| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Honorio Torres-Aguilar | + 4195 word(s) | 4195 | 2020-11-17 11:04:32 | | | |
| 2 | Rita Xu | -2114 word(s) | 2081 | 2020-11-18 07:01:05 | | |
Video Upload Options
This entry summarizes the current knowledge of T cells subsets contribution to the SS immunopathology, focusing on the cellular and biomolecular properties allowing them to infiltrate and to harm target tissues and, that simultaneously make them key therapeutic targets for SS treatment.
1. Introduction
Sjogren’s syndrome (SS) is a complex, inflammatory, autoimmune disorder characterized by damage to the salivary and lacrimal glands, which may lead to the loss of appropriate tear and saliva production, resulting in symptoms of severe dry eyes and mouth. The pathology in SS may additionally extend from sicca symptoms and complications of mucosal dryness, as a result of exocrine gland involvement, to a systemic disease or even to malignant B cell lymphoproliferation. SS is called “primary” (pSS) when it occurs alone, or “secondary” (sSS) if it is associated to the presence of another autoimmune disease [1]. Current evidence suggests that T cells form a large part of the lymphocytic infiltrated at earlier stages of the disease, involved in tolerance loss to self-antigens and in the secretion of many pro-inflammatory cytokines associated to local inflammation [2][3].
T cells comprise the helper T cell populations (CD4+), which differentiate in several subsets such as Th1, Th2, Th17, regulatory T cells (Treg), and T follicular helper cells (Tfh), as well as CD8+ T cells, also called Cytotoxic T Lymphocytes (CTL) [4]. Increased T cells infiltration into salivary glands (SG) from pSS patients has been evidenced accomplished by decreased levels in periphery blood, supporting the hypothesis that lymphopenia, a frequent finding in pSS patients associated with higher disease activity and increased mortality, might be owed to T cells migration [5].
Both Th1 and Th17 cells subsets infiltrating the SG at an early disease stage have been evidenced by detection of interferon (IFN)-γ and interleukin (IL)-17 respectively, being highly associated with the inflammatory damage [6]. In regard to regulatory T cells (Treg), some studies show conflicting data about their frequency in blood and target organs, displaying uncertain effects. Although circulating Treg cells have not been shown to be significantly decreased with impaired clonal expansion and functionality [7], their physiological and pathological role in SS is unclear yet. Tfh cells are taking special attention for their essential roles in ectopic lymphoid structures (ELS) development in pSS patients, due to their germinal center (GC)-like organization that allows a potent B cell response [8]. On the other hand, CTL have also been implicated into SS pathology, since data from a murine SS model and human biopsies reveal the pathogenic significance of CD8+ T cells in the development and progression of SS in the SG [9]. Strikingly, a novel T cells subset is emerging, namely a kind of regulatory T cell localized in the GC to limit the humoral response, called follicular regulatory T cells (Tfr), which might play a critical protective role, since their deficiency affects the salivary glands with lymphocyte infiltration and antibody deposition in a mouse experimental model of SS [10].
2. T Cell: Targeted Shots in Sjogren´s Syndrome
Mechanistically, many factors are largely and recently associated with the involvement of T cells and the detrimental conditions developed in SS patients. Genetic associations have pointed to the aberrant biology of T cells that can carry weight in SS development [11][12]. In pSS, the lymphocytic infiltration of the salivary and lacrimal glands may organize into B and T cell areas, where different T cell subsets may be involved in the pathology by infiltration, secretion of pro-inflammatory cytokine, damage to epithelium, and also by regulating the function of other immunological cells. On the other hand, some biological processes such as autophagy are acquiring a novel interest in the intracellular events that regulate the immunobiology of T cells since it is crucial for their development, proliferation, survival and functions [13]. Autophagy is a mechanism that has been recently involved in the etiology and development of autoimmune/anti-inflammatory disease as systemic lupus erythematosus, inflammatory bowel disease, rheumatoid arthritis, psoriasis and multiple sclerosis [14]. Although there are few evidences depicting the association of autophagy and SS, some studies suggest that dysregulated autophagy could be involved in the aberrant activation of T cells [15][16]. On the other hand, regarding the break of self-tolerance at the T cell level, the activation of autoreactive T cells by commensal microbiota-derived antigens has been explored as molecular mimicry, prompting SS onset [17]. Additionally, a persistent viral infection has been related to the high frequency of CTL in pSS patients [18]. Likewise, T cells hyperactivity related to dysregulated immune checkpoint signaling pathways could play an important role in pSS pathogenesis. The above is owing to a regulatory pathway for T cell activation associated to autoimmune diseases: the axis T cell Ig and ITIM domain (TIGIT) and CD226 (TIGIT/CD226) [19], was overexpressed in T cells from pSS patients. This suggests that this axis might be involved as an unsuccessful negative immune regulation, making it a potential therapeutic target for this disease [20].
