Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Shi, C. Perspective for High-Value C2 Chemicals. Encyclopedia. Available online: https://encyclopedia.pub/entry/16457 (accessed on 08 February 2026).
Shi C. Perspective for High-Value C2 Chemicals. Encyclopedia. Available at: https://encyclopedia.pub/entry/16457. Accessed February 08, 2026.
Shi, Chuan. "Perspective for High-Value C2 Chemicals" Encyclopedia, https://encyclopedia.pub/entry/16457 (accessed February 08, 2026).
Shi, C. (2021, November 27). Perspective for High-Value C2 Chemicals. In Encyclopedia. https://encyclopedia.pub/entry/16457
Shi, Chuan. "Perspective for High-Value C2 Chemicals." Encyclopedia. Web. 27 November, 2021.
Copy Citation
The oxidative coupling of methane (OCM) to C2 hydrocarbons (C2H4 and C2H6) has aroused worldwide interest over the past decade due to the rise of vast new shale gas resources. However, obtaining higher C2 selectivity can be very challenging in a typical OCM process in the presence of easily oxidized products such as C2H4 and C2H6. Regarding this, different types of catalysts have been studied to achieve desirable C2 yields.
oxidative coupling of methane
ethylene
lattice oxygen
pyrochlore
La2Ce2O7
1. High-Value C2 Chemicals
Methane, being an abundant hydrocarbon resource, provides a kind of comparably cheaper and environmentally friendly fuel [1][2]. The use of natural gas as an important industrial feedstock has increased as compared to petroleum over the past few decades due to the extraction of natural gas from unconventional sources, for example shale deposits [3]. Figure 1 reveals the recoverable shale gas reserves in the world in trillion cubic feet (TCF).
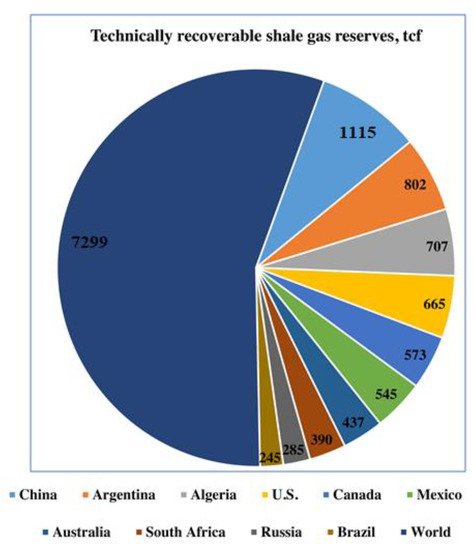 Figure 1. Global projection of technically recoverable shale gas reserves in trillion cubic feet (TCF). The leading global countries are shown in different colors. Reproduced with permission from [3] Copyright 2016 Elsevier.
Ethylene is used as a raw material in the manufacture of polymers such as polyethylene, polystyrene, polyvinyl chloride and polyethylene terephthalate, as well as fibers, petrochemical products and organic chemicals. It is also used as the main feedstock (~50%) in the chemical industry. Hence, many global chemical companies are planning to develop economical and feasible processes to produce ethylene directly from methane [3][4].
In general, steam and the thermal cracking of naphtha processes are used for ethylene production in petroleum refineries. Global ethylene production is mainly dependent on these processes [5][6]. However, some of the key problems identified here are energy intensiveness (up to 40 GJ heat per tonne ethylene) and environmental unsustainability [7]. High temperatures (>1200 °C) are required for breaking the C-H bond (bond energy, 440 kJ/mol) in this process and approximately three tons of CO2 is released per ton of ethylene production [8]. Therefore, another way ethylene can be produced from natural gas is via indirect paths by the formation of syngas (CO/H2) through the catalytic Fischer-Tropsch synthesis (FTS) process [9][10]. Alternatively, syngas can be transformed into methanol and then methanol to ethylene [11][12]. In addition to this, methane can also be converted directly to value-added chemicals and fuels through oxidative and non-oxidative catalytic reaction pathways. The non-oxidative processes involve the coupling of methane to olefins; this process suffers from intrinsic thermodynamic limitations and needs higher energy for large-scale applications [13]. Therefore, most of the research for direct conversion has emphasized a process called oxidative coupling of methane (OCM) [14][15][16][17][18][19].
