Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sahng G Kim | + 2448 word(s) | 2448 | 2021-05-09 12:54:08 | | | |
| 2 | Lily Guo | Meta information modification | 2448 | 2021-06-03 03:50:35 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kim, S.G. Dental Pulp Regeneration. Encyclopedia. Available online: https://encyclopedia.pub/entry/10443 (accessed on 08 February 2026).
Kim SG. Dental Pulp Regeneration. Encyclopedia. Available at: https://encyclopedia.pub/entry/10443. Accessed February 08, 2026.
Kim, Sahng G. "Dental Pulp Regeneration" Encyclopedia, https://encyclopedia.pub/entry/10443 (accessed February 08, 2026).
Kim, S.G. (2021, June 02). Dental Pulp Regeneration. In Encyclopedia. https://encyclopedia.pub/entry/10443
Kim, Sahng G. "Dental Pulp Regeneration." Encyclopedia. Web. 02 June, 2021.
Copy Citation
Dental pulp regeneration requires an integrated use of three key elements, including cells, biomaterial scaffolds, and signaling molecules, which enables the recapitulation of biological processes for normal tissue development.
Dental Pulp Regeneration
mesenchymal stem cells
1. Introduction
Dental pulp regeneration requires an integrated use of three key elements, including cells, biomaterial scaffolds, and signaling molecules, which enables the recapitulation of biological processes for normal tissue development [1][2][3][4][5][6]. Various combinations of this triad have been used to regenerate the pulp–dentin complex [3][4][5]. Among them, transplantation of mesenchymal stem cells with the aid of biomaterial scaffolds or signaling molecules has been used as a major tissue engineering strategy [5]. This approach is based on the beneficial effects of transplanted stem cells, which can augment the regenerative processes of a variety of tissues [7][8][9][10][11]. The transplanted stem cells regenerate the parenchyma of a tissue as a building block following cell differentiation [12][13]. Furthermore, the stem cells release a multitude of biological trophic factors [14], which can modulate the immune function and promote regenerative cellular events, such as mobilization, proliferation, and differentiation of resident cells, in addition to enhancing angiogenesis and neurogenesis [15][16].
Due to stem cell plasticity induced by cell reprogramming in response to local instructive cues [17][18], various types of stem cells can be candidates for dental pulp regeneration. However, there still remains a degree of lineage commitment and differentiation associated with adult mesenchymal stem cells [18]. Tissue-specific mesenchymal stem cells such as dental pulp stem cells (DPSC) and their subpopulations have been most widely used to regenerate the pulp–dentin complex [19][20][21][22][23][24][25][26][27][28][29][30][31][32]. Other mesenchymal stem cells such as bone marrow stem cells (BMSC) [26][28], adipose-derived stem cells (ADSC) [20][26][28], and stem cells from human exfoliated deciduous teeth (SHED) [33][34] have also been tested for dental pulp regeneration.
2. Overview
Dental pulp regeneration has been a clinically approved therapy by the American Dental Association since January 2011 [4]. However, there is still an unmet clinical need for regeneration of pulp tissue, which is particularly important for patients with various degrees of pulpal and periapical infection. There have been a multitude of studies showing that pulp regeneration therapy with evoked bleeding and no stem cell transplantation has yielded tissues mimicking periodontal tissue, such as cementum, bone, and periodontal ligament [35][36][37]. The goal of pulp regeneration therapy for diseased teeth is to reconstitute the pulp–dentin complex [3][4], and the current clinical protocols may fail to achieve this goal [5].
There have been several systematic reviews concerning stem-cell-based pulp regeneration [38][39][40]. The previous systematic reviews identified pulp and dentin regeneration in their included studies using in situ stem cell transplantation strategies [38][39][40], as in this study. The present scoping review included several more current studies based on eligibility criteria than the previous systematic reviews. With the data analysis involving more studies, this review could provide more up-to-date information focusing on tissue engineering protocols for the clinical translation and applicability of a cell-based approach to dental pulp regeneration. A meta-analysis was not performed in the present review due to the significant heterogeneity of animal models, transplanted cells, scaffolds, and growth factors among the included studies.
