
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Davide Sartini | + 1334 word(s) | 1334 | 2021-08-23 08:07:40 | | | |
| 2 | Amina Yu | Meta information modification | 1334 | 2021-08-26 05:22:03 | | |
Video Upload Options
Nicotinamide N-methyltransferase (NNMT) catalyzes the N-methylation reaction of nicotinamide, using S-adenosyl-L-methionine as the methyl donor. Enzyme overexpression has been described in many non-neoplastic diseases, as well as in a wide range of solid malignancies. This entry aims to report and discuss evidence available in scientific literature, dealing with NNMT expression and the potential involvement in main urologic neoplasms, namely, renal, bladder and prostate cancers.
1. Introduction
NNMT is mainly expressed in the liver and belongs to phase II metabolizing enzymes [1]. Although the enzyme is described to be involved in the biotransformation and detoxification of many xenobiotics [1][2][3], porcine liver NNMT was only described to be active towards pyridine derivatives, such as quinoline, isoquinoline, and 1,2,3,4-tetrahydroisoquinoline, in addition to structural NA analogs, such as thionicotinamide and 3-acetylpyridine [4]. The resolution of tridimensional (3D) structures of purified human recombinant NNMT, together with the detection of the main amino acid residues involved in the catalysis [5], led to the discovery of the kinetic mechanism of NNMT [6], the identification of alternate enzyme substrates [7][8][9], as well as the design and assay of a great number of inhibitors [10][11][12][13][14][15][16][17][18][19][20][21].
NNMT upregulation, as well as its involvement, has been described for several non-neoplastic disorders, such as Parkinson’s disease [22][23][24][25], metabolic disfunctions [26][27][28][29], chronic obstructive pulmonary disease [30][31], atherosclerosis [32] and pulmonary arterial hypertension [33].
However, the bulk of scientific literature on NNMT is greatly focused on speculating and clarifying the role played by the enzyme in cancer. In fact, NNMT overexpression has been reported for many solid tumors, including gastrointestinal neoplasms [34][35][36][37][38][39][40][41][42], lung [43][44][45][46], oral [47][48][49][50][51][52][53], esophageal [54][55], nasopharyngeal [56] and thyroid [57][58][59] cancers, as well as ameloblastoma [60] and glioblastoma multiforme [61]. Significant NNMT upregulation was recently found in epithelial neoplasms [62][63][64][65][66][67] and in association with cancer stem cells (CSCs) [68][69][70][71][72]. Regarding women’s cancers, enzyme overexpression was detected in breast [73][74][75], endometrial [76], cervical [77] and ovarian [78] cancer.
The aim of this review was to give an update on data concerning NNMT expression levels in urologic malignancies, such as renal, bladder and prostate cancer, as well as to report evidence showing enzyme contributions in malignant transformations featuring these neoplasms, and the role played by NNMT in tumor cell metabolism and phenotypes.
2. NNMT and Renal Cancer
Among urological neoplasms, renal cell carcinoma (RCC) was the first to be examined in regard to potential NNMT involvement, in which enzyme overexpression was described.
Although several studies described NNMT upregulation in RCC, the molecular events leading to enzyme overexpression, as well as the effect induced by such dysregulation in cancer cell phenotype, remain partly understood. In the last decade, a few studies have been carried out in order to elucidate these aspects.
Most of the studies devoted to elucidating NNMT contributions to the malignant transformation of kidney cells, by promoting proliferation, migration, invasiveness and resistance to therapy, had been carried out on primary (769-P and 786-O) and metastatic (Caki-1) ccRCC cell lines, as well as in primary (Caki-2) pRCC cells.
Caki-1 treatment with a sulfonamide analogue of alkaloid cryptopleurine led to a significant reduction in proliferation and cell cycle progression, as well as to NNMT downregulation. Interestingly, antiproliferative activity and cell cycle arrest exerted by this pharmacological treatment were inhibited or abolished upon siRNA-mediated NNMT silencing. Further analyses demonstrated that NNMT knockdown was associated with decreased levels of phosphorylated c-Jun, whose accumulation is responsible for G0/G1 cell cycle arrest. This evidence seems to suggest a potential involvement of NNMT in molecular events connected with the antiproliferative and cell cycle-regulating effects induced by Caki-1 cell treatment with a methanesulfonamide analogue of cryptopleurine [79].
3. NNMT and Bladder Cancer
In the context of studies aiming to disclose the potential involvement of NNMT in urothelial neoplasms, array analyses were used to profile the expression of tumor and normal-looking tissue samples obtained from patients affected with bladder cancer (BC) [80][81].
