoptosis via the activation of cellular endonucleases leading to the cleavage of chromatin DNA into internucleosomal fragments [14]. Long cfDNA fragments of >10 kbp are thought to be generated by necrosis. Another source of cfDNA is neutrophil extracellular traps (NETs), which play an active role in the innate immune system response to both pathogens and physical exercise [15]. Physiologically, low concentrations of cfDNA (2.5–27.0 ng/mL) circulate in the plasma/serum from healthy individuals before being cleansed by the liver [16]. Elevated concentration of circulating cfDNA in the bloodstream is considered to be a novel molecular biomarker, a hallmark manifestation of inflammatory response (not only associated with diseases, but also physical exhaustion)—whether acute or chronic. This has prompted sports medical researchers to investigate the role of cDNA as a potential marker of exercise induced-metabolic changes in a healthy human body after—i.e., resistance training, marathon run, continuous treadmill running, incremental exercise, rowing, soccer, or strength training [17][18][19][20]. It is already known that high levels of physical activity are associated with metabolic and mechanical muscular damage, leukocyte inflammatory response and DNA damage caused by oxidative stress, which in turn may induce an increase in cfDNA concentration [10]. Elevated concentrations of cfDNA have already been observed immediately after acute exercise and exhibiting a rapid return to baseline levels, whereas typical biomarkers of skeletal muscle injury (C-reactive protein, uric acid, creatine kinase) display delayed kinetics compared with the cfDNA peak response [10].
Additionally, exercise parameters such as the duration and intensity of aerobic running have already been positively correlated with selected markers of muscle damage [21], which indicates the enormous potential of cfDNA as a biomarker for exercise load in the aerobic and the anaerobic state.
Strong data can be found in the literature on the effect of exercise on changes in cfDNA, but little attention has been paid to the study of internal and extracellular mechanisms resulting from adaptive changes in the body caused by many years of training, as well as the type of exercise [9][10][13][18]. Therefore, the aim of the research was to assess the changes of circulating cfDNA in association with intensive aerobic and anaerobic exercises. These changes may be associated with exercise intensity and may indicate the physical effort exerted during the type of exercise. In conclusion, measurement of cfDNA may be a good predictor of specific activity preparation; therefore, measurement of its changes may indicate the precise stage preparation and may be a useful tool in athlete preparation diagnosis.
2. cfDNA Changes in Maximal Exercises as a Sport Adaptation Predictor
The blood count characteristics are presented in Supplementary Table S1. The results of the Student’s t-test showed a significantly lower level of MPV (4.58%) and higher level of HDL (19.12%) in the athlete group compared to the control group. There were no significant differences in the rest of the examined blood count parameters (Table S1). All men in both groups successfully completed two different maximal physical tests. The Student’s t-test analysis showed the significant differences in MAnE between groups (Table S2). The athlete group had a 7.86% and 6.11% higher relative mean and peak power in comparison to the control group during the first WAnT, respectively. In the second WAnT, after 30 s of rest, a similar decrease in the relative mean and maximum power was noted in both groups. During MAE, the athlete group had a 15.24% and 9.07% higher maximum oxygen uptake and a maximum ventilation compared to the control group, respectively (Table S3).
Serum levels of cfDNA before and after MAnE and MAE are presented in Figure 1. When analysing the time up to 5 min after MAnE, 88% of the athletes were responders, while in the control group there were only 17% (overall: 56%). In the case of MAE, 64% of athletes and 56% of the control group were responders (overall: 62%) to the applied effort in terms of cfDNA increase. The two-way ANOVA revealed a significant effect of group and RM factors in MAnE on serum cfDNA concentrations (Table S4). For the main group, the factor showed a 32.15% higher concentration of cfDNA in the athlete group compared to the control group. In turn, a significant influence of the RM factor was shown by the greatest increase (38.19%) in cfDNA concentration up to 5 min after exercise compared to the baseline values and 60 min after collection (61.16%). The analysis of variance also showed a significant interaction of the group and RM factor in MAnE. The results of the post hoc test showed a 102.62% increase in the concentration of cfDNA up to 5 min after MAnE compared to the baseline values in the athlete group, while in the control group there was a gradual decrease, achieving about 49.79% lower concentrations of cfDNA 60 min after MAnE in compared to the baseline. As a result of the above changes, 68.47% higher cfDNA concentrations were also noticed up to 5 min after MAnE in the athlete group compared to the control group.