CTLA-4/CD28 axis remains the most studied pathway working as a major immune checkpoint regulating T cells activation in SS and other autoimmune diseases [21]. For this reason, abatacept (a humanized cytotoxic T-lymphocyte–associated antigen 4 (CTLA4)–IgG1 fusion protein binding CD80 or CD86 and inhibiting the CD28 co-stimulatory pathway on T cell therapy) has been evaluated for SS treatment [22][23][24]. The focus towards TIGIT/CD226 pathway similarly relies on the CTLA-4/CD28 pathway owing to the TIGIT/CD226 pathway that exerts its immunomodulatory effects by competing for the same ligand. Moreover, additional pathways like PD-1/PD-L and ICOS/ICOSL have also been considered as defective immune checkpoints associated with T cells hyperactivity in SS [21].
Thus, regarding all those aforementioned aspects, here, we discuss the current knowledge of T cells subset involvement in SS, focusing on their properties for the infiltration of exocrine glands and their pathologic mechanisms, as well as the novel emerging factors making them potential therapeutic targets for the treatment of SS.
3. Th1/Th17 Cells: Primordial Effectors Coordinating the Inflammatory and Detrimental Environment
Th1 and Th17 cells have long been implicated as crucial mediators at early stages of SS pathology, contributing in shape up the inflammatory microenvironment (Figure 1). Such notion was emphasized by the fact that both pSS-infiltrating activated Th1 and Th17 subsets presented restricted clonal diversities with TCR recognizing common autoantigen unique to pSS [25]. Th1 cells have also been narrowly associated to development and disease severity, owing to their selective infiltration into target tissues, analyzed by the presence of the transcription factor T-bet and IFN-γ [26]. Alternatively, some studies have found an increased expression of predominantly Th1-cytokines, such as IL-2, IL-12, IFN- γ, and IP-10, in liquids like tears and saliva from pSS patients correlating with clinical manifestations [27][28]. Consistent with the underlying mechanism to shape the setting of the inflammatory and detrimental environment, IFN-γ might disrupt tight junction structure in glandular tissue from SS patients [29], indicating that the local cytokine production may contribute to the observed glandular dysfunction. In addition, it is very well known that IFN-γ regulates the activity of certain innate immune cells, whose overactivation may further contribute to SS pathology [30][31][32][33]. On the other hand, TNF-α is another Th1 cytokine associated to SS development in murine model, detected at high levels in serum [34] and exhibiting enhanced expression in saliva fluid and salivary glands from SS patients [28][35]. Although TNF-α can be produced not only by T cells, this cytokine effectively acts on both naïve and effector T cells by regulating their proliferation and survival [36]. Further, TNF-α can mediate inflammatory activity via special effects on structural cells through regulating the reorganization of epithelial junctions and inducing the expression of adhesion molecules, such as E-selectin, facilitating leukocytes adhesion [37]. Alike IFN-γ, the detrimental roles of TNF-α on glandular tissues from SS patients are due to its tight junctions-disrupting effect [29]. Furthermore, TNF-α can impact at the inflammatory microenvironment by stimulating secretion of pro-inflammatory cytokines like IL-8, which in turn increases leukocyte infiltration, particularly neutrophils, whose extracellular traps have been related to SS pathogenesis [38][39]. Additionally, TNF-α induce amphiregulin (AREG) secretion by epidermal cells, a growth factor whose presence has been demonstrated to play critical roles in pro-inflammatory cytokine secretion in SG from SS patients [40][41].