Zhao et al. [14] prepared hierarchically structured porous flower-like materials such as La2O3-H- and Sr-doped (Sr0.1LaOx-H) compounds and highly dense non-porous (La2O3-D and Sr0.1LaOx-D) microspheres. The porous materials showed higher catalytic performances in OCM than non-porous catalysts, presumably due to the creation of the active sites in porous materials, which could enhance the activation of oxygen. Sr0.1LaOx-H showed better OCM results than La2O3-H and reached the optimum selectivity (~48%) and C2 yield (~19%) at 550 °C due to Sr isolating the active sites of the catalyst, which can act as a strong basic site. However, deactivation occurred in the Sr0.1LaOx-H catalyst during OCM in ~30 h, but adding a small amount of Zr can produce better stability in OCM. Lopes et al. [15] prepared La2Ti2O7-based catalysts (LaTi1−xMgxO3+δ) with varied Ti/Mg molar ratios through the partial substitution of Ti by Mg to regulate the quantities of alkaline sites and surface oxygen species, properties crucial for OCM reaction. The characterization data testifies that alkalinity and the surface oxygen vacancy concentration increase as Mg increases. The presence of high amounts of Mg on the surface can increase the incidence of defective sites in the interface formed by La-O-Mg. In general, the rich defects are active and selective toward C2 production in the OCM reaction. Hence, the catalytic results showed that methane conversion and C2 selectivity increased as a function of the Mg amount, and also this catalyst exhibited high thermal stability after 24 h on stream. Sato et al. [16] proposed that, in the presence of an electric field, the CePO4 nanorod catalysts showed high activity and stability even at low temperatures for OCM (C2 yield = 18%) without any external heating. Because, in the electric field, these catalysts can be produced and regenerate the surface-active oxygen species even at low temperatures, which are mainly responsible for OCM, Schmack et al. proposed a meta-analysis method. It is often useful ascertain the links between a catalyst’s physicochemical characteristics and its performance in an OCM reaction [17]. Pirro et al. [18] proposed the process simulator and illustrated it for the OCM to determine the catalyst-dependent kinetics for industrial implementation. For the case study, the feeding of oxygen and cooling were operated under a multi-stage adiabatic configuration. Three catalysts (Sn-Li/MgO, NaMnW/SiO2 and Sr/La2O3) were introduced to estimate the methane conversion and C2+ yield compared to a single stage. They revealed that multi-phase configuration is advantageous compared to a single phase.
The OCM process follows the value-added transformation of natural gas through the catalytic reaction between methane and oxygen for ethylene production. Although more than four decades have passed since the initial work on OCM, this reaction has still not been able to be used as a successful industrial process for ethylene production because of the lower yield (<30%) of C2 products.
In the reaction mechanism of OCM, the first step of abstraction of a hydrogen atom from methane with the help of surface-active oxygen results in the formation of hydroxyl groups, which react with each other to produce H2O, and at the same time create an oxygen vacancy on the catalyst surface. Meanwhile, methyl radicals will combine to form ethane which further undergoes a dehydrogenation reaction to produce the desired product of ethylene, as shown in Figure 2. According to previous reports, active oxygen species on the surface of catalyst consist of peroxide (O22−), lattice oxygen (O2−), carbonate (CO32−), superoxide (O2−) and hydroxide (OH−) ions. Among all, surface lattice oxygen (O2−) anions play a crucial role in attaining the C2 product selectively, whereas surface electrophilic anions (O−, O2− and O22−) usually result in an over-oxidation of the COx product [20][21].
Figure 1. Global projection of technically recoverable shale gas reserves in trillion cubic feet (TCF). The leading global countries are shown in different colors. Reproduced with permission from [3] Copyright 2016 Elsevier.
Ethylene is used as a raw material in the manufacture of polymers such as polyethylene, polystyrene, polyvinyl chloride and polyethylene terephthalate, as well as fibers, petrochemical products and organic chemicals. It is also used as the main feedstock (~50%) in the chemical industry. Hence, many global chemical companies are planning to develop economical and feasible processes to produce ethylene directly from methane [3][4].
