
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Martin Raspor | + 1854 word(s) | 1854 | 2021-08-17 06:19:52 | | | |
| 2 | Martin Raspor | + 3 word(s) | 1857 | 2021-08-17 17:36:47 | | | | |
| 3 | Martin Raspor | -20 word(s) | 1837 | 2021-08-17 17:44:16 | | | | |
| 4 | Nora Tang | + 484 word(s) | 2321 | 2021-08-18 07:34:09 | | |
Video Upload Options
The role of CK and auxin signalization is central to the entire process of DNSO, whereby the early stages of DNSO are dominated by auxin and the later stages by CK signaling. Taking into account the early observation by Skoog and Miller that a high auxin/CK ratio stimulates the development of roots, while a high CK/auxin ratio is favorable to the development of shoot tissue, the general need for a sequence of two regeneration media can be explained by the differences in morphogenic requirements between early and late stages of DNSO. In the early stages, a high auxin/CK ratio is required not only for the development of calli but also of lateral root-like primordia within the calli; later, a high CK/auxin ratio will be required to convert the developmental fate of these primordia into taking on a shoot identity.
1. Introduction
Thanks to their totipotency, plant cells and tissues cultured in vitro are capable of regenerating into complete, fertile plants under appropriate cultivation conditions. Thus, in vitro cultures represent one of the most important tools in plant biotechnology. Plant in vitro culture techniques are rapidly evolving to optimize the efficiency of procedures in order to take full advantage of plant phenotype plasticity for agricultural, industrial or conservation purposes, as well as for applied and fundamental research. The regeneration of morphologically and physiologically true-to-type shoots from explants derived from a variety of tissues—referred to as caulogenesis or de novo shoot organogenesis (DNSO)—is being widely employed, relying on the addition of appropriate plant hormones, notably cytokinin (CK) and auxin, into the regeneration media. The amenability of plant species to shoot regeneration varies greatly, with recalcitrance to shoot regeneration in certain species presenting a major obstacle to genetic modifications for the improvement of yield, nutritional value or resistance to stress [1]. It has been reported that the targeted manipulation of CK [2] or auxin [3] signaling pathways can considerably enhance shoot regeneration, even from recalcitrant tissues.
Extensive knowledge is already available about DNSO and its hormonal regulation, but some of its aspects are constantly left out of the picture. While, for instance, the complicated signaling events related to the differentiation and spatial organization of the shoot apical meristem (SAM) have been thoroughly characterized, simple questions, such as the relationship between exogenous application of growth regulators, their uptake and effect on hormone levels in plant tissues, have not been adequately addressed. Here we focus on the molecular aspects of the involvement of CK and auxin in DNSO, with special emphasis on the questions on hormone uptake from the regeneration media and their crosstalk with sucrose present in the media—questions that have so far remained unanswered or poorly answered.
2. The Course of DNSO: From Pluripotent Primordia to Developing Shoots
Two-step shoot regeneration is subdivided into several stages, depending on the authors’ perspective, but is best described by a sequence of five main morphogenic processes: (1) founder cell specification (requires CIM); (2) formation of pluripotent primordia (requires CIM); (3) acquisition of organogenic competence (requires both CIM and SIM); (4) acquisition of shoot identity (requires SIM); (5) shoot outgrowth (occurs spontaneously after shoot identity is acquired) [4][5][6] (Figure 1). The formation of pluripotent primordia may or may not involve the formation of a mass of apparently disorganized plant tissue—the callus. There are various types of callus tissues in plants, but in general, calli are formed when intense cell proliferation is not followed by proper tissue patterning associated with normal morphogenic processes in plants [7][8]. Depending on the presence or absence of callus tissue, shoot organogenesis can be classified as either indirect (proceeding through the stage of callus formation) or direct (callus-independent).