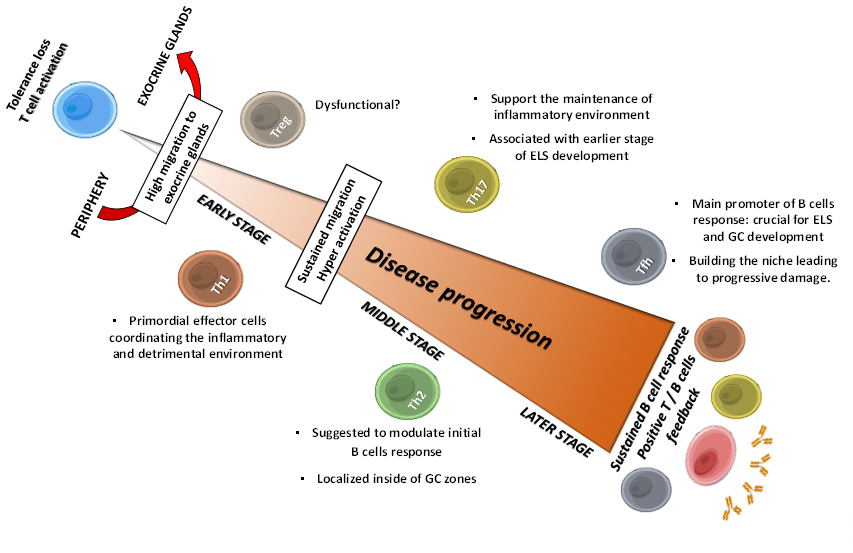
Figure 1. Sequential roles of T cells in SS progression: flares of main implications. Tolerance loss drives T cell activation and migration to exocrine glands, where they provide several factor that make the auspicious environment for the pathology establishment: Th1 and Th17 cells contribute for inflammatory and detrimental environment by secreting cytokines like IFN-γ, TNF-α and IL-17, triggering sustained T cells migration and hyperactivation. Th2 cells are suggested to modulate early B cells response, and to contribute with Th17 cells in initial ESL development, allowing Tfh cells to establish and to provide the necessary factors to trigger B cells response; thus, leading to progressive tissue damage. Treg cells dysfunctionality and low frequency are suggested to contribute to periphery loss tolerance. Positive T and B cells feedback trough intercellular contact and soluble factor allow a sustained B cells response and the onset of clinical features. GC, Germinal Center; ELS, Ectopic Lymphoid Structures.
Th1 cells can be attracted at SG by exacerbated secretion of specific chemokines; therefore, knowledge of those mechanisms allowing such migration is essential to elucidate and to treat SS. IL-7 is a cytokine promoting T cells development and survival. Additionally, it may induce expression of several chemokine favoring massive T cells homing towards many tissues [42]. Interesting data shows that Th1 cells may promote development of SS-like autoimmune exocrinopathy in murine model, supported by enhanced expression of CXCR3 ligands in a IL-7-dependent manner [43]. This notion is relevant owing to an increased expression of IL-7 correlates with increased inflammation in SS [44] and the expression of CXCR3 ligands (including CXCL9, CXCL10, and CXCL11) are elevated in SG from SS patients [45].
Regarding TH17 cells involvement, both experimental and clinical data have pointed their crucial role in development and progression of SS by supporting autoreactive B cells responses. pSS patients show increased levels of circulating Th17 cells, as well as in salivary glands correlating with clinical parameter [46][47]. Th17 cells involvement in SS can be also analyzed by measuring their associated cytokines, particularly IL-17. This cytokine has been found significantly augmented in several exocrine glands from pSS patients, correlating with the severity of the pathology [48][49]. Moreover, the Th17/Treg cell imbalance ratio in peripheral blood from SS patients with extra glandular complication, relates the possible role of Th17 cells in the development of systemic manifestations [50]. The ontogenesis of Th17/Treg cells imbalance has recently been implicated to transcriptional regulators. The dysregulated expression of the transcriptional co-activator TAZ (transcriptional coactivator with PDZ (postsynaptic density 65-discs large-zonula occludens 1-binding)) leads to autoimmune diseases such as SS by promoting Th17 differentiation and attenuating Treg development in a mouse model. Further, a higher expression of TAZ has been demonstrated in circulating CD4+ memory T cells from pSS patients [51]. Thereby, the aforementioned statements highlight the interest for depicting underlying intracellular events concerning to Th17/Treg imbalanced driving to development of SS.