In general, steam and the thermal cracking of naphtha processes are used for ethylene production in petroleum refineries. Global ethylene production is mainly dependent on these processes [5][6]. However, some of the key problems identified here are energy intensiveness (up to 40 GJ heat per tonne ethylene) and environmental unsustainability [7]. High temperatures (>1200 °C) are required for breaking the C-H bond (bond energy, 440 kJ/mol) in this process and approximately three tons of CO2 is released per ton of ethylene production [8]. Therefore, another way ethylene can be produced from natural gas is via indirect paths by the formation of syngas (CO/H2) through the catalytic Fischer-Tropsch synthesis (FTS) process [9][10]. Alternatively, syngas can be transformed into methanol and then methanol to ethylene [11][12]. In addition to this, methane can also be converted directly to value-added chemicals and fuels through oxidative and non-oxidative catalytic reaction pathways. The non-oxidative processes involve the coupling of methane to olefins; this process suffers from intrinsic thermodynamic limitations and needs higher energy for large-scale applications [13]. Therefore, most of the research for direct conversion has emphasized a process called oxidative coupling of methane (OCM) [14][15][16][17][18][19].
Zhao et al. [14] prepared hierarchically structured porous flower-like materials such as La2O3-H- and Sr-doped (Sr0.1LaOx-H) compounds and highly dense non-porous (La2O3-D and Sr0.1LaOx-D) microspheres. The porous materials showed higher catalytic performances in OCM than non-porous catalysts, presumably due to the creation of the active sites in porous materials, which could enhance the activation of oxygen. Sr0.1LaOx-H showed better OCM results than La2O3-H and reached the optimum selectivity (~48%) and C2 yield (~19%) at 550 °C due to Sr isolating the active sites of the catalyst, which can act as a strong basic site. However, deactivation occurred in the Sr0.1LaOx-H catalyst during OCM in ~30 h, but adding a small amount of Zr can produce better stability in OCM. Lopes et al. [15] prepared La2Ti2O7-based catalysts (LaTi1−xMgxO3+δ) with varied Ti/Mg molar ratios through the partial substitution of Ti by Mg to regulate the quantities of alkaline sites and surface oxygen species, properties crucial for OCM reaction. The characterization data testifies that alkalinity and the surface oxygen vacancy concentration increase as Mg increases. The presence of high amounts of Mg on the surface can increase the incidence of defective sites in the interface formed by La-O-Mg. In general, the rich defects are active and selective toward C2 production in the OCM reaction. Hence, the catalytic results showed that methane conversion and C2 selectivity increased as a function of the Mg amount, and also this catalyst exhibited high thermal stability after 24 h on stream. Sato et al. [16] proposed that, in the presence of an electric field, the CePO4 nanorod catalysts showed high activity and stability even at low temperatures for OCM (C2 yield = 18%) without any external heating. Because, in the electric field, these catalysts can be produced and regenerate the surface-active oxygen species even at low temperatures, which are mainly responsible for OCM, Schmack et al. proposed a meta-analysis method. It is often useful ascertain the links between a catalyst’s physicochemical characteristics and its performance in an OCM reaction [17]. Pirro et al. [18] proposed the process simulator and illustrated it for the OCM to determine the catalyst-dependent kinetics for industrial implementation. For the case study, the feeding of oxygen and cooling were operated under a multi-stage adiabatic configuration. Three catalysts (Sn-Li/MgO, NaMnW/SiO2 and Sr/La2O3) were introduced to estimate the methane conversion and C2+ yield compared to a single stage. They revealed that multi-phase configuration is advantageous compared to a single phase.
The OCM process follows the value-added transformation of natural gas through the catalytic reaction between methane and oxygen for ethylene production. Although more than four decades have passed since the initial work on OCM, this reaction has still not been able to be used as a successful industrial process for ethylene production because of the lower yield (<30%) of C2 products.