NNMT levels were also detected in exfoliated cells collected from urine samples obtained from BC patients and healthy subjects. The data reported showed that enzyme expression was significantly higher in the urine of patients suffering from BC compared with that detected in control specimens. Subsequent statistical analyses revealed a significant inverse correlation between tumor NNMT levels and histological grade. Moreover, ROC analysis, as well as AUC evaluation, demonstrated the excellent diagnostic accuracy of a potential urine-based NNMT test. Such evidence strongly indicates that NNMT level determination could be used for early and non-invasive BC detection [82].
The pivotal study, that started to elucidate the role of NNMT in bladder cancer cells, used the cDNA array technique to profile the expression of stress-related and DNA repair genes in both radioresistant MGH-U1 BC cell line and its radiosensitive subclone S40b. Among dysregulated genes, the NNMT displayed marked decreased levels in S40b with respect to those detected in MGH-U1 cells. This result, further confirmed by Real-Time PCR assay, represented the first evidence suggesting a potential role played by the enzyme in cell responses to radiation treatment [83].
Wu et al. were the first to report NNMT involvement in BC cell metabolism, focusing on events featuring cell migration. The goal of this in vitro study was to detect genes promoting invasion and metastasis. To this aim, numerous human BC cell lines were both subjected to radial migration assay and then microarray-profiled. NNMT was identified among genes whose expression was directly correlated with cell migration rate, and therefore selected for further investigation. Data obtained from analyses carried out in BC tissue samples revealed that enzyme expression was also positively associated with the tumor stage, and was found to be higher in muscle-invasive forms compared with that of superficial neoplasms. Given these results, further in vitro experiments were performed in order to speculate the potential mechanistic role of NNMT in cell migration. Interestingly, siRNA-mediated enzyme downregulation significantly reduced cell proliferation, as well as chemotaxis and chemokinesis in the 253J human BC cell line [84].
4. Conclusions
To the best of our knowledge, this is the first review reporting the concerns regarding the role played by NNMT in urological tumors, focusing on enzyme expression levels in tissues and body fluids, as well as its involvement in molecular and cellular events promoting malignant transformation.
For almost twenty years, NNMT has been described to be upregulated in several solid cancers, participating in crucial mechanisms leading to cancer progression, metastasis and resistance to chemo- and radiotherapy.
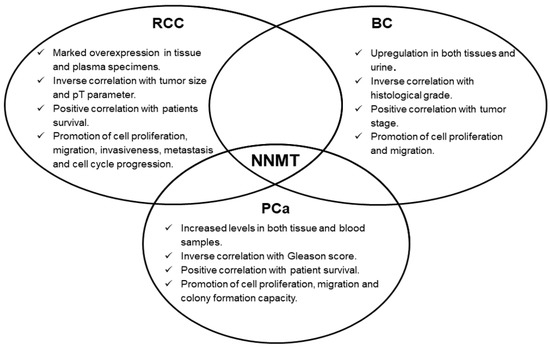
Concerning urologic malignancies, enzyme overexpression emerged in renal, bladder and prostate cancers. In these neoplasms, NNMT seems to act as an interesting diagnostic biomarker, whose measurement might even be suitable for non-invasive and early cancer detection. Moreover, the prognostic potential of NNMT level determination was also demonstrated, with enzyme intratumor expression significantly correlated with important parameters, such as tumor stage, size, grade, as well as with patient survival. Subsequent studies clearly demonstrated NNMT involvement in fundamental events featuring tumorigenesis, namely, cell proliferation, migration and invasiveness (Figure 1), thus attributing to the enzyme a promising role as a molecular target for effective treatment of these tumors. NNMT 3D structure resolution [5], together with the identification of related kinetic mechanism-of-action [6], have triggered the design, synthesis and validation of a wide range of molecules that seem to act as potent inhibitors of enzyme activity [10][11][12][13][14][15][16][17][18][19][20][21], that could be used as novel anti-cancer therapeutics.
In addition to this, recent studies showed that the enrichment of tumor cell lines with CSCs was significantly associated with the enhanced expression of NNMT [70][72]. Acquisition of stem-like phenotype by cancer cells was demonstrated to have detrimental effects in terms of tumor aggressiveness and its resistance to therapy, thus leading to the relapse of neoplasm and metastatic spread [85]. In the light of these considerations and based on data reported in this review, it is conceivable to hypothesize NNMT involvement in those molecular processes and cellular events featuring poor clinical outcomes of urological malignancies. Once again, targeting NNMT could represent a powerful molecular strategy to effectively treat cancer.
References
- Rini, J.; Szumlanski, C.; Guerciolini, R.; Weinshilboum, R.M. Human liver nicotinamide N-methyltransferase: Ion-pairing radiochemical assay, biochemical properties and individual variation. Clin. Chim. Acta 1990, 186, 359–374.
- Aksoy, S.; Szumlanski, C.L.; Weinshilboum, R.M. Human liver nicotinamide N-methyltransferase. cDNA cloning, expression, and biochemical characterization. J. Biol. Chem. 1994, 269, 14835–14840.