Even though low evidence supporting this role until now, IL-17 has been described as a novel Th17 mechanism associated with the development of ELS in SS. [52]. Th17 cells are more effective than Th1 cells in supporting B cells responses under autoimmunity conditions like SS, and an increased numbers of circulating RORγ+CD161+CD4+Th17 subset positively correlated with humoral manifestations [46][53]. IL-17 might contribute by modulating the immune response, since this cytokine induces secretion of inflammatory factors such as TNF, IL-6 and IL-1β and promotes immune cells recruitment by inducing secretion of chemokine like IL-8, CXCL9 and ligand for CCR3 receptors, as well as by modulating the integrity of epithelial barrier trough secretion of proteins like metalloproteinase [54][55]. Additionally, there are six IL-17 isotypes, (IL-17A–IL-17F), with IL-17A and IL-17F being the more prevalent isotypes. Available data display that both IL-17A and IL-17F are more associated to SS, since high levels of IL-17A are found in salivary glands from pSS patients [56], and IL-17F was correlated with the humoral and disease activity [57]. On the other hand, IL-22 (another TH17 cytokine) favors B cells recruitment and lymphoid aggregation to form ELS, as well as the production of autoantibodies by inducing the expression of several cytokines such as CXCL12 and CXCL13 [58]. This aspect is relevant owing to higher IL-22 levels correlate with SS parameters such as lower saliva flow, autoantibodies profile of anti-SSB, anti SSA/SSB combined, rheumatoid factor and hypergammaglobulinemia [59]. Thus, current evidence shows how Th17 cytokines have become crucial orchestrators of the aberrant immunity response in SS.
References
- Fox, R.I. Sjögren’s syndrome. Lancet 2005, 366, 321–331, doi:10.1016/S0140-6736(05)66990-5.
- Aqrawi, L.A.; Ivanchenko, M.; Bjork, A.; Ramírez Sepúlveda, J.I.; Imgenberg-Kreuz, J.; Kvarntröm,M.; Haselmayer, P.; Jensem, J.L.; Nordmark, G.; Chemin, K.; Skarstein, K.; Wahren-Herlenius, M. Diminished CXCR5 expression in peripheral blood of patients with Sjögren’s Syndrome may relate to both genotype and salivary gland homing. Exp. Immunol. 2018, 192, 259–270, doi:10.1111/cei.13118.
- Mingueneau, M.; Boudaoud, S.; Haskett, S.; Reynolds, T.L.; Norton, E.; Zhang, X.; Constanty, M.; Park, D.; Wang, W.; Lazure, T.; et al. Cytometry by time-of-flight immunophenotyping identifies a blood Sjögren’s signature correlating with disease activity and glandular inflammation. J. Allergy Clin. Immunol. 2016, 137, 1809–1821, doi:10.1016/j.jaci.2016.01.024.
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213, doi:10.1016/j.immuni.2018.01.007.
- Fessler, J.; Fasching, P.; Raicht, A.; Hammerl, S.; Weber, J.; Lackner, A.; Hermann, J.; Dejaco, C.; Graninger, W.B.; Schwinger, W.; et al. Lymphopenia in primary Sjögren’s syndrome is associated with premature aging of naïve CD4+ T cells. Rheumaology 2020, doi:10.1093/rheumatology/keaa105.
- Verstappen, G.M.; Kroese, F.G.M.; Bootsma, H. T cells in primary Sjogren´s syndrome: Targets for early intervention. Rheumatolology 2019, 15, kez004, doi:10.1093/rheumatology/kez004.
- Luo, J.; Ming, B.; Zhang, C.; Deng, X.; Li, P.; Wei, Z.; Xia, Y.; Jiang, K.; Ye, H.; Ma, W. IL-2 Inhibition of Th17 Generation Rather Than Induction of Treg Cells Is Impared Induction of Primary Sjögren’s syndrome Patients. Immunol. 2018, 9, 1755, doi:10.3389/fimmu.2018.01755.
- Saito, M.; Otsuka, K.; Ushio, A.; Yamada, A.; Arakaki, R.; Kudo, Y.; Ishimaru, N. Unique Phenotypes and Functions of Follicular Helper T Cells and Regulatory T Cells in Sjögren’s syndrome. Rheumatol. Rev. 2018, 12, 239–245, doi:10.2174/1573397113666170125122858.