Successful tissue regeneration is driven by the tissue engineering triad, including stem cells, biomaterial scaffolds, and signaling molecules [6]. The usage of mesenchymal stem cell transplantation for pulp regeneration has recently attracted increasing attention due to promising reported outcomes. This scoping review has focused on orthotopic de novo pulp regeneration in animal studies and clinical trials because ectopic animal models using cell transplantation in renal capsules [41] or subcutaneous tissues [42][43] cannot simulate local microenvironments that significantly contribute to the regenerative processes of transplanted stem cells, and thus are less clinically relevant. A total of 17 studies that used de novo orthotopic pulp regeneration models, including 15 animal studies and two clinical trials, were identified through the selection processes. All animal studies using stem cell transplantation except for two studies that used a PRP scaffold [29][30] revealed the regeneration of vascularized pulp-like tissue. Autologous cell transplantation in two clinical studies [32][34] also successfully regenerated vascularized pulp-like tissue, as revealed by the findings from pulp vitality testing, CBCT, or MRI.
The presence of the previous infection may affect stem cell functions and regeneration outcomes because it significantly alters regenerative processes by devastating essential regenerative microenvironments and constructive stem or progenitor cells [44]. Indeed, the histologic observations from human and animal studies have shown that tissues formed in the previously infected canals are mostly of periodontal origin, such as cementum, bone, and periodontal ligament, rather than of pulpal origin [35][36][37][45][46]. In the present review, however, one animal study using an infection model with the induction of apical periodontitis showed robust pulp and dentin regeneration when autologous DPSC were transplanted [19]. More animal studies with an infection model should be conducted to prove whether the same efficacy of pulp regeneration identified in the present review could be achieved in the infection model.
Large animal models have been preferred in biomedical research due to better suitability for clinical translation [47][48]. Compared with a rodent model, dogs and pigs are considered more translatable models based on their biochemical, genetic, and physiological similarities to humans [31][47][48][49][50]. A dog model was identified to be the most common, followed by a pig model for orthotopic pulp regeneration in this review. Only one study used a rat model for cell transplantation in molars [27]. Incisors and premolars are most widely used in all large animal studies because these tooth types have more anatomical resemblance to human teeth and are more accessible when performing endodontic therapy than more posteriorly located molars.
The use of autologous cells is ideal for stem cell transplantation therapy because it circumvents safety issues, such as possible immune rejection and pathogen transmission between donors and recipients [51]. As shown in this review, autologous stem cells have been used in most animal studies and all clinical trials. However, autologous cell transplantation still encounters major hurdles for clinical translation, such as donor site morbidity, difficulties with cell isolation and ex vivo expansion, loss of cells and stemness during cryopreservation or banking, need for a GMP facility, and regulatory and economic barriers [4][52]. Allogeneic stem cell transplantation is proposed as an alternative strategy, and the efficacy of allogeneic stem cells for orthotopic pulp regeneration has been reported in several animal studies [24][25][31]. However, allogeneic stem cell transplantation suffers from immune-related problems as well as difficulties and barriers related to autologous cell transplantation [4][52]. No clinical trials with allogeneic stem cells for pulp regeneration have been identified yet.