- Aksoy, S.; Brandriff, B.F.; Ward, A.; Little, P.F.; Weinshilboum, R.M. Human nicotinamide N-methyltransferase gene: Molecular cloning, structural characterization and chromosomal localization. Genomics 1995, 29, 555–561.
- Alston, T.A.; Abeles, R.H. Substrate specificity of nicotinamide methyltransferase isolated from porcine liver. Arch. Biochem. Biophys. 1988, 260, 601–608.
- Peng, Y.; Sartini, D.; Pozzi, V.; Wilk, D.; Emanuelli, M.; Yee, V.C. Structural basis of substrate recognition in human nicotinamide N-methyltransferase. Biochemistry 2011, 50, 7800–7808.
- Loring, H.S.; Thompson, P.R. Kinetic Mechanism of Nicotinamide N-Methyltransferase. Biochemistry 2018, 57, 5524–5532.
- van Haren, M.J.; Sastre Toraño, J.; Sartini, D.; Emanuelli, M.; Parsons, R.B.; Martin, N.I. A Rapid and Efficient Assay for the Characterization of Substrates and Inhibitors of Nicotinamide N-Methyltransferase. Biochemistry 2016, 55, 5307–5315.
- Thomas, M.G.; Sartini, D.; Emanuelli, M.; van Haren, M.J.; Martin, N.I.; Mountford, D.M.; Barlow, D.J.; Klamt, F.; Ramsden, D.B.; Reza, M.; et al. Nicotinamide N-methyltransferase catalyses the N-methylation of the endogenous β-carboline norharman: Evidence for a novel detoxification pathway. Biochem. J. 2016, 473, 3253–3267.
- van Haren, M.J.; Thomas, M.G.; Sartini, D.; Barlow, D.J.; Ramsden, D.B.; Emanuelli, M.; Klamt, F.; Martin, N.I.; Parsons, R.B. The kinetic analysis of the N-methylation of 4-phenylpyridine by nicotinamide N-methyltransferase: Evidence for a novel mechanism of substrate inhibition. Int. J. Biochem. Cell. Biol. 2018, 98, 127–136.
- Neelakantan, H.; Wang, H.Y.; Vance, V.; Hommel, J.D.; McHardy, S.F.; Watowich, S.J. Structure-Activity Relationship for Small Molecule Inhibitors of Nicotinamide N-Methyltransferase. J. Med. Chem. 2017, 60, 5015–5028.
- van Haren, M.J.; Taig, R.; Kuppens, J.; Sastre Toraño, J.; Moret, E.E.; Parsons, R.B.; Sartini, D.; Emanuelli, M.; Martin, N.I. Inhibitors of nicotinamide N-methyltransferase designed to mimic the methylation reaction transition state. Org. Biomol. Chem. 2017, 15, 6656–6667.
- Neelakantan, H.; Vance, V.; Wetzel, M.D.; Wang, H.L.; McHardy, S.F.; Finnerty, C.C.; Hommel, J.D.; Watowich, S.J. Selective and membrane-permeable small molecule inhibitors of nicotinamide N-methyltransferase reverse high fat diet-induced obesity in mice. Biochem. Pharmacol. 2018, 147, 141–152.
- Kannt, A.; Rajagopal, S.; Kadnur, S.V.; Suresh, J.; Bhamidipati, R.K.; Swaminathan, S.; Hallur, M.S.; Kristam, R.; Elvert, R.; Czech, J.; et al. A small molecule inhibitor of Nicotinamide N-methyltransferase for the treatment of metabolic disorders. Sci. Rep. 2018, 8, 3660.
- Ruf, S.; Hallur, M.S.; Anchan, N.K.; Swamy, I.N.; Murugesan, K.R.; Sarkar, S.; Narasimhulu, L.K.; Putta, V.P.R.K.; Shaik, S.; Chandrasekar, D.V.; et al. Novel nicotinamide analog as inhibitor of nicotinamide N-methyltransferase. Bioorg. Med. Chem. Lett. 2018, 28, 922–925.
- Lee, H.Y.; Suciu, R.M.; Horning, B.D.; Vinogradova, E.V.; Ulanovskaya, O.A.; Cravatt, B.F. Covalent inhibitors of nicotinamide N-methyltransferase (NNMT) provide evidence for target engagement challenges in situ. Bioorg. Med. Chem. Lett. 2018, 28, 2682–2687.
- Neelakantan, H.; Brightwell, C.R.; Graber, T.G.; Maroto, R.; Wang, H.L.; McHardy, S.F.; Papaconstantinou, J.; Fry, C.S.; Watowich, S.J. Small molecule nicotinamide N-methyltransferase inhibitor activates senescent muscle stem cells and improves regenerative capacity of aged skeletal muscle. Biochem. Pharmacol. 2019, 163, 481–492.