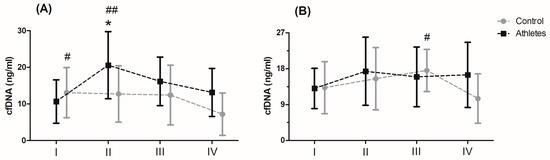
Figure 1. Concentration of cell-free DNA (cfDNA) after the maximal anaerobic (A) and aerobic effort (B) in training and control group (means and standard deviations). I, baseline; II, up to 5 min after the effort; III, 30 min after the effort; IV, 60 min after the effort. Significant difference at p < 0.05 vs: * II-Controls; # IV-Control; ## I, IV—Athletes.
Analysis of the variance of cfDNA concentrations before and after the MAE showed a significant influence of the RM factor and its interaction with the group factor (Table S4). The RM post hoc test results showed a significant increase in cfDNA concentration up to 5 min after (27.84%) and 30 min after exercise (27.77%) compared to baseline values. In turn the results of the post hoc test for the interaction of the RM factor and the group showed that while the concentration of cfDNA decreased by 40.10% in the period from 30 min to 60 min after exercise in the control group, the concentration of cfDNA in the group of athletes remained at a similar level (Figure 1).
The changes in cfDNA from the baseline visit to up to 5 min after and 30 min and 60 min after maximum exercise are shown in Figure 2. Analysis of variance showed a significant effect of the group factor on changes in cfDNA concentration up to 5 min after and 60 min after maximum exercise (Table S5). Regardless of the type of exercise, the difference between the groups was 49% for the change in cfDNA concentration up to 5 min after exercise. A two-fold difference between the groups was noted for the change in cfDNA concentration 60 min after exercise (104.34%). The significant interaction of the group factor and the type of effort was noted only for the change in cfDNA concentration up to 5 min after exercise (Figure 2A). The results of the post hoc test showed a 94.03% smaller increase in the control group in cfDNA concentration immediately after MAnE compared to the athlete group.
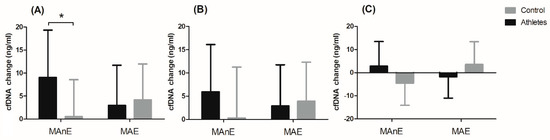
Figure 2. Changes in cell-free DNA (cfDNA) from baseline to u to 5 min after (A) and 30 min (B) and 60 min (C) after maximal anaerobic (MAnE) and aerobic effort (MAE) in the athletes and controls. * significant difference vs. control group at p < 0.01.
3. Discussion
The main goal of the current study was to define whether cfDNA changes were associated with maximal aerobic and anaerobic effort, and whether those changes indicate specific sport adaptation and/or physical activity preparation for different types of activity.
Both analysed groups successfully completed two different maximal physical tests—double repeated WanT and Bruce protocol. Test analysis showed that the athlete group had a 7.86% and 6.11% higher relative mean and peak power in comparison to control group during the first WAnT. Similar conclusions were observed in the work of researchers like Rotstein et al. [22], Reaburn et al. [23], and many others. In the second WAnT, after 30 s of rest, a similar decrease in the relative mean and maximum power was noted in both groups. This is probably due to the fact that the energy expenditure spent on the implementation of the first task in both populations was analogous. During MAE, the athlete group had a 15.24% higher maximum oxygen uptake and a 9.07% higher maximum ventilation compared to the control group. Such conclusions are summed up in the meta-analysis [24], where training experience is directly correlated with maximal oxygen intake and higher results, which are mostly observed in training individuals.