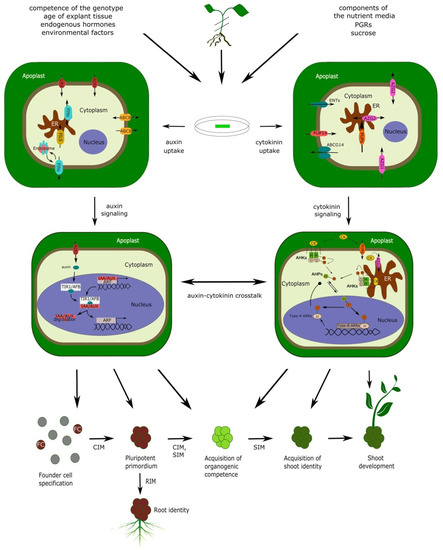
Both direct and indirect shoot organogenesis proceed through a similar series of cellular and genetic events associated with the corresponding morphogenic stages, regardless of the presence or absence of visible callus tissue [9][10][11]. In either case, shoot organogenesis is dependent on the formation of primordia, containing a pre-existing population of stem cells that never cease to exist even in differentiated tissues [12]. Indeed, the long-standing assumption that DNSO requires tissue dedifferentiation in order to subsequently acquire organogenic competence has been recently disproved—DNSO has been shown to occur from xylem pole pericycle cells of Arabidopsis root and hypocotyl explants, involving the stage in which shoot primordia develop from structures present in calli that are similar to lateral root primordia (LRP) [13]. Calli derived from explants from above-ground organs like cotyledons and petals also regenerate shoots through primordia resembling LRP; moreover, mutants incapable of developing lateral roots are also unable to form calli on CIM, confirming that the organogenic primordia present within the calli share both structural and functional homology with LRP [14]. The same LR-like primordia are formed during direct shoot organogenesis without the proliferation of callus tissue [9]. These primordia, which, in the case of ontogenic root development grow to become normal lateral roots [15], can be induced within a certain developmental window to trans-differentiate into shoot primordia [16] (Figure 1). A population of stem cells exists within these primordia, ensuring their pluripotency and capability of developing a meristem [12] able to switch identity through a timely induction of changes in gene expression [16]. Such degree of developmental plasticity suggests that the cellular, genetic and developmental organization of LRP still shares considerable commonality with the most primitive traits of shoot development, which likely predate the modern organ identities of higher plants. Thus, although the primordia which appear in the early stages of DNSO share all the histological and genetic features of LRP and readily differentiate into root tissue, referring to them as “pluripotent primordia” or even simply “primordia” instead of “LRP” is more appropriate in the context of DNSO.
The role of CK and auxin signalization is central to the entire process of DNSO, whereby the early stages of DNSO are dominated by auxin and the later stages by CK signaling. Taking into account the early observation by Skoog and Miller [17] that a high auxin/CK ratio stimulates the development of roots, while a high CK/auxin ratio is favorable to the development of shoot tissue, the general need for a sequence of two regeneration media can be explained by the differences in morphogenic requirements between early and late stages of DNSO. In the early stages, a high auxin/CK ratio is required not only for the development of calli but also of lateral root-like primordia within the calli; later, a high CK/auxin ratio will be required to convert the developmental fate of these primordia into taking on a shoot identity [4][5][16][18]. The genetic basis of DNSO is extraordinarily complex and involves the participation of a tremendous number of genes, among which the most prominent include transcription factors, hormonal response regulators, genes involved in hormonal metabolism and transport, and cell-cycle-related genes.
3. Sucrose Interferes with Auxin and Cytokinin Signaling in the Regulation of Shoot Organogenesis
During their in vitro development, plant shoots express a low capability for photosynthesis; hence, the presence of a carbon foundation is necessary in the growth media to compensate for the reduced carbon fixation [19]. Sucrose is most commonly used as carbon source in plant regeneration systems, being taken up from the growth medium by explants and hydrolyzed into glucose and fructose that further enter plant metabolism [20][21].
Numerous reports have demonstrated the existence of crosstalk between phytohormone signaling and sugar sensing in higher plants, regulating developmental processes on transcriptional, posttranscriptional and posttranslational levels. Sugars can affect phytohormone response by altering the levels, localization and/or transport of different phytohormones [22][23][24].
Further indication of crosstalk between sugar and phytohormones in DNSO recently came from our research group [25]. Significant influence of both CK and sucrose treatment, as well as their interaction, was observed during various stages of DNSO from kohlrabi seedlings. Results demonstrated a remarkable increase in endogenous CK levels when 2 mg L −1 CK trans -zeatin ( t Z) and high sucrose concentration (9%) were applied together, suggesting that sucrose may interact with CK uptake and/or homeostasis. In addition, higher concentration of sucrose significantly affected organogenesis-related genes involved in auxin transport, CK response, SAM formation and cell division, while correlation analysis suggested that sucrose could affect endogenous CK levels and their impact on the transcriptional activity of analyzed genes during callus and shoot formation in kohlrabi shoot organogenesis [25].
Taken together, these reports offer only partial insight into a highly complex network of phytohormone and sugar signaling, which underlies the balanced regulation of callus formation and shoot regeneration. The case of sucrose, which is routinely added to the regeneration media as a carbon source and was never intended to interfere with shoot regeneration, suggests that any component of nutrient media, just like any factor in general present in the environment of a cultured plant, can interact with the signaling pathways that regulate shoot regeneration or any other process being studied in cultured plants.