The selection of appropriate stem cells for cell transplantation is critical for successful pulp regeneration (Figure 1). The majority of the studies included in this review used DPSC subpopulations to augment the regenerative processes. Of the eight studies using DPSC subpopulations [20][21][22][23][24][25][26][28], MDPSC were used most commonly [21][22][23][24][25][28][32]. In five dog studies by Iohara et al. [21][22][23][24][25], MDPSC were isolated from autologous DPSC by G-CSF-induced chemotaxis for cell transplantation. The transplantation of MDPSC is thought to be advantageous for pulp regeneration because they release trophic factors that promote angiogenesis and neurogenesis and have high immunosuppressive properties [21][22][23][24][25]. Histological analysis showed that pulp-like tissue with vasculatures and nerves and dentin-like tissue with odontoblast-like cells were regenerated when MDPSC were transplanted [21][22][24][25]. The efficacy of regeneration was found to be significantly higher when MDPSC were transplanted with G-CSF compared with MDPSC alone or G-CSF alone [21]. Attenuation in inflammation and apoptosis was also observed when MDPSC and G-CSF were delivered together [21]. Another dog study by Murakami et al. [28] transplanting MDPSC also showed similar robust pulp–dentin regeneration with vasculature and nerves. In a clinical trial by Nakashima et al. [32], MRI revealed complete pulp regeneration in teeth with irreversible pulpitis when MDPSC were transplanted with G-CSF. The safety and feasibility of the use of autologous MDPSC and G-CSF for pulp regeneration were demonstrated in a pilot clinical trial [32]. Pulp CD105+ cells and CD31- SP cells were also shown to release a higher amount of angiogenic and neurotrophic factors and regenerated significantly higher volumes of vascularized and innervated pulp-like tissue than stem cells from other tissue origins [20][26]. In a dog study by Iohara et al. [20], greater pulp tissue regeneration was observed when pulp CD105+ cells were delivered compared with adipose CD105+ cells. In another dog study by Ishizaka et al. [26], pulp CD31- SP cell transplantation yielded significantly higher pulp regeneration than bone marrow CD31-SP cell transplantation. The transplantation of autologous, deciduous pulp stem cells for pulp regeneration has been shown to be safe and effective in an animal study and a randomized controlled trial. Other mesenchymal stem cells, such as subpopulations of BMSC and ADSC, have been suggested as alternative stem cell sources for pulp regeneration [20][26][28].
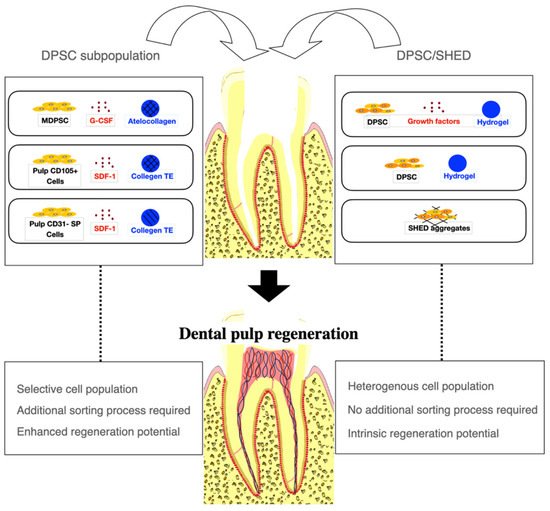
Figure 1. A cell-based approach to dental pulp regeneration. Dental pulp stem cell (DPSC) subpopulations have been most commonly used for dental pulp regeneration to augment regenerative processes. However, an additional cell sorting process is required. DPSC and Stem cells from human exfoliated deciduous teeth (SHED) have been used with or without growth factors or scaffold materials. Robust pulp and dentin regeneration has been observed in animal studies using either DPSC subpopulations or DPSC, although the efficacy of regeneration may be enhanced with DPSC subpopulations. MDPSC: mobilized dental pulp stem cells; G-CSF: granulocyte-colony stimulating factor; SDF-1: stromal-derived factor-1; Collagen TE: collagen tissue equivalent (mixture of collagen type I and type III).