In conclusion, this is the first report demonstrating that maximal aerobic and anaerobic exercise significantly increases plasma levels of cfDNA, possibly resulting from early stages of tissue inflammation reaction and as a predictor of further activity processes and the stimulating effect of physical activity on body metabolism. These observations imply that formation of cfDNA is associated with occurrences of physical activity but only in maximal physical activity. Plasma cfDNA measurement similar to exerkine analyses is additionally non-invasive and requires limited time and personnel to receive specific data. Further investigation of the origin of plasma cfDNA in the blood stream associated with muscle work should be undertaken.
References
- Mieszkowski, J.; Borkowska, A.; Stankiewicz, B.; Kochanowicz, A.; Niespodziński, B.; Surmiak, M.; Waldziński, T.; Rola, R.; Petr, M.; Antosiewicz, J. Single High-Dose Vitamin D Supplementation as an Approach for Reducing Ultramarathon-Induced Inflammation: A Double-Blind Randomized Controlled Trial. Nutrients 2021, 13, 1280.
- De Salles, B.F.; Simão, R.; Fleck, S.J.; Dias, I.; Kraemer-Aguiar, L.G.; Bouskela, E. Effects of Resistance Training on Cytokines. Int. J. Sports Med. 2010, 31, 441–450.
- Mars, M.; Govender, S.; Weston, A.; Naicker, V.; Chuturgoon, A. High intensity exercise: A cause of lymphocyte apoptosis? Biochem. Biophys. Res. Commun. 1998, 249, 366–370.
- Kreider, R.B.; O’Toole, M.L.; Fry, A.C.; Kibler, W.B.; Nieman, D.C.; Whelan, J.P.; Lehmann, M. Overtraining in Sport; Human Kinetics: Champaign, IL, USA, 1998.
- Mieszkowski, J.; Kochanowicz, M.; Żychowska, M.; Kochanowicz, A.; Grzybkowska, A.; Anczykowska, K.; Sawicki, P.; Borkowska, A.; Niespodziński, B.; Antosiewicz, J. Ferritin Genes Overexpression in PBMC and a Rise in Exercise Performance as an Adaptive Response to Ischaemic Preconditioning in Young Men. BioMed Res. Int. 2019, 2019, 1–9.
- Maciejewska-Skrendo, A.; Mieszkowski, J.; Kochanowicz, A.; Stankiewicz, B.; Cieszczyk, P.; Switala, K.; de Assis, G.; Kecler, K.; Tarnowski, M.; Sawczuk, M. TNFA expression level changes observed in response to the Wingate Anaerobic Test in non-trained and trained individuals. Balt. J. Health Phys. Act. 2019, 11, 1–10.
- Mooren, F.C.; Lechtermann, A.; Völker, K. Exercise-Induced Apoptosis of Lymphocytes Depends on Training Status. Med. Sci. Sports Exerc. 2004, 36, 1476–1483.
- Margeli, A.; Skenderi, K.; Tsironi, M.; Hantzi, E.; Matalas, A.-L.; Vrettou, C.; Kanavakis, E.; Chrousos, G.; Papassotiriou, I. Dramatic Elevations of Interleukin-6 and Acute-Phase Reactants in Athletes Participating in the Ultradistance Foot Race Spartathlon: Severe Systemic Inflammation and Lipid and Lipoprotein Changes in Protracted Exercise. J. Clin. Endocrinol. Metab. 2005, 90, 3914–3918.
- Fatouros, I.G.; Destouni, A.; Margonis, K.; Jamurtas, A.Z.; Vrettou, C.; Kouretas, D.; Mastorakos, G.; Mitrakou, A.; Taxildaris, K.; Kanavakis, E.; et al. Cell-Free Plasma DNA as a Novel Marker of Aseptic Inflammation Severity Related to Exercise Overtraining. Clin. Chem. 2006, 52, 1820–1824.
- Breitbach, S.; Tug, S.; Simon, P. Circulating cell-free DNA: An up-coming molecular marker in exercise physiology. Sports Med. 2012, 42, 565–586.