- Gao, Y.; van Haren, M.J.; Moret, E.E.; Rood, J.J.M.; Sartini, D.; Salvucci, A.; Emanuelli, M.; Craveur, P.; Babault, N.; Jin, J.; et al. Bisubstrate Inhibitors of Nicotinamide N-Methyltransferase (NNMT) with Enhanced Activity. J. Med. Chem. 2019, 62, 6597–6614.
- Policarpo, R.L.; Decultot, L.; May, E.; Kuzmič, P.; Carlson, S.; Huang, D.; Chu, V.; Wright, B.A.; Dhakshinamoorthy, S.; Kannt, A.; et al. High-Affinity Alkynyl Bisubstrate Inhibitors of Nicotinamide N-Methyltransferase (NNMT). J. Med. Chem. 2019, 62, 9837–9873.
- Chen, D.; Li, L.; Diaz, K.; Iyamu, I.D.; Yadav, R.; Noinaj, N.; Huang, R. Novel Propargyl-Linked Bisubstrate Analogues as Tight-Binding Inhibitors for Nicotinamide N-Methyltransferase. J. Med. Chem. 2019, 62, 10783–10797.
- Kannt, A.; Rajagopal, S.; Hallur, M.S.; Swamy, I.; Kristam, R.; Dhakshinamoorthy, S.; Czech, J.; Zech, G.; Schreuder, H.; Ruf, S. Novel Inhibitors of Nicotinamide-N-Methyltransferase for the Treatment of Metabolic Disorders. Molecules 2021, 26, 991.
- Akar, S.; Duran, T.; Azzawri, A.A.; Koçak, N.; Çelik, Ç.; Yıldırım, H.İ. Small molecule inhibitor of nicotinamide N-methyltransferase shows anti-proliferative activity in HeLa cells. J. Obstet. Gynaecol. 2021, 1, 1–9.
- Aoyama, K.; Matsubara, K.; Kondo, M.; Murakawa, Y.; Suno, M.; Yamashita, K.; Yamaguchi, S.; Kobayashi, S. Nicotinamide-N-methyltransferase is higher in the lumbar cerebrospinal fluid of patients with Parkinson’s disease. Neurosci. Lett. 2001, 298, 78–80.
- Parsons, R.B.; Smith, M.L.; Williams, A.C.; Waring, R.H.; Ramsden, D.B. Expression of nicotinamide N-methyltransferase (E.C. 2.1.1.1) in the Parkinsonian brain. J. Neuropathol. Exp. Neurol. 2002, 61, 111–124.
- Parsons, R.B.; Smith, S.W.; Waring, R.H.; Williams, A.C.; Ramsden, D.B. High expression of nicotinamide N-methyltransferase in patients with idiopathic Parkinson’s disease. Neurosci. Lett. 2003, 342, 13–16.
- Williams, A.C.; Ramsden, D.B. Autotoxicity, methylation and a road to the prevention of Parkinson’s disease. J. Clin. Neurosci. 2005, 12, 6–11.
- Liu, M.; Li, L.; Chu, J.; Zhu, B.; Zhang, Q.; Yin, X.; Jiang, W.; Dai, G.; Ju, W.; Wang, Z.; et al. Serum N(1)-Methylnicotinamide Is Associated With Obesity and Diabetes in Chinese. J. Clin. Endocrinol. Metab. 2015, 100, 3112–3117.
- Kannt, A.; Pfenninger, A.; Teichert, L.; Tönjes, A.; Dietrich, A.; Schön, M.R.; Klöting, N.; Blüher, M. Association of nicotinamide-N-methyltransferase mRNA expression in human adipose tissue and the plasma concentration of its product, 1-methylnicotinamide, with insulin resistance. Diabetologia 2015, 58, 799–808.
- Giuliante, R.; Sartini, D.; Bacchetti, T.; Rocchetti, R.; Klöting, I.; Polidori, C.; Ferretti, G.; Emanuelli, M. Potential involvement of nicotinamide N-methyltransferase in the pathogenesis of metabolic syndrome. Metab. Syndr. Relat. Disord. 2015, 13, 165–170.
- Ehebauer, F.; Ghavampour, S.; Kraus, D. Glucose availability regulates nicotinamide N-methyltransferase expression in adipocytes. Life Sci. 2020, 248, 117474.
- Debigaré, R.; Maltais, F.; Côté, C.H.; Michaud, A.; Caron, M.; Mofarrahi, M.; Leblanc, P.; Hussain, S.N. Profiling of mRNA expression in quadriceps of patients with COPD and muscle wasting. COPD 2008, 5, 75–84.
- Kim, H.C.; Mofarrahi, M.; Vassilakopoulos, T.; Maltais, F.; Sigala, I.; Debigare, R.; Bellenis, I.; Hussain, S.N. Expression and functional significance of nicotinamide N-methyl transferase in skeletal muscles of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 181, 797–805.