The incorporation of biomaterial scaffolds can facilitate the attachment, proliferation, and differentiation of transplanted stem cells. Injectable scaffolds such as hydrogels are highly desirable for successful pulp regeneration therapy because of their excellent conformity to the complex root anatomy and readiness of stem cell and growth factor delivery, while providing a structural framework for cellular functions [53]. Most of the included studies used collagen-derived scaffolds, among which atelocollagen was the most common type [21][22][23][24][25][28][32]. Atelocollagen is a collagen solution that can be easily introduced into root canals with stem cells and growth factors, forming a hydrogel under physiological conditions [21]. A clinical-grade atelocollagen scaffold was safely used in a pilot clinical trial [32]. Chitosan, which can be manufactured in the form of hydrogels, has been used for various regenerative applications in skin, cartilage, bone, fat, and nerve due to its favorable physical properties, such as its porosity for cellular growth and nutrient transport and its fluid absorption capacity, biodegradability, biocompatibility, non-immunogenicity, and antimicrobial activity [54]. Lyophilized chitosan hydrogels used in animal studies for pulp regeneration [19][55] employed the benefits of chitosan’s physicochemical properties in tissue regeneration, while allowing vascularization within the scaffold material [54]. PRP has been used for pulp regeneration therapy [29][30][56][57]. It can be useful for tissue regeneration since it contains multiple growth factors, such as PDGF, fibroblast growth factor, transforming growth factor β, and vascular endothelial growth factor [56]. However, the use of PRP as a scaffold did not aid in the regeneration of the pulp–dentin complex, as evidenced by the ectopic tissue formation in animal studies [29][30][58]. Therefore, caution should be exercised with the use of a PRP scaffold for pulp regeneration.
The use of signaling molecules is beneficial for regenerative therapy, since it can enhance the cellular activities of transplanted stem cells toward regeneration. In the present review, G-CSF was found to be most commonly used for pulp regeneration [20][21][22][23][24][25][32]. G-CSF has been originally approved by the United States Food and Drug Administration for use to decrease infection in patients with immunosuppressive cancer therapy [59]. For dental pulp regeneration, the use of G-CSF was proposed because regenerative biological events such as angiogenesis, neurogenesis, cell migration and proliferation, antiapoptosis, and immunosuppression were augmented by G-CSF [60][61]. The efficacy of the use of G-CSF coupled with MDPSC for pulp regeneration was shown in a pilot clinical trial [32] and several animal studies [20][21][22][23][24][25]. One animal study used a cocktail of growth factors including VEGF-2, bFGF, PDGF, NGF, and BMP7 for pulp regeneration by orchestrating cellular behaviors of transplanted DPSC and showed successful regeneration of vascularized pulp-like and dentin-like tissue [19]. Six studies did not use any exogenous signaling molecule because it was presumed that trophic factors released by transplanted stem cells, as well as local biological cues from conditioned dentin and resident cells, could serve as endogenous signaling molecules [27][29][30][31][33][34].
Complete pulp regeneration after stem cell transplantation may take approximately three months in animal models [23], although it appears to heavily depend on the age of the animals [22]. Trypsin pretreatment or use of nanobubbles may rescue age-dependent decline in regeneration potential [25]. Hypoxia priming of transplanted stem cells may be beneficial in promoting pulp regeneration by increasing vasculogenesis and odontoblastic differentiation [27].
This scoping review demonstrated that the regeneration of pulp-like tissue, dentin-like tissue, and odontoblast-like cells lining the dentin, blood vessels, and nerves could be achieved using the stem cell transplantation strategy in animal models. Furthermore, similar regeneration may be achieved in patients, as shown in clinical trials using autologous stem cells. From a clinical perspective, the tissue engineering protocols used in de novo orthotopic pulp regeneration models in the present review may be useful for translation into therapeutic applications. The analysis of data from the two clinical trials may also provide clinicians with more information to better implement a cell-based approach to dental pulp regeneration and bring this strategy to the forefront of patient care. However, the findings from this scoping review should be interpreted in the context of the following limitations. First, the overall risk of bias observed in animal studies was unclear and the overall risk of bias in clinical trials was moderate or judged to raise some concerns. Second, the long-term safety and efficacy of stem cell transplantation have not been reported yet. The longest follow-up period in the included studies was 24 months [34] for clinical trials and nine months [20] for animal studies. High-quality animal studies are needed to facilitate the clinical translation of this stem cell transplantation approach. More well-designed randomized controlled trials are also required to determine the safety and efficacy of the therapeutic applications of mesenchymal stem cells for dental pulp regeneration.