- Ispirlidis, I.; Fatouros, I.G.; Jamurtas, T.; Nikolaidis, M.G.; Michailidis, Y.; Douroudos, I.; Margonis, K.; Chatzinikolaou, A.; Kalistratos, E.; Katrabasas, I.; et al. Time-course of Changes in Inflammatory and Performance Responses Following a Soccer Game. Clin. J. Sport Med. 2008, 18, 423–431.
- Fackler, M.J.; Bujanda, Z.L.; Umbricht, C.; Teo, W.W.; Cho, S.; Zhang, Z.; Visvanathan, K.; Jeter, S.; Argani, P.; Wang, C.; et al. Novel Methylated Biomarkers and a Robust Assay to Detect Circulating Tumor DNA in Metastatic Breast Cancer. Cancer Res. 2014, 74, 2160–2170.
- Hummel, E.M.; Hessas, E.; Müller, S.; Beiter, T.; Fisch, M.; Eibl, A.; Wolf, O.T.; Giebel, B.; Platen, P.; Kumsta, R.; et al. Cell-free DNA release under psychosocial and physical stress conditions. Transl. Psychiatry 2018, 8, 1–10.
- Zhivotosky, B.; Orrenius, S. Assessment of Apoptosis and Necrosis by DNA Fragmentation and Morphological Criteria. Curr. Protoc. Cell Biol. 2001, 12, 18.3.1–18.3.23.
- Beiter, T.; Fragasso, A.; Hudemann, J.; Schild, M.; Steinacker, J.M.; Mooren, F.C.; Nieß, A.M. Neutrophils release extracellular DNA traps in response to exercise. J. Appl. Physiol. 2014, 117, 325–333.
- Lou, X.; Hou, Y.; Liang, D.; Peng, L.; Chen, H.; Ma, S.; Zhang, L. A novel Alu-based real-time PCR method for the quantitative detection of plasma circulating cell-free DNA: Sensitivity and specificity for the diagnosis of myocardial infarction. Int. J. Mol. Med. 2015, 35, 72–80.
- Tug, S.; Mehdorn, M.; Helmig, S.; Breitbach, S.; Ehlert, T.; Simon, P. Exploring the Potential of Cell-Free-DNA Measurements After an Exhaustive Cycle-Ergometer Test as a Marker for Performance-Related Parameters. Int. J. Sports Physiol. Perform. 2017, 12, 597–604.
- Breitbach, S.; Sterzing, B.; Magallanes, C.; Tug, S.; Simon, P. Direct measurement of cell-free DNA from serially collected capillary plasma during incremental exercise. J. Appl. Physiol. 2014, 117, 119–130.
- Helmig, S.; Frühbeis, C.; Krämer-Albers, E.-M.; Simon, P.; Tug, S. Release of bulk cell free DNA during physical exercise occurs independent of extracellular vesicles. Eur. J. Appl. Physiol. 2015, 115, 2271–2280.
- Velders, M.; Treff, G.; Machus, K.; Bosnyák, E.; Steinacker, J.M.; Schumann, U. Exercise is a potent stimulus for enhancing circulating DNase activity. Clin. Biochem. 2014, 47, 471–474.
- Vittori, L.N.; Tarozzi, A.; Latessa, P.M. Circulating Cell-Free DNA in Physical Activities. In Cell-free DNA as Diagnostic Markers; Humana Press: New York, NY, USA, 2019; pp. 183–197.
- Rotstein, A.; Dotan, R.; Bar-Or, O.; Tenenbaum, G. Effect of Training on Anaerobic Threshold, Maximal Aerobic Power and Anaerobic Performance of Preadolescent Boys. Int. J. Sports Med. 1986, 7, 281–286.
- Reaburn, P.; Dascombe, B. Anaerobic performance in masters athletes. Eur. Rev. Aging Phys. Act. 2008, 6, 39–53.
- Wilson, T.M.; Tanaka, H. Meta-analysis of the age-associated decline in maximal aerobic capacity in men: Relation to training status. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H829–H834.
More
Information
Subjects:
Cell Biology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
805
Revisions:
2 times
(View History)
Update Date:
26 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