- Gao, C.Y.; Yao, Y.; Li, L.; Yang, S.H.; Chu, H.; Tsuneyama, K.; Li, X.M.; Gershwin, M.E.; Lian, Z.X. Tissue-Resident Memory CD8+ T Cells Acting as Mediators of Salivary Gland Damage in a Murine Model of Sjögren’s Syndrome. Arthritis Rheumatol. 2019, 7, 121–132, doi:10.1002/art.40676.
- Fu, W.; Liu, X.; Lin, X.; Feng, H.; Sun, L.; Li, S.; Chen, H.; Tang, H.; Lu, L.; Jin, W.; et al. Deficiency in T follicular regulatory cells promotes autoimmunity. Exp. Med. 2018, 215, 815–825, doi:10.1084/jem.20170901.
- Harris, V.M.; Scofield, R.H.; Sivils, K.L. Genetics in Sjögren’s syndrome: Where we are and where we go. Exp. Rheumatol. 2019, 37 (Suppl. 118), 234–239.
- Imgenberg-Kreuz, J.; Rasmussen, A.; Sivils, K.; Nordmark, G. Genetics and epigenetics in primary Sjögren’s syndrome. Rheumatology 2019, doi:10.1093/rheumatology/key330.
- Macian, F. Autophagy in T Cell Function and Aging. Cell Dev. Biol. 2019, 7, 213, doi:10.3389/fcell.2019.00213.
- Gianchecchi, E.; Delfino, D.V.; Fierabracci, A. Recent insights on the putative role of autophagy in autoimmune diseases. Rev. 2014, 13, 231–241, doi:10.1016/j.autrev.2013.10.007.
- Alessandri, C.; Ciccia, F.; Priori, R.; Astorri, E.; Guggino, G.; Alessandro, R.; Rizzo, A.; Conti, F.; Minniti, A., Barbati, C. CD4 T lymphocyte autophagy is upregulated in the salivary glands of primary Sjögren’s syndrome patients and correlates with focus score and disease activity. Res. Ther. 2017, 19, 178, doi:10.1186/s13075-017-1385-y.
- Voynova, E.; Lefebvre, F.; Qadri, A.; Muller, S. Correction of autophagy impairment inhibits pathology in the NOD.H-2h4 mouse model of primary Sjögren’s syndrome. Autoimmun. 2020, 108, 102418, doi:10.1016/j.jaut.2020.102418.
- Szymula, A.; Rosenthal, J.; Szczerba, B.M.; Bagavant, H.; Fu, S.M.; Deshmukh, U.S. T cell epitope mimicry between Sjögren’s syndrome Antigen A (SSA)/Ro60 and oral, gut, skin and vaginal bacteria. Immunol. 2014, 152, 1–9, doi:10.1016/j.clim.2014.02.004.
- Narkeviciute, I.; Sudzius, G.; Mieliauskaite, D.; Mackiewicz, Z.; Butrimiene, I.; Viliene, R.; Dumalakiene, I. Are cytotoxic effector cells changes in peripheral blood of patients with Sjögren’s syndrome related to persistent virus infection: Suggestions and conundrums. Immunol. 2016, 310, 123–130, doi:10.1016/j.cellimm.2016.08.013.
- Lozano, E.; Dominguez-Villar, M.; Kuchroo, V.; Hafler, D.A. The TIGIT/CD226 axis regulates human T cell function. Immunol. 2012, 188, 3869–3875, doi:10.4049/jimmunol.1103627.
- Deng, C.; Chen, Y.; Li, W.; Peng, L.; Luo, X.; Peng, Y.; Zhao, L.; Wu, Q.; Zhang, W.; Zhang, X.; et al. Alteration of CD226/TIGIT immune checkpoint on T cells in the pathogenesis of primary Sjögren’s syndrome. Autoimmun. 2020, 113, 102485, doi:10.1016/j.jaut.2020.102485.
- Ceeraz, S.; Nowak, E.C.; Burns, C.M.; Noelle, R.J. Immune checkpoint receptors in regulating immune reactivity in rheumatic disease. Arthitis Res. Ther. 2014, 16, 469, doi:10.1186/s13075-014-0469-1.