3. Conclusions
Stem cell transplantation is a major in vivo pulp regeneration approach. DPSC and their subpopulations have served as the main mesenchymal stem cell sources for pulp regeneration due to their high trophic and immunomodulatory effects, as well as their tissue specificity. The use of autologous stem cells has been suggested to be more clinically translatable, as it avoids possible immune-related issues. The safety and efficacy of autologous and allogeneic stem cell transplantation have been shown in animal studies. In two clinical trials, autologous pulp stem cell transplantation generated vascularized and innervated pulp-like tissues, as shown by pulp vitality testing and imaging analyses, and their safe application was confirmed with follow-ups for up to two years. There still remain barriers, such as difficulty with cell isolation and ex vivo expansion, the need for a cell banking system and a GMP facility, and regulatory approval, which are yet to be overcome for clinical translation. More high-quality randomized clinical trials are needed to further confirm the safety and efficacy of the stem cell transplantation strategy for dental pulp regeneration.
References
- Pashley, D. Dynamics of the Pulpo-Dentin Complex. Crit. Rev. Oral Biol. Med. 1996, 7, 104–133.
- Mao, J.J.; Prockop, D.J. Stem Cells in the Face: Tooth Regeneration and Beyond. Cell Stem Cell 2012, 11, 291–301.
- He, L.; Zhou, J.; Chen, M.; Lin, C.-S.; Kim, S.G.; Zhou, Y.; Xiang, L.; Xie, M.; Bai, H.; Yao, H.; et al. Parenchymal and stromal tissue regeneration of tooth organ by pivotal signals reinstated in decellularized matrix. Nat. Mater. 2019, 18, 627–637.
- Mao, J.J.; Kim, S.G.; Zhou, J.; Ye, L.; Cho, S.; Suzuki, T.; Fu, S.Y.; Yang, R.; Zhou, X. Regenerative endodontics: Barriers and strategies for clinical translation. Dent. Clin. N. Am. 2012, 56, 639–649.
- Kim, S.G.; Malek, M.; Sigurdsson, A.; Lin, L.M.; Kahler, B. Regenerative endodontics: A comprehensive review. Int. Endod. J. 2018, 51, 1367–1388.
- Vacanti, J.P.; Langer, R. Tissue engineering: The design and fabrication of living replacement devices for surgical recon-struction and transplantation. Lancet 1999, 354, SI32–SI34.
- Bruder, S.P.; Kurth, A.A.; Shea, M.; Hayes, W.C.; Jaiswal, N.; Kadiyala, S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J. Orthop. Res. 1998, 16, 155–162.
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human mesenchymal stem cells differentiate to a cardio-myocyte phenotype in the adult murine heart. Circulation 2002, 105, 93–98.
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228.
- Young, R.G.; Butler, D.L.; Weber, W.; Caplan, A.I.; Gordon, S.L.; Fink, D.J. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J. Orthop. Res. 1998, 16, 406–413.
- Black, I.B.; Woodbury, D. Adult Rat and Human Bone Marrow Stromal Stem Cells Differentiate into Neurons. Blood Cells, Mol. Dis. 2001, 27, 632–636.
- Sacco, A.; Doyonnas, R.; Kraft, P.; Vitorovic, S.; Blau, H.M. Self-renewal and expansion of single transplanted muscle stem cells. Nature 2008, 456, 502–506.
- Fox, I.J.; Daley, G.Q.; Goldman, S.A.; Huard, J.; Kamp, T.J.; Trucco, M. Use of differentiated pluripotent stem cells in replacement therapy for treating disease. Science 2014, 345, 1247391.
- Fu, Y.; Karbaat, L.; Wu, L.; Leijten, J.; Both, S.K.; Karperien, M. Trophic effects of mesenchymal stem cells in tissue regeneration. Tissue Eng. Part B Rev. 2017, 23, 515–528.
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 1–4.
- Blanpain, C.; Fuchs, E. Plasticity of epithelial stem cells in tissue regeneration. Science 2014, 344, 1242281.
- Frisén, J. Stem Cell Plasticity? Neuron 2002, 35, 415–418.
- Saidova, A.A.; Vorobjev, I.A. Lineage Commitment, Signaling Pathways, and the Cytoskeleton Systems in Mesenchymal Stem Cells. Tissue Eng. Part B: Rev. 2020, 26, 13–25.