In the reaction mechanism of OCM, the first step of abstraction of a hydrogen atom from methane with the help of surface-active oxygen results in the formation of hydroxyl groups, which react with each other to produce H2O, and at the same time create an oxygen vacancy on the catalyst surface. Meanwhile, methyl radicals will combine to form ethane which further undergoes a dehydrogenation reaction to produce the desired product of ethylene, as shown in Figure 2. According to previous reports, active oxygen species on the surface of catalyst consist of peroxide (O22−), lattice oxygen (O2−), carbonate (CO32−), superoxide (O2−) and hydroxide (OH−) ions. Among all, surface lattice oxygen (O2−) anions play a crucial role in attaining the C2 product selectively, whereas surface electrophilic anions (O−, O2− and O22−) usually result in an over-oxidation of the COx product [20][21].
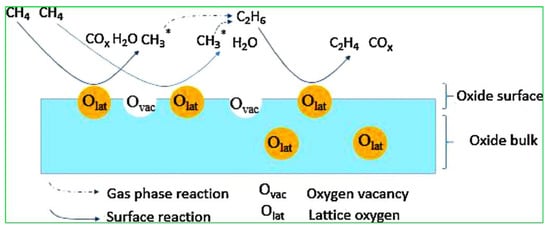 Figure 2. Diagrammatic representation of oxidative coupling of methane (OCM) reaction on a metal oxide catalyst surface. Reproduced with permission from [19]. Copyright 2018 Elsevier.
From the pioneering work of Keller and Bhasin [22] and Ito and Lunsford [23], various catalysts have been investigated to attain adequate methane conversion and C2 selectivity/yield. Most of the metal oxides in the periodic table, such as transition metal oxides, rare earth metal oxides, alkali or alkaline earth metal oxides, perovskites and pyrochlore compounds, have been individually or in various combinations tested for OCM reaction [3][24][25][26][27][28]. Zavyalova et al. [29] conducted a comprehensive statistical analysis of about ~1000 research articles related to OCM. They pointed out that both rare-earth metal oxides and alkaline-earth metal oxides, along with alkali metal oxides, are good candidates for OCM reaction. In addition, binary and ternary combinations of metal oxides are also crucial for high and sustainable catalytic activity in the OCM reaction. The selectivity of the catalysts can be further improved by using various dopants such as alkali (Cs, Na) and alkaline earth (Sr, Ba) metals, whereas Mn, W and the Cl− anion dopants can be used to enhance the catalytic activity. Figure 3 represents the most promising OCM catalytic systems with C2 yields greater than 25%.
Figure 2. Diagrammatic representation of oxidative coupling of methane (OCM) reaction on a metal oxide catalyst surface. Reproduced with permission from [19]. Copyright 2018 Elsevier.
From the pioneering work of Keller and Bhasin [22] and Ito and Lunsford [23], various catalysts have been investigated to attain adequate methane conversion and C2 selectivity/yield. Most of the metal oxides in the periodic table, such as transition metal oxides, rare earth metal oxides, alkali or alkaline earth metal oxides, perovskites and pyrochlore compounds, have been individually or in various combinations tested for OCM reaction [3][24][25][26][27][28]. Zavyalova et al. [29] conducted a comprehensive statistical analysis of about ~1000 research articles related to OCM. They pointed out that both rare-earth metal oxides and alkaline-earth metal oxides, along with alkali metal oxides, are good candidates for OCM reaction. In addition, binary and ternary combinations of metal oxides are also crucial for high and sustainable catalytic activity in the OCM reaction. The selectivity of the catalysts can be further improved by using various dopants such as alkali (Cs, Na) and alkaline earth (Sr, Ba) metals, whereas Mn, W and the Cl− anion dopants can be used to enhance the catalytic activity. Figure 3 represents the most promising OCM catalytic systems with C2 yields greater than 25%.
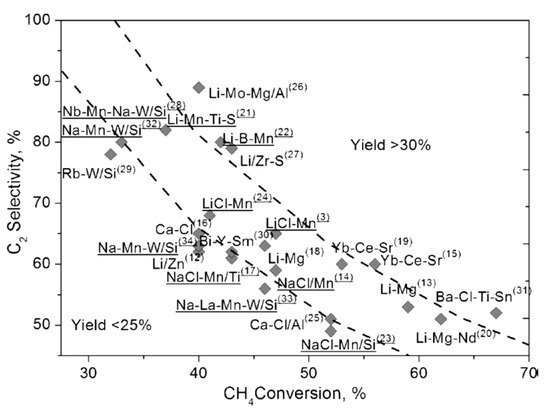 Figure 3. Various OCM catalysts with C2 yields (≥25%). Reproduced with permission from [29]. Copyright 2011 Wiley-VCH.