- Van Nimwegen, J.F.; Mossel, E.; van Zuiden, G.S.; Wijnsma, R.F., Delli, K.; Stel, A.J.; van der Vegt, B.; Haacke, E.A.; Olie, S.; Los, L.I.; et al. Abatacept treatment for patients with early active primary Sjögren’s syndrome: A single-centre, randomised, double-blind, placebo-controlled, phase 3 trial (ASAP-III study). Rheumatol. 2020, doi:10.1016/S2665-9913(19)30160-2.
- Chu, L.L.; Cui, K.; Pope, J.E. Meta-Analysis of Treatment for Primary Sjögren’s Syndrome. Arthritis Care Res. (Hoboken) 2020, 72, 1011–1021, doi:10.1002/acr.23917.
- Verstappen, G.M.; Meiners, P.M.; Corneth, O.B.J.; Visser, A.; Arends, S.; Abdulahad, W.H.; Hendriks, R.W.; Vissink, A.; Kroese, F.G.M.; Bootsma, H. Attenuation of Follicular Helper T Cell-Dependent B Cell Hyperactivity by Abatacept Treatment in Primary Sjögren’s Syndrome. Arthritis Rheumatol. 2017, 69, 1850–1861, doi:10.1002/art.40165.
- Voigt, A.; Bohn, K.; Sukumaran, S.; Stewart, C.M.; Bhattacharya, I.; Nguyen, C.Q. Unique glandular ex-vivo Th1 and Th17 receptor motifs in Sjögren’s syndrome patients using single-cell analysis. Immunol. 2018, 192, 58–67, doi:10.1016/j.clim.2018.04.009.
- Maehara, T.; Moriyama, M.; Hayashida, J.N.; Tanaka, A.; Shinozaki, S.; Kubo, Y.; Matsumura, K.; Nakamura, S. Selective localization of T helper subsets in labial salivary glands from primary Sjögren’s syndrome patients. Exp. Immunol. 2012, 169, 89–99, doi:10.1111/j.1365-2249.2012.04606.x.
- Zhao, H.; Li, Q.; Ye, M.; Yu, J. Tear Luminex Analysis in Dry Eye Patients. Sci. Monit. 2018, 24, 7595–7602, doi:10.12659/MSM.912010.
- Chen, X.; Aqrawi, L.A.; Utheim, T.P.;Tashbayev, B.; Utheim, Ø.A.; Reppe, S.; Hove, L.H.; Herlofson, B.B.; Sing, P.B.; Palm, Ø; et al. Elevated cytokine levels in tears and saliva of patients with primary Sjögren’s syndrome correlate with clinical ocular and oral manifestations. Rep. 2019, 9, 7319, doi:10.1038/s41598-019-43714-5.
- Ewert, P.; Aguilera, S.; Alliende, C.; Kwon, Y.J.; Albornoz, A.; Molina, C.; Urzúa, U.; Quest, A.F.; Olea, N.; Pérez, P.; et al. Disruption of tight junction structure in salivary glands from Sjögren’s syndrome patients is linked to proinflammatory cytokine exposure. Arthritis Rheum. 2010, 62, 1280–1289, doi:10.1002/art.27362.
- Lees, J.R. Interferon gamma in autoimmunity: A complicated player on a complex stage. Cytokine 2015, 74, 18–26, doi:10.1016/j.cyto.2014.10.014.
- Ushio, A.; Arakaki, R.; Otsuka, K.; Yamada, A.; Tsunematu, T.; Kudo, Y.; Azuma, M.; Ishimaru, N. CCL22-Producing Resident Macrophages Enhance T Cell Response in Sjögren’s Syndrome. Immunol. 2018, 9, 2594, doi:10.3389/fimmu.2018.02594.
- Ciccia, F.; Guggino, G.; Giardina, A.; Ferrante, A.; Carrubbi, F.; Giacomelli, R.; Triolo, G. The role of innate and lymphoid IL-22-producing cells in the immunopathology of primary Sjögren’s syndrome. Rev. Clin. Immunol. 2014, 10, 533–541, doi:10.1586/1744666X.2014.884461.
- Torres-Aguilar, H.; Sosa-Luis, S.A.; Aguilar-Ruiz, S.R. Infections as triggers of flares in systemic autoimmune diseases: Novel innate immunity mechanisms. Opin. Rheumatol. 2019, 31, 525–531, doi:10.1097/BOR.0000000000000630.