- Mateuszuk, Ł.; Khomich, T.I.; Słomińska, E.; Gajda, M.; Wójcik, L.; Łomnicka, M.; Gwóźdź, P.; Chłopicki, S. Activation of nicotinamide N-methyltrasferase and increased formation of 1-methylnicotinamide (MNA) in atherosclerosis. Pharmacol. Rep. 2009, 61, 76–85.
- Fedorowicz, A.; Mateuszuk, Ł.; Kopec, G.; Skórka, T.; Kutryb-Zając, B.; Zakrzewska, A.; Walczak, M.; Jakubowski, A.; Łomnicka, M.; Słomińska, E.; et al. Activation of the nicotinamide N-methyltransferase (NNMT)-1-methylnicotinamide (MNA) pathway in pulmonary hypertension. Respir. Res. 2016, 17, 108.
- Jang, J.S.; Cho, H.Y.; Lee, Y.J.; Ha, W.S.; Kim, H.W. The differential proteome profile of stomach cancer: Identification of the biomarker candidates. Oncol. Res. 2004, 14, 491–499.
- Lim, B.H.; Cho, B.I.; Kim, Y.N.; Kim, J.W.; Park, S.T.; Lee, C.W. Overexpression of nicotinamide N-methyltransferase in gastric cancer tissues and its potential post-translational modification. Exp. Mol. Med. 2006, 38, 455–465.
- Chen, C.; Wang, X.; Huang, X.; Yong, H.; Shen, J.; Tang, Q.; Zhu, J.; Ni, J.; Feng, Z. Nicotinamide N-methyltransferase: A potential biomarker for worse prognosis in gastric carcinoma. Am. J. Cancer Res. 2016, 6, 649–663.
- Roessler, M.; Rollinger, W.; Palme, S.; Hagmann, M.L.; Berndt, P.; Engel, A.M.; Schneidinger, B.; Pfeffer, M.; Andres, H.; Karl, J.; et al. Identification of nicotinamide N-methyltransferase as a novel serum tumor marker for colorectal cancer. Clin. Cancer Res. 2005, 11, 6550–6557.
- Xie, X.; Yu, H.; Wang, Y.; Zhou, Y.; Li, G.; Ruan, Z.; Li, F.; Wang, X.; Liu, H.; Zhang, J. Nicotinamide N-methyltransferase enhances the capacity of tumorigenesis associated with the promotion of cell cycle progression in human colorectal cancer cells. Arch. Biochem. Biophys. 2014, 15, 52–66.
- Xie, X.; Liu, H.; Wang, Y.; Zhou, Y.; Yu, H.; Li, G.; Ruan, Z.; Li, F.; Wang, X.; Zhang, J. Nicotinamide N-methyltransferase enhances resistance to 5-fluorouracil in colorectal cancer cells through inhibition of the ASK1-p38 MAPK pathway. Oncotarget 2016, 7, 45837–45848.
- Song, M.; Li, Y.; Miao, M.; Zhang, F.; Yuan, H.; Cao, F.; Chang, W.; Shi, H.; Song, C. High stromal nicotinamide N-methyltransferase (NNMT) indicates poor prognosis in colorectal cancer. Cancer Med. 2020, 9, 2030–2038.
- Rogers, C.D.; Fukushima, N.; Sato, N.; Shi, C.; Prasad, N.; Hustinx, S.R.; Matsubayashi, H.; Canto, M.; Eshleman, J.R.; Hruban, R.H.; et al. Differentiating pancreatic lesions by microarray and QPCR analysis of pancreatic juice RNAs. Cancer Biol. Ther. 2006, 5, 1383–1389.
- Yu, T.; Wang, Y.T.; Chen, P.; Li, Y.H.; Chen, Y.X.; Zeng, H.; Yu, A.M.; Huang, M.; Bi, H.C. Effects of nicotinamide N-methyltransferase on PANC-1 cells proliferation, metastatic potential and survival under metabolic stress. Cell Physiol. Biochem. 2015, 35, 710–721.
- Tomida, M.; Mikami, I.; Takeuchi, S.; Nishimura, H.; Akiyama, H. Serum levels of nicotinamide N-methyltransferase in patients with lung cancer. J. Cancer Res. Clin. Oncol. 2009, 135, 1223–1229.
- Sartini, D.; Morganti, S.; Guidi, E.; Rubini, C.; Zizzi, A.; Giuliante, R.; Pozzi, V.; Emanuelli, M. Nicotinamide N-methyltransferase in non-small cell lung cancer: Promising results for targeted anti-cancer therapy. Cell Biochem. Biophys. 2013, 67, 865–873.