Figure 3. Various OCM catalysts with C2 yields (≥25%). Reproduced with permission from [29]. Copyright 2011 Wiley-VCH.

Figure 1. Global projection of technically recoverable shale gas reserves in trillion cubic feet (TCF). The leading global countries are shown in different colors. Reproduced with permission from [3] Copyright 2016 Elsevier.

Figure 2. Diagrammatic representation of oxidative coupling of methane (OCM) reaction on a metal oxide catalyst surface. Reproduced with permission from [19]. Copyright 2018 Elsevier.

Figure 3. Various OCM catalysts with C2 yields (≥25%). Reproduced with permission from [29]. Copyright 2011 Wiley-VCH.
References
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic dehydrogenation of light alkanes on metals and metal oxides. Chem. Rev. 2014, 114, 10613–10653.
- Wood, D.A.; Nwaoha, C.; Towler, B.F. Gas-to-liquids (GTL): A review of an industry offering several routes for monetizing natural gas. J. Nat. Gas Sci. Eng. 2012, 9, 196–208.
- Galadima, A.; Muraza, O. Revisiting the oxidative coupling of methane to ethylene in the golden period of shale gas: A review. J. Ind. Eng. Chem. 2016, 37, 1–13.
- Guo, B.; Shan, J.; Feng, Y. Productivity of blast-fractured wells in liquid-rich shale gas formations. J. Nat. Gas Sci. Eng. 2014, 18, 360–367.
- Seifzadeh Haghighi, S.; Rahimpour, M.R.; Raeissi, S.; Dehghani, O. Investigation of ethylene production in naphtha thermal cracking plant in presence of steam and carbon dioxide. Chem. Eng. J. 2013, 228, 1158–1167.
- Le Van Mao, R.; Yan, H.; Muntasar, A.; Al-Yassir, N. Chapter 7—Blending of non-petroleum compounds with current hydrocarbon feeds to use in the thermo-catalytic steam-cracking process for the selective production of light olefins. In New and Future Developments in Catalysis; Suib, S.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 143–173.
- Keyvanloo, K.; Towfighi, J.; Sadrameli, S.M.; Mohamadalizadeh, A. Investigating the effect of key factors, their interactions and optimization of naphtha steam cracking by statistical design of experiments. J. Anal. Appl. Pyrolysis 2010, 87, 224–230.
- Corma, A.; Mengual, J.; Miguel, P.J. IM-5 zeolite for steam catalytic cracking of naphtha to produce propene and ethene. An alternative to ZSM-5 zeolite. Appl. Catal. A Gen. 2013, 460–461, 106–115.
- Dry, M.E. The Fischer–Tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241.
- Schulz, H. Short history and present trends of Fischer–Tropsch synthesis. Appl. Catal. A Gen. 1999, 186, 3–12.
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to olefins (MTO): From fundamentals to commercialization. ACS Catal. 2015, 5, 1922–1938.
- Olsbye, U.; Svelle, S.; Bjørgen, M.; Beato, P.; Janssens, T.V.W.; Joensen, F.; Bordiga, S.; Lillerud, K.P. Conversion of methanol to hydrocarbons: How zeolite cavity and pore size controls product selectivity. Angew. Chem. Int. Ed. 2012, 51, 5810–5831.
- Guo, X.; Fang, G.; Li, G.; Ma, H.; Fan, H.; Yu, L.; Ma, C.; Wu, X.; Deng, D.; Wei, M.; et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 2014, 344, 616–619.
- Zhao, M.; Ke, S.; Wu, H.; Xia, W.; Wan, H. Flower-like Sr-La2O3 microspheres with hierarchically porous structures for oxidative coupling of methane. Ind. Eng. Chem. Res. 2019, 58, 22847–22856.