- Zhou, J.; Kawai, T.; Yu, Q. Pathogenic role of endogenous TNF-α in the development of Sjögren’s-like sialadenitis and secretory dysfunction in non-obese diabetic mice. Investig. 2017, 97, 458–467, doi:10.1038/labinvest.2016.141.
- Yoshimura, S.; Nakamura, H.; Horai, Y.; Nakajima, H.; Shiraishi, H.; Hayashi, T.; Takahashi, T.; Kawakami, A. Abnormal distribution of AQP5 in labial salivary glands is associated with poor saliva secretion in patients with Sjögren’s syndrome including neuromyelitis optica complicated patients. Rheumatol. 2016, 26, 384–390, doi:10.3109/14397595.2015.1083146.
- Mehta, A.K.; Gracias, D.T.; Croft, M. TNF activity and T cells. Cytokine 2018, 101, 14–18, doi:10.1016/j.cyto.2016.08.003.
- Zelová, H.; Hošek, J. TNF-α signalling and inflammation: Interactions between old acquaintances. Res. 2013, 62, 641–651, doi:10.1007/s00011-013-0633-0.
- Gonzalez-Aparicio, M.; Alfaro, C. Influence of Interleukin-8 and Neutrophil Extracellular Trap (NET) Formation in the Tumor Microenvironment: Is There a Pathogenic Role? Immunol. Res. 2019, 2019, 6252138, doi:10.1155/2019/6252138.
- de Bont, C.M.; Stokman, M.E.M.; Faas, P.; Thurlings, R.M.; Boelens, W.C.; Wright, H.L.; Prugin, G.J.M. Autoantibodies to neutrophil extracellular traps represent a potential serological biomarker in rheumatoid arthritis. Autoimmun. 2020, 113, 102484, doi:10.1016/j.jaut.2020.102484.
- Sisto, M.; Lisi, S.; Lofrumento, D.D.; Ingravallo, G.; Mitolo, V.; D’Amore, M. Expression of pro-inflammatory TACE-TNF-α-amphiregulin axis in Sjögren’s syndrome salivary glands. Cell. Biol. 2010, 134, 345–353, doi:10.1007/s00418-010-0735-5.
- Sisto, M.; Lisi, S.; D’Amore, M.; Lofrumento, D.D. The metalloproteinase ADAM17 and the epidermal growth factor receptor (EGFR) signaling drive the inflammatory epithelial response in Sjögren’s syndrome. Exp. Med. 2015, 15, 215–225, doi:10.1007/s10238-014-0279-4.
- Ponte, R.; Rancez, M.; Figueiredo-Morgado, S.; Dutrieux, J.; Fabre-Mersseman, V.; Charmeteaude-Muylder, B.; Guilbert, T.; Routy, J.P.; Cherynier, R.; Couëdel-Courteille, A. Acute Simian Immunodeficiency Virus Infection Triggers Early and Transient Interleukin-7 Production in the Gut, Leading to Enhanced Local Chemokine Expression and Intestinal Immune Cell Homing. Immunol. 2017, 8, 588, doi:10.3389/fimmu.2017.00588.
- Jin, J.O.; Kawai, T.; Cha, S.; Yu, Q. Interleukin-7 enhances the Th1 response to promote the development of Sjögren’s syndrome-like autoimmune exocrinopathy in mice. Rheum. 2013, 65, 2132–2142, doi:10.1002/art.38007.
- Bikker, A.; van Woerkom, J.M.; Kruize, A.A.; Wenting-van Wijk, M.; de Jager, W.; Bijlsma, J.W.; Lafeber, F.P.; van Roon, J.A. Increased expression of interleukin-7 in labial salivary glands of patients with primary Sjögren’s syndrome correlates with increased inflammation. Rheum. 2010, 62, 969–977, doi:10.1002/art.27318.
- Zhou, J.; Yu, Q. Disruption of CXCR3 function impedes the development of Sjögren’s syndrome-like xerostomia in non-obese diabetic mice. Investig. 2018, 98, 620–628, doi:10.1038/s41374-017-0013-4.