- El Ashiry, E.A.; AlAmoudi, N.M.; El Ashiry, M.K.; Bastawy, H.A.; El Derwi, D.A.; Atta, H.M. Tissue Engineering of Necrotic Dental Pulp of Immature Teeth with Apical Periodontitis in Dogs: Radiographic and Histological Evaluation. J. Clin. Pediatr. Dent. 2018, 42, 373–382.
- Iohara, K.; Imabayashi, K.; Ishizaka, R.; Watanabe, A.; Nabekura, J.; Ito, M.; Matsushita, K.; Nakamura, H.; Nakashima, M. Complete Pulp Regeneration After Pulpectomy by Transplantation of CD105+ Stem Cells with Stromal Cell-Derived Factor-1. Tissue Eng. Part A 2011, 17, 1911–1920.
- Iohara, K.; Murakami, M.; Takeuchi, N.; Osako, Y.; Ito, M.; Ishizaka, R.; Utunomiya, S.; Nakamura, H.; Matsushita, K.; Nakashima, M. A novel combinatorial therapy with pulp stem cells and granulocyte colony-stimulating factor for total pulp regeneration. Stem Cells Trans. Med. 2013, 2, 521–533.
- Iohara, K.; Murakami, M.; Nakata, K.; Nakashima, M. Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp. Gerontol. 2014, 52, 39–45.
- Iohara, K.; Fujita, M.; Ariji, Y.; Yoshikawa, M.; Watanabe, H.; Takashima, A.; Nakashima, M. Assessment of pulp regeneration induced by stem cell therapy by magnetic resonance imaging. J. Endod. 2016, 42, 397–401.
- Iohara, K.; Utsunomiya, S.; Kohara, S.; Nakashima, M. Allogeneic transplantation of mobilized dental pulp stem cells with the mismatched dog leukocyte antigen type is safe and efficacious for total pulp regeneration. Stem Cell Res. Ther. 2018, 9, 1–16.
- Iohara, K.; Zayed, M.; Takei, Y.; Watanabe, H.; Nakashima, M. Treatment of Pulpectomized Teeth with Trypsin Prior to Transplantation of Mobilized Dental Pulp Stem Cells Enhances Pulp Regeneration in Aged Dogs. Front. Bioeng. Biotechnol. 2020, 8, 983.
- Ishizaka, R.; Iohara, K.; Murakami, M.; Fukuta, O.; Nakashima, M. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials 2012, 33, 2109–2118.
- Kuang, R.; Zhang, Z.; Jin, X.; Hu, J.; Shi, S.; Ni, L.; Ma, P.X. Nanofibrous spongy microspheres for the delivery of hypox-ia-primed human dental pulp stem cells to regenerate vascularized dental pulp. Acta Biomater. 2016, 33, 225–234.
- Murakami, M.; Hayashi, Y.; Iohara, K.; Osako, Y.; Hirose, Y.; Nakashima, M. Trophic effects and regenerative potential of mobilized mesenchymal stem cells from bone marrow and adipose tissue as alternative cell sources for pulp/dentin regeneration. Cell Transplant. 2015, 24, 1753–1765.
- Zhu, X.; Zhang, C.; Huang, G.T.-J.; Cheung, G.S.; Dissanayaka, W.L.; Zhu, W. Transplantation of Dental Pulp Stem Cells and Platelet-rich Plasma for Pulp Regeneration. J. Endod. 2012, 38, 1604–1609.
- Zhu, X.; Wang, Y.; Liu, Y.; Huang, G.T.; Zhang, C. Immunohistochemical and histochemical analysis of newly formed tis-sues in root canal space transplanted with dental pulp stem cells plus platelet-rich plasma. J. Endod. 2014, 40, 1573–1578.
- Zhu, X.; Liu, J.; Yu, Z.; Chen, C.-A.; Aksel, H.; Azim, A.A.; Huang, G.T.-J. A Miniature Swine Model for Stem Cell-Based De Novo Regeneration of Dental Pulp and Dentin-Like Tissue. Tissue Eng. Part C Methods 2018, 24, 108–120.