- Sartini, D.; Seta, R.; Pozzi, V.; Morganti, S.; Rubini, C.; Zizzi, A.; Tomasetti, M.; Santarelli, L.; Emanuelli, M. Role of nicotinamide N-methyltransferase in non-small cell lung cancer: In vitro effect of shRNA-mediated gene silencing on tumorigenicity. Biol. Chem. 2015, 396, 225–234.
- Bach, D.H.; Kim, D.; Bae, S.Y.; Kim, W.K.; Hong, J.Y.; Lee, H.J.; Rajasekaran, N.; Kwon, S.; Fan, Y.; Luu, T.T.; et al. Targeting Nicotinamide N-Methyltransferase and miR-449a in EGFR-TKI-Resistant Non-Small-Cell Lung Cancer Cells. Mol. Ther. Nucleic Acids 2018, 11, 455–467.
- Sartini, D.; Santarelli, A.; Rossi, V.; Goteri, G.; Rubini, C.; Ciavarella, D.; Lo Muzio, L.; Emanuelli, M. Nicotinamide N-methyltransferase upregulation inversely correlates with lymph node metastasis in oral squamous cell carcinoma. Mol. Med. 2007, 13, 415–421.
- Emanuelli, M.; Santarelli, A.; Sartini, D.; Ciavarella, D.; Rossi, V.; Pozzi, V.; Rubini, C.; Lo Muzio, L. Nicotinamide N-methyltransferase upregulation correlates with tumour differentiation in oral squamous cell carcinoma. Histol. Histopathol. 2010, 25, 15–20.
- Pozzi, V.; Mazzotta, M.; Lo Muzio, L.; Sartini, D.; Santarelli, A.; Renzi, E.; Rocchetti, R.; Tomasetti, M.; Ciavarella, D.; Emanuelli, M. Inhibiting proliferation in KB cancer cells by RNA interference-mediated knockdown of nicotinamide N-methyltransferase expression. Int. J. Immunopathol. Pharmacol. 2011, 24, 69–77.
- Sartini, D.; Pozzi, V.; Renzi, E.; Morganti, S.; Rocchetti, R.; Rubini, C.; Santarelli, A.; Lo Muzio, L.; Emanuelli, M. Analysis of tissue and salivary nicotinamide N-methyltransferase in oral squamous cell carcinoma: Basis for the development of a noninvasive diagnostic test for early-stage disease. Biol. Chem. 2012, 393, 505–511.
- Pozzi, V.; Sartini, D.; Morganti, S.; Giuliante, R.; Di Ruscio, G.; Santarelli, A.; Rocchetti, R.; Rubini, C.; Tomasetti, M.; Giannatempo, G.; et al. RNA-mediated gene silencing of nicotinamide N-methyltransferase is associated with decreased tumorigenicity in human oral carcinoma cells. PLoS ONE 2013, 8, e71272.
- Ishibashi, K.; Ishii, K.; Sugiyama, G.; Sumida, T.; Sugiura, T.; Kamata, Y.U.; Seki, K.; Fujinaga, T.; Kumamaru, W.; Kobayashi, Y.; et al. Deregulation of Nicotinamide N-Methyltransferase and Gap Junction Protein Alpha-1 Causes Metastasis in Adenoid Cystic Carcinoma. Anticancer Res. 2018, 38, 187–197.
- Seta, R.; Mascitti, M.; Campagna, R.; Sartini, D.; Fumarola, S.; Santarelli, A.; Giuliani, M.; Cecati, M.; Lo Muzio, L.; Emanuelli, M. Overexpression of Nicotinamide N-Methyltransferase in HSC-2 OSCC cell line: Effect on apoptosis and cell proliferation. Clin. Oral. Investig. 2019, 23, 829–838.
- Cui, Y.; Zhang, L.; Wang, W.; Ma, S.; Liu, H.; Zang, X.; Zhang, Y.; Guan, F. Downregulation of nicotinamide N-methyltransferase inhibits migration and epithelial-mesenchymal transition of esophageal squamous cell carcinoma via Wnt/beta-catenin pathway. Mol. Cell. Biochem. 2019, 460, 93–103.
- Cui, Y.; Yang, D.; Wang, W.; Zhang, L.; Liu, H.; Ma, S.; Guo, W.; Yao, M.; Zhang, K.; Li, W.; et al. Nicotinamide N-methyltransferase decreases 5-fluorouracil sensitivity in human esophageal squamous cell carcinoma through metabolic reprogramming and promoting the Warburg effect. Mol. Carcinog. 2020, 59, 940–954.
- Win, K.T.; Lee, S.W.; Huang, H.Y.; Lin, L.C.; Lin, C.Y.; Hsing, C.H.; Chen, L.T.; Li, C.F. Nicotinamide N-methyltransferase overexpression is associated with Akt phosphorylation and indicates worse prognosis in patients with nasopharyngeal carcinoma. Tumour Biol. 2013, 34, 3923–3931.