4. Hormone Uptake: The Missing Link for the Integrative Interpretation of DNSO
Ever since the first protocols for shoot regeneration were developed, their efficiency in inducing callus formation and shoot regeneration were interpreted in relation to the composition of plant growth regulators. However, in an in vitro shoot organogenesis system, the hormonal composition of CIM and SIM represents only the first input parameter in the complex regeneration process. The output, in terms of shoot regeneration efficiency, is the resultant of hormone uptake by the explants, transport to target tissues, alterations in hormonal homeostasis, and downstream signaling processes leading to shoot regeneration. Thus, interpreting shoot regeneration efficiency as a simple output of the media composition represents a rough, albeit often practical, simplification.
Of the several steps linking the hormonal composition of the media to the final shoot regeneration output, the first one—hormone uptake—is the most neglected and the least studied. DNSO occurs from a callus mass and/or a starting tissue explant of various origin (root, hypocotyl, cotyledon, etc.) that is in contact with the regeneration medium. The mechanisms of hormone uptake from the regeneration medium have not been studied sufficiently, and nothing is known about the differences in the mechanisms of uptake between tissues of various origin. However, it is conceivable that these mechanisms are “borrowed” from other developmental programs; thus, the uptake of growth regulators from the nutrient media is presumably carried out similarly to the hormone uptake from the intercellular spaces within an intact plant. For auxin, uptake likely relies on hormone transporters, membrane-associated proteins responsible for phytohormone transport and uptake within the plant body. For CK, the term “uptake” has a broader meaning. If CK signaling occurred entirely from the plasma membrane, CK molecules would not need to be taken up by the cells; however, they would still need to join the pool of apoplastic CK to be available to their target cells for signalization. Hence, CK “uptake” by the explant in the broader sense can be considered as the moment when an exogenous growth regulator becomes indistinguishable from the endogenous phytohormone. Additionally, recent research has suggested the importance of ER-located CK signalization, implying that the intracellular uptake of CK is also an essential step in CK perception [26][27].
References
- Yildiz, M. The prerequisite of the success in plant tissue culture: High frequency shoot regeneration. In Recent Advances in Plant In Vitro Culture; Leva, A., Rinaldi, L.M.R., Eds.; IntechOpen Limited: London, UK, 2012; pp. 63–90. ISBN 978-953-51-0787-3.
- Hill, K.; Schaller, G.E. Enhancing plant regeneration in tissue culture. Plant Signal. Behav. 2013, 8, e25709.
- Ckurshumova, W.; Berleth, T. Overcoming recalcitrance—Auxin response factor functions in plant regeneration. Plant Signal. Behav. 2015, 10, e993293.
- Motte, H.; Vereecke, D.; Geelen, D.; Werbrouck, S. The molecular path to in vitro shoot regeneration. Biotechnol. Adv. 2014, 32, 107–121.
- Ikeuchi, M.; Favero, D.S.; Sakamoto, Y.; Iwase, A.; Coleman, D.; Rymen, B.; Sugimoto, K. Molecular mechanisms of plant regeneration. Annu. Rev. Plant Biol. 2019, 70, 377–406.
- Shin, J.; Bae, S.; Seo, P.J. De novo shoot organogenesis during plant regeneration. J. Exp. Bot. 2020, 71, 63–72.
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant callus: Mechanisms of induction and repression. Plant Cell 2013, 25, 3159–3173.
- Fehér, A. Callus, dedifferentiation, totipotency, somatic embryogenesis: What these terms mean in the era of molecular plant biology? Front. Plant Sci. 2019, 10, 536.
- Kareem, A.; Radhakrishnan, D.; Wang, X.; Bagavathiappan, S.; Trivedi, Z.B.; Sugimoto, K.; Xu, J.; Mähönen, A.P.; Prasad, K. Protocol: A method to study the direct reprogramming of lateral root primordia to fertile shoots. Plant Methods 2016, 12, 27.
- Ikeuchi, M.; Shibata, M.; Rymen, B.; Iwase, A.; Bågman, A.M.; Watt, L.; Coleman, D.; Favero, D.S.; Takahashi, T.; Ahnert, S.E.; et al. A gene regulatory network for cellular reprogramming in plant regeneration. Plant Cell Physiol. 2018, 59, 770–782.