- Lopes, L.B.; Vieira, L.H.; Assaf, J.M.; Assaf, E.M. Effect of Mg substitution on LaTi1−xMgxO3+δ catalysts for improving the C2 selectivity of the oxidative coupling of methane. Catal. Sci. Technol. 2021, 11, 283–296.
- Sato, A.; Ogo, S.; Kamata, K.; Takeno, Y.; Yabe, T.; Yamamoto, T.; Matsumura, S.; Hara, M.; Sekine, Y. Ambient-temperature oxidative coupling of methane in an electric field by a cerium phosphate nanorod catalyst. Chem. Commun. 2019, 55, 4019–4022.
- Schmack, R.; Friedrich, A.; Kondratenko, E.V.; Polte, J.; Werwatz, A.; Kraehnert, R. A meta-analysis of catalytic literature data reveals property-performance correlations for the OCM reaction. Nat. Commun. 2019, 10, 441.
- Pirro, L.; Mendes, P.S.F.; Kemseke, B.; Vandegehuchte, B.D.; Marin, G.B.; Thybaut, J.W. From catalyst to process: Bridging the scales in modeling the OCM reaction. Catal. Today 2021, 365, 35–45.
- Gambo, Y.; Jalil, A.A.; Triwahyono, S.; Abdulrasheed, A.A. Recent advances and future prospect in catalysts for oxidative coupling of methane to ethylene: A review. J. Ind. Eng. Chem. 2018, 59, 218–229.
- Grant, J.T.; Venegas, J.M.; McDermott, W.P.; Hermans, I. Aerobic Oxidations of Light Alkanes over Solid Metal Oxide Catalysts. Chem. Rev. 2018, 118, 2769–2815.
- Lomonosov, V.I.; Sinev, M.Y. Oxidative coupling of methane: Mechanism and kinetics. Kinet. Catal. 2016, 57, 647–676.
- Keller, G.E.; Bhasin, M.M. Synthesis of ethylene via oxidative coupling of methane: I. Determination of active catalysts. J. Catal. 1982, 73, 9–19.
- Ito, T.; Lunsford, J.H. Synthesis of ethylene and ethane by partial oxidation of methane over lithium-doped magnesium oxide. Nature 1985, 314, 721–722.
- Jiang, T.; Song, J.; Huo, M.; Yang, N.; Liu, J.; Zhang, J.; Sun, Y.; Zhu, Y. La2O3 catalysts with diverse spatial dimensionality for oxidative coupling of methane to produce ethylene and ethane. RSC Adv. 2016, 6, 34872–34876.
- Papa, F.; Luminita, P.; Osiceanu, P.; Birjega, R.; Akane, M.; Balint, I. Acid–base properties of the active sites responsible for C2+ and CO2 formation over MO–Sm2O3 (M = Zn, Mg, Ca and Sr) mixed oxides in OCM reaction. J. Mol. Catal. A Chem. 2011, 346, 46–54.
- Wang, P.; Zhao, G.; Wang, Y.; Lu, Y. MnTiO3-driven low-temperature oxidative coupling of methane over TiO2-doped Mn2O3-Na2WO4/SiO2 catalyst. Sci. Adv. 2017, 3, e1603180.
- Bai, L.; Polo-Garzon, F.; Bao, Z.; Luo, S.; Moskowitz, B.M.; Tian, H.; Wu, Z. Impact of Surface Composition of SrTiO3 Catalysts for Oxidative Coupling of Methane. ChemCatChem 2019, 11, 2107–2117.
- Lee, J.S.; Oyama, S.T. Oxidative Coupling of Methane to Higher Hydrocarbons. Catal. Rev. 1988, 30, 249–280.
- Zavyalova, U.; Holena, M.; Schlögl, R.; Baerns, M. Statistical analysis of past catalytic data on oxidative methane coupling for new insights into the composition of high-performance catalysts. ChemCatChem 2011, 3, 1935–1947.
More
Information
Subjects:
Biochemical Research Methods
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.0K
Revisions:
3 times
(View History)
Update Date:
29 Nov 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