- Xu, J.; Moatamed, F.; Caldwell, J.S.; Walker, J.R.; Kraiem, Z.; Taki, K.; Brent, G.A.; Hershman, J.M. Enhanced expression of nicotinamide N-methyltransferase in human papillary thyroid carcinoma cells. J. Clin. Endocrinol. Metab. 2003, 88, 4990–4996.
- Xu, J.; Capezzone, M.; Xu, X.; Hershman, J.M. Activation of nicotinamide N-methyltransferase gene promoter by hepatocyte nuclear factor-1beta in human papillary thyroid cancer cells. Mol. Endocrinol. 2005, 19, 527–539.
- Xu, J.; Hershman, J.M. Histone deacetylase inhibitor depsipeptide represses nicotinamide N-methyltransferase and hepatocyte nuclear factor-1beta gene expression in human papillary thyroid cancer cells. Thyroid 2006, 16, 151–160.
- Mascitti, M.; Sartini, D.; Togni, L.; Pozzi, V.; Rubini, C.; Santarelli, A.; Emanuelli, M. Differential expression of nicotinamide N-methyltransferase in primary and recurrent ameloblastomas and odontogenic keratocysts. Eur. J. Clin. Invest. 2020, 50, e13220.
- Markert, J.M.; Fuller, C.M.; Gillespie, G.Y.; Bubien, J.K.; McLean, L.A.; Hong, R.L.; Lee, K.; Gullans, S.R.; Mapstone, T.B.; Benos, D.J. Differential gene expression profiling in human brain tumors. Physiol. Genomics 2001, 5, 21–33.
- Ganzetti, G.; Sartini, D.; Campanati, A.; Rubini, C.; Molinelli, E.; Brisigotti, V.; Cecati, M.; Pozzi, V.; Campagna, R.; Offidani, A.; et al. Nicotinamide N-methyltransferase: Potential involvement in cutaneous malignant melanoma. Melanoma Res. 2018, 28, 82–88.
- Mascitti, M.; Santarelli, A.; Sartini, D.; Rubini, C.; Colella, G.; Salvolini, E.; Ganzetti, G.; Offidani, A.; Emanuelli, M. Analysis of nicotinamide N-methyltransferase in oral malignant melanoma and potential prognostic significance. Melanoma Res. 2019, 29, 151–156.
- Pompei, V.; Salvolini, E.; Rubini, C.; Lucarini, G.; Molinelli, E.; Brisigotti, V.; Pozzi, V.; Sartini, D.; Campanati, A.; Offidani, A.; et al. Nicotinamide N-methyltransferase in nonmelanoma skin cancers. Eur. J. Clin. Investig. 2019, 49, e13175.
- Hah, Y.S.; Cho, H.Y.; Jo, S.Y.; Park, Y.S.; Heo, E.P.; Yoon, T.J. Nicotinamide N-methyltransferase induces the proliferation and invasion of squamous cell carcinoma cells. Oncol. Rep. 2019, 42, 1805–1814.
- Sartini, D.; Pompei, V.; Lucarini, G.; Rubini, C.; Molinelli, E.; Brisigotti, V.; Salvolini, E.; Campanati, A.; Offidani, A.; Emanuelli, M. Differential expression of nicotinamide n-methyltransferase in cutaneous keratoacanthoma and squamous cell carcinoma: An immunohistochemical study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e121–e123.
- Campagna, R.; Salvolini, E.; Pompei, V.; Pozzi, V.; Salvucci, A.; Molinelli, E.; Brisigotti, V.; Sartini, D.; Campanati, A.; Offidani, A.; et al. Nicotinamide N-Methyltransferase Gene Silencing Enhances Chemosensitivity of Melanoma Cell Lines. Pigment Cell Melanoma Res. 2021, in press.
- D’Andrea, F.P.; Safwat, A.; Kassem, M.; Gautier, L.; Overgaard, J.; Horsman, M.R. Cancer stem cell overexpression of nicotinamide N-methyltransferase enhances cellular radiation resistance. Radiother. Oncol. 2011, 99, 373–378.
- Ulanovskaya, O.A.; Zuhl, A.M.; Cravatt, B.F. NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat. Chem. Biol. 2013, 9, 300–306.
- Pozzi, V.; Sartini, D.; Rocchetti, R.; Santarelli, A.; Rubini, C.; Morganti, S.; Giuliante, R.; Calabrese, S.; Di Ruscio, G.; Orlando, F.; et al. Identification and Characterization of Cancer Stem Cells from Head and Neck Squamous Cell Carcinoma Cell Lines. Cell. Physiol. Biochem. 2015, 36, 784–798.
- Jung, J.; Kim, L.J.; Wang, X.; Wu, Q.; Sanvoranart, T.; Hubert, C.G.; Prager, B.C.; Wallace, L.C.; Jin, X.; Mack, S.C.; et al. Nicotinamide metabolism regulates glioblastoma stem cell maintenance. JCI Insight 2017, 2, e90019.