- Alvarez, J.M.; Bueno, N.; Cuesta, C.; Feito, I.; Ordás, R.J. Hormonal and gene dynamics in de novo shoot meristem formation during adventitious caulogenesis in cotyledons of Pinus pinea. Plant Cell Rep. 2020, 39, 527–541.
- Muñoz, A.; Mangano, S.; González-García, M.P.; Contreras, R.; Sauer, M.; De Rybel, B.; Weijers, D.; Sánchez-Serrano, J.J.; Sanmartín, M.; Rojo, E. RIMA-dependent nuclear accumulation of IYO triggers auxin-irreversible cell differentiation in arabidopsis. Plant Cell 2017, 29, 575–588.
- Atta, R.; Laurens, L.; Boucheron-Dubuisson, E.; Guivarc’h, A.; Carnero, E.; Giraudat-Pautot, V.; Rech, P.; Chriqui, D. Pluripotency of arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J. 2009, 57, 626–644.
- Sugimoto, K.; Jiao, Y.; Meyerowitz, E.M. Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev. Cell 2010, 18, 463–471.
- Torres-Martínez, H.H.; Rodríguez-Alonso, G.; Shishkova, S.; Dubrovsky, J.G. Lateral root primordium morphogenesis in angiosperms. Front. Plant Sci. 2019, 10, 206.
- Rosspopoff, O.; Chelysheva, L.; Saffar, J.; Lecorgne, L.; Gey, D.; Caillieux, E.; Colot, V.; Roudier, F.; Hilson, P.; Berthomé, R.; et al. Direct conversion of root primordium into shoot meristem relies on timing of stem cell niche development. Development 2017, 144, 1187–1200.
- Skoog, F.; Miller, C.O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–130.
- Pernisova, M.; Grochova, M.; Konecny, T.; Plackova, L.; Harustiakova, D.; Kakimoto, T.; Heisler, M.G.; Novak, O.; Hejatko, J. Cytokinin signalling regulates organ identity via the AHK4 receptor in arabidopsis. Development 2018, 145, dev163907.
- Desjardins, Y.; Hdider, C.; de Riek, J. Carbon nutrition in vitro—Regulation and manipulation of carbon assimilation in micropropagated systems. In Automation and Environmental Control in Plant Tissue Culture; Aitken-Christie, J., Kozai, T., Smith, M.A.L., Eds.; Springer Nature: Cham, Switzerland, 1995; pp. 441–471. ISBN 978-94-015-8461-6.
- Huang, W.L.; Liu, L.F. Carbohydrate Metabolism in rice during callus induction and shoot regeneration induced by osmotic stress. Bot. Bull. Acad. Sin. 2002, 43, 107–113.
- Lee, S.T.; Huang, W.L. Cytokinin, auxin, and abscisic acid affects sucrose metabolism conduce to de novo shoot organogenesis in rice (Oryza sativa L.) callus. Bot. Stud. 2013, 54, 5.
- Kushwah, S.; Laxmi, A. The interaction between glucose and cytokinin signaling in controlling Arabidopsis thaliana seedling root growth and development. Plant Signal. Behav. 2017, 12, e1312241.
- Sakr, S.; Wang, M.; Dédaldéchamp, F.; Perez-Garcia, M.D.; Ogé, L.; Hamama, L.; Atanassova, R. The sugar-signaling hub: Overview of regulators and interaction with the hormonal and metabolic network. Int. J. Mol. Sci. 2018, 19, 2506.
- Kotov, A.A.; Kotova, L.M.; Romanov, G.A. Signaling Network regulating plant branching: Recent advances and new challenges. Plant Sci. 2021, 307, 110880.
- Ćosić, T.; Motyka, V.; Savić, J.; Raspor, M.; Marković, M.; Dobrev, P.I.; Ninković, S. Sucrose interferes with endogenous cytokinin homeostasis and expression of organogenesis-related genes during de novo shoot organogenesis in kohlrabi. Sci. Rep. 2021, 11, 6494.
- Lomin, S.N.; Myakushina, Y.A.; Arkhipov, D.V.; Leonova, O.G.; Popenko, V.I.; Schmülling, T.; Romanov, G.A. Studies of cytokinin receptor-phosphotransmitter interaction provide evidences for the initiation of cytokinin signalling in the endoplasmic reticulum. Funct. Plant Biol. 2018, 45, 192–202.
- Romanov, G.A.; Lomin, S.N.; Schmülling, T. Cytokinin signaling: From the ER or from the PM? That is the question! New Phytol. 2018, 218, 41–53.