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplan-tation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 1–3.
- Guo, H.; Zhao, W.; Liu, A.; Wu, M.; Shuai, Y.; Li, B.; Huang, X.; Liu, X.; Yang, X.; Guo, X.; et al. SHED promote angiogenesis in stem cell-mediated dental pulp regeneration. Biochem. Biophys. Res. Commun. 2020, 529, 1158–1164.
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227.
- Becerra, P.; Ricucci, D.; Loghin, S.; Gibbs, J.L.; Lin, L.M. Histologic Study of a Human Immature Permanent Premolar with Chronic Apical Abscess after Revascularization/Revitalization. J. Endod. 2014, 40, 133–139.
- Lei, L.; Chen, Y.; Zhou, R.; Huang, X.; Cai, Z. Histologic and immunohistochemical findings of a human immature permanent tooth with apical periodontitis after regenerative endodontic treatment. J. Endod. 2015, 41, 1172–1179.
- Saoud, T.M.A.; Zaazou, A.; Nabil, A.; Moussa, S.; Aly, H.M.; Okazaki, K.; Rosenberg, P.A.; Lin, L.M. Histological observations of pulpal replacement tissue in immature dog teeth after revascularization of infected pulps. Dent. Traumatol. 2015, 31, 243–249.
- Conde, M.C.M.; Chisini, L.A.; Demarco, F.F.; Nör, J.E.; Casagrande, L.; Tarquinio, S.B.C. Stem cell-based pulp tissue engineering: Variables enrolled in translation from the bench to the bedside, a systematic review of literature. Int. Endod. J. 2015, 49, 543–550.
- Fawzy El-Sayed, K.M.; Ahmed, G.M.; Abouauf, E.A.; Schwendicke, F. Stem/progenitor cell-mediated pulpal tissue regeneration: A systematic review and meta-analysis. Int. Endod. J. 2019, 52, 1573–1585.
- El-Sayed, K.M.F.; Jakusz, K.; Jochens, A.; Dörfer, C.; Schwendicke, F. Stem Cell Transplantation for Pulpal Regeneration: A Systematic Review. Tissue Eng. Part B Rev. 2015, 21, 451–460.
- Wang, L.; Yan, M.; Wang, Y.; Lei, G.; Yu, Y.; Zhao, C.; Tang, Z.; Zhang, G.; Tang, C.; Yu, J.; et al. Proliferation and os-teo/odontoblastic differentiation of stem cells from dental apical papilla in mineralization-inducing medium containing additional KH(2)PO(4). Cell Prolif. 2013, 46, 214–222.
- Dissanayaka, W.L.; Hargreaves, K.M.; Jin, L.; Samaranayake, L.P.; Zhang, C. The interplay of dental pulp stem cells and endothelial cells in an injectable peptide hydrogel on angiogenesis and pulp regeneration in vivo. Tissue Eng. Part A 2015, 21, 550–563.
- Horibe, H.; Murakami, M.; Iohara, K.; Hayashi, Y.; Takeuchi, N.; Takei, Y.; Kurita, K.; Nakashima, M. Isolation of a Stable Subpopulation of Mobilized Dental Pulp Stem Cells (MDPSCs) with High Proliferation, Migration, and Regeneration Potential Is Independent of Age. PLoS ONE 2014, 9, e98553.
- Kim, S.G. Infection and pulp regeneration. Dent. J. 2016, 4, 4.
- Wang, X.; Thibodeau, B.; Trope, M.; Lin, L.M.; Huang, G.T.-J. Histologic Characterization of Regenerated Tissues in Canal Space after the Revitalization/Revascularization Procedure of Immature Dog Teeth with Apical Periodontitis. J. Endod. 2010, 36, 56–63.
- Gomes-Filho, J.E.; Duarte, P.C.T.; Ervolino, E.; Bomfim, S.R.M.; Abimussi, C.J.X.; Santos, L.M.D.S.; Lodi, C.S.; De Oliveira, S.H.P.; Dezan, E.; Cintra, L.T.A. Histologic Characterization of Engineered Tissues in the Canal Space of Closed-apex Teeth with Apical Periodontitis. J. Endod. 2013, 39, 1549–1556.