- Pozzi, V.; Salvolini, E.; Lucarini, G.; Salvucci, A.; Campagna, R.; Rubini, C.; Sartini, D.; Emanuelli, M. Cancer stem cell enrichment is associated with enhancement of nicotinamide N-methyltransferase expression. IUBMB Life 2020, 72, 1415–1425.
- Zhang, J.; Wang, Y.; Li, G.; Yu, H.; Xie, X. Down-regulation of nicotinamide N-methyltransferase induces apoptosis in human breast cancer cells via the mitochondria-mediated pathway. PLoS ONE 2014, 9, e89202.
- Wang, Y.; Zeng, J.; Wu, W.; Xie, S.; Yu, H.; Li, G.; Zhu, T.; Li, F.; Lu, J.; Wang, G.Y.; et al. Nicotinamide N-methyltransferase enhances chemoresistance in breast cancer through SIRT1 protein stabilization. Breast Cancer Res. 2019, 21, 64.
- Yu, H.; Zhou, X.; Wang, Y.; Huang, X.; Yang, J.; Zeng, J.; Li, G.; Xie, X.; Zhang, J. Nicotinamide N-methyltransferase inhibits autophagy induced by oxidative stress through suppressing the AMPK pathway in breast cancer cells. Cancer Cell Int. 2020, 20, 191.
- Akar, S.; Harmankaya, İ.; Uğraş, S.; Çelik, Ç. Nicotinamide N-methyltransferase expression and its association with phospho-Akt, p53 expression, and survival in high-grade endometrial cancer. Turk. J. Med. Sci. 2019, 49, 1547–1554.
- Akar, S.; Harmankaya, İ.; Uğraş, S.; Çelik, Ç. Expression and Clinical Significance of Nicotinamide N-Methyltransferase in Cervical Squamous Cell Carcinoma. Int. J. Gynecol. Pathol. 2020, 39, 289–295.
- Harmankaya, İ.; Akar, S.; Uğraş, S.; Güler, A.H.; Ezveci, H.; Aydoğdu, M.; Çelik, Ç. Nicotinamide N-methyltransferase overexpression may be associated with poor prognosis in ovarian cancer. J. Obstet. Gynaecol. 2020, 14, 1–6.
- Kwon, Y.; Song, J.; Lee, H.; Kim, E.Y.; Lee, K.; Lee, S.K.; Kim, S. Design, Synthesis, and Biological Activity of Sulfonamide Analogues of Antofine and Cryptopleurine as Potent and Orally Active Antitumor Agents. J. Med. Chem. 2015, 58, 7749–7762.
- Sartini, D.; Muzzonigro, G.; Milanese, G.; Pozzi, V.; Vici, A.; Morganti, S.; Rossi, V.; Mazzucchelli, R.; Montironi, R.; Emanuelli, M. Upregulation of tissue and urinary nicotinamide N-methyltransferase in bladder cancer: Potential for the development of a urine-based diagnostic test. Cell Biochem. Biophys. 2013, 65, 473–483.
- Riester, M.; Taylor, J.M.; Feifer, A.; Koppie, T.; Rosenberg, J.E.; Downey, R.J.; Bochner, B.H.; Michor, F. Combination of a novel gene expression signature with a clinical nomogram improves the prediction of survival in high-risk bladder cancer. Clin. Cancer Res. 2012, 18, 1323–1333.
- Pozzi, V.; Di Ruscio, G.; Sartini, D.; Campagna, R.; Seta, R.; Fulvi, P.; Vici, A.; Milanese, G.; Brandoni, G.; Galosi, A.B.; et al. Clinical performance and utility of a NNMT-based urine test for bladder cancer. Int. J. Biol. Mark. 2018, 33, 94–101.
- Kassem, H.S.; Sangar, V.; Cowan, R.; Clarke, N.; Margison, G.P. A potential role of heat shock proteins and nicotinamide N-methyl transferase in predicting response to radiation in bladder cancer. Int. J. Cancer 2002, 101, 454–460.
- Wu, Y.; Siadaty, M.S.; Berens, M.E.; Hampton, G.M.; Theodorescu, D. Overlapping gene expression profiles of cell migration and tumor invasion in human bladder cancer identify metallothionein 1E and nicotinamide N-methyltransferase as novel regulators of cell migration. Oncogene 2008, 27, 6679–6689.
- Novak Kujundžić, R.; Prpić, M.; Đaković, N.; Dabelić, N.; Tomljanović, M.; Mojzeš, A.; Fröbe, A.; Trošelj, K.G. Nicotinamide N-Methyltransferase in Acquisition of Stem Cell Properties and Therapy Resistance in Cancer. Int. J. Mol. Sci. 2021, 22, 5681.