- Zhao, L.; Nocturne, G.; Haskett, S.; Boudaoud, S.; Lazure, T.; Le Pajolec, C.; Mariette, X.; Mingueneau, M.; Banerjee, D. Clinical relevance of RORγ positive and negative subsets of CD161+CD4+ T cells in primary Sjögren’s syndrome. Rheumatology 2017, 56, 303–312, doi:10.1093/rheumatology/kew360.
- Verstappen, G.M.; Corneth, O.B.J.; Bootsma, H.; Kroese, F.G.M. Th17 cells in primary Sjögren’s syndrome: Pathogenicity and plasticity. Autoimmun. 2018, 87, 16–25, doi:10.1016/j.jaut.2017.11.003.
- Liu, R.; Gao, C.; Chen, H.; Li, Y.; Jin, Y.; Qi, H. Analysis of Th17-associated cytokines and clinical correlations in patients with dry eye disease. PLoS ONE 2017, 12, e0173301, doi:10.1371/journal.pone.0173301.
- Zhang, L.W.; Zhou, P.R.; Wei, P.; Cong, X.; Wu, L.L.; Hua, H. Expression of interleukin-17 in primary Sjögren’s syndrome and the correlation with disease severity: A systematic review and meta-analysis. Scand. Immunol. 2018, 87, e12649, doi:10.1111/sji.12649.
- Hao, L.R.; Li, X.F.; Gao, C.; Cao, L.; Han, Z.Y.; Gao, H. Th17/Treg cell level and clinical characteristics of peripheral blood of patients with Sjogren’s syndrome complicated with primary biliary cirrhosis. Medicine 2019, 98, e15952, doi:10.1097/MD.0000000000015952.
- Geng, J.; Yu, S.; Zhao, H.; Sun, X.; Li, X.; Wang, P.; Xiong, X.; Hong, L.; Xie, C.; Gao, J.; et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of TH17 cells and Treg cells. Immunol. 2017, 18, 800–812, doi:10.1038/ni.3748.
- Pontarini, E.; Lucchesi, D.; Bombardieri, M. Current views on the pathogenesis of Sjögren’s syndrome. Opin. Rheumatol. 2018, 30, 215–221, doi:10.1097/BOR.0000000000000473.
- Subbarayal, B.; Chauhan, S.K.; Di Zazzo, A.; Dana, R. IL-17 Augments B Cell Activation in Ocular Surface Autoimmunity. Immunol. 2016, 197, 3464–3470, doi:10.4049/jimmunol.1502641.
- Veldhoen, M. Interleukin 17 is a chief orchestrator of immunity. Immunol. 2017, 18, 612–621, doi:10.1038/ni.3742.
- Ruiz de Morales, J.M.G.; Puig, L.; Daudén, E.; Cañete, J.D.; Pablos, J.L.; Martín, A.O.; Juanatey, C.G.; Adán, A.; Montalbán, X.; Borruel, N. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Rev. 2020, 19, 102429, doi:10.1016/j.autrev.2019.102429.
- Fei, Y.; Zhang, W.; Lin, D.; Wu, C.; Li, M.; Zhao, Y.; Zeng, X.; Zhang, F. Clinical parameter and Th17 related to lymphocytes infiltrating degree of labial salivary gland in primary Sjögren’s syndrome. Rheumatol. 2014, 33, 523–529, doi:10.1007/s10067-013-2476-z.
- Gan, Y.; Zhao, X.; He, J.; Liu, X.; Li, Y.; Sun, X.; Li, Z. Increased Interleukin-17F is Associated with Elevated Autoantibody Levels and More Clinically Relevant than Interleukin-17A in Primary Sjögren’s Syndrome. Immunol. Res. 2017, 2017, 4768408, doi:10.1155/2017/4768408.
- Barone, F.; Nayar, S.; Campos, J.B.; Cloake, T.; Withers, D.R.; Toellner, K.M.; Zhang, Y.; Fouser, L.; Fisher, B.; Bowman, S. IL-22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Natl. Acad. Sci. USA 2015, 112, 11024–11029, doi:10.1073/pnas.1503315112.
- Lavoie, T.N.; Stewart, C.M.; Berg, K.M.; Li, Y.; Nguyen, C.Q. Expression of interleukin-22 in Sjögren’s syndrome: Significant correlation with disease parameters. J. Immunol. 2011, 74, 377–382, doi:10.1111/j.1365-3083.2011.02583.x.