- Parker, H.G.; Shearin, A.L.; Ostrander, E.A. Man’s best friend becomes biology’s best in show: Genome analyses in the domestic dog. Ann. Rev. Genet. 2010, 44, 309–336.
- Volk, S.W.; Theoret, C. Translating stem cell therapies: The role of companion animals in regenerative medicine. Wound Repair Regen. 2013, 21, 382–394.
- Nakashima, M.; Iohara, K.; Bottino, M.C.; Fouad, A.F.; Nör, J.E.; Huang, G.T. Animal models for stem cell-based pulp re-generation: Foundation for human clinical applications. Tissue Eng. Part B Rev. 2019, 25, 100–113.
- Štembírek, J.; Kyllar, M.; Putnová, I.; Stehlík, L.; Buchtová, M. The pig as an experimental model for clinical craniofacial research. Lab. Anim. 2012, 46, 269–279.
- Prasongchean, W.; Ferretti, P. Autologous stem cells for personalised medicine. New Biotechnol. 2012, 29, 641–650.
- Kim, S.G.; Zheng, Y.; Zhou, J.; Chen, M.; Embree, M.C.; Song, K.; Jiang, N.; Mao, J.J. Dentin and dental pulp regeneration by the patient’s endogenous cells. Endod. Top. 2013, 28, 106–117.
- Erisken, C.; Kalyon, D.M.; Zhou, J.; Kim, S.G.; Mao, J.J. Viscoelastic Properties of Dental Pulp Tissue and Ramifications on Biomaterial Development for Pulp Regeneration. J. Endod. 2015, 41, 1711–1717.
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. Biomed. Res. Int. 2015, 2015, 821279.
- Palma, P.J.; Ramos, J.C.; Martins, J.B.; Diogenes, A.; Figueiredo, M.H.; Ferreira, P.; Viegas, C.; Santos, J.M. Histologic evaluation of regenerative endodontic procedures with the use of chitosan scaffolds in immature dog teeth with apical periodontitis. J. Endod. 2017, 43, 1279–1287.
- Xu, J.; Gou, L.; Zhang, P.; Li, H.; Qiu, S. Platelet-rich plasma and regenerative dentistry. Aust. Dent. J. 2020, 65, 131–142.
- Elsheshtawy, A.S.; Nazzal, H.; El Shahawy, O.I.; El Baz, A.A.; Ismail, S.M.; Kang, J.; Ezzat, K.M. The effect of platelet-rich plasma as a scaffold in regeneration/revitalization endodontics of immature permanent teeth assessed using 2-dimensional radiographs and cone beam computed tomography: A randomized controlled trial. Int. Endod. J. 2020, 53, 905–921.
- Torabinejad, M.; Milan, M.; Shabahang, S.; Wright, K.R.; Faras, H. Histologic Examination of Teeth with Necrotic Pulps and Periapical Lesions Treated with 2 Scaffolds: An Animal Investigation. J. Endod. 2015, 41, 846–852.
- Mehta, H.M.; Malandra, M.; Corey, S.J. G-CSF and GM-CSF in Neutropenia. J. Immunol. 2015, 195, 1341–1349.
- Lee, M.; Aoki, M.; Kondo, T.; Kobayashi, K.; Okumura, K.; Komori, K.; Murohara, T. Therapeutic angiogenesis with intra-muscular injection of low-dose recombinant granulocyte-colony stimulating factor. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2535–2541.
- Pan, H.-C.; Wu, H.-T.; Cheng, F.-C.; Chen, C.-H.; Sheu, M.-L.; Chen, C.-J. Potentiation of angiogenesis and regeneration by G-CSF after sciatic nerve crush injury. Biochem. Biophys. Res. Commun. 2009, 382, 177–182.
More
Information
Subjects:
Dentistry, Oral Surgery & Medicine
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.7K
Revisions:
2 times
(View History)
Update Date:
03 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




