Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohsen Fallah Vostakola | + 2990 word(s) | 2990 | 2021-09-08 08:19:52 | | | |
| 2 | Rita Xu | Meta information modification | 2990 | 2021-09-09 05:09:40 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fallah Vostakola, M. High-Temperature Polymer Electrolyte Membranes. Encyclopedia. Available online: https://encyclopedia.pub/entry/14012 (accessed on 08 February 2026).
Fallah Vostakola M. High-Temperature Polymer Electrolyte Membranes. Encyclopedia. Available at: https://encyclopedia.pub/entry/14012. Accessed February 08, 2026.
Fallah Vostakola, Mohsen. "High-Temperature Polymer Electrolyte Membranes" Encyclopedia, https://encyclopedia.pub/entry/14012 (accessed February 08, 2026).
Fallah Vostakola, M. (2021, September 08). High-Temperature Polymer Electrolyte Membranes. In Encyclopedia. https://encyclopedia.pub/entry/14012
Fallah Vostakola, Mohsen. "High-Temperature Polymer Electrolyte Membranes." Encyclopedia. Web. 08 September, 2021.
Copy Citation
Sufficient access to clean energy sources is one of the ongoing key challenges for global development that directly impacts industrial development, economic growth, and human well-being. Historically, the energy sector is widely dominated based on fossil fuels (such as petroleum fuels, natural gas, coal, etc.), which are the primary sources of carbon dioxide (CO2) and other greenhouse gases emissions in the environment.
polymer electrolyte membranes
high temperature
proton conductivity
polybenzimidazole
1. Introduction
Sufficient access to clean energy sources is one of the ongoing key challenges for global development that directly impacts industrial development, economic growth, and human well-being. Historically, the energy sector is widely dominated based on fossil fuels (such as petroleum fuels, natural gas, coal, etc.), which are the primary sources of carbon dioxide (CO2) and other greenhouse gases emissions in the environment. This has fundamentally driven a global climate change that has been accelerated over the past few decades and hence needs significant and immediate actions in order to alter both the energy sources and energy conversion techniques. There is a growing movement by the research and manufacturing communities to alleviate the impact of the petroleum-based economy by developing clean energy sources for implementing an alternative hydrogen-based economy [1][2][3]. Developing new clean energy supplies requires abundant access to sustainable energy sources. Thus, the energy storage systems such as lithium-ion batteries, redox flow batteries, and other fuel-cell-based power-to-gas technologies should be well integrated side by side, while developing the new capabilities of renewable energy sources. The lithium-ion battery is one of the most promising energy storage candidates in the portable and auxiliary device markets due to its high power density, environmental friendliness, and long service life [4]. By contrast, redox flow (cell) batteries can be used for large-scale energy storage applications. Their unique design leads to a higher power-to-capacity ratio from about 1:10 to 1:4. However, their toxicity, corrosivity, and high costs of the electrolyte solutions as well as low charge/discharge rate (1–10 h) and relatively low energy density have limited their applications [5][6][7]. Given the current environmental challenges associated with fossil fuels, fuel cell technology has been introduced as a promising, cleaner, high energy density, and more efficient power generation system [2][8][9]. The first fuel cell to operate with hydrogen and oxygen was developed about 150 years ago by Sir William Grove and was then further studied by developing many other sorts of fuel cells within the 19th century [8][10]. Lately, polymer electrolyte membrane (PEM) fuel cells, which directly convert the chemical energy of hydrogen into electrical energy, were predominantly developed as the most common commercial fuel cell [11][12]. Hydrogen gas (as a fuel with the highest energy density) and oxygen (usually taken from the air) are being used in a PEM fuel cells over the surface of electrodes to produce water through a few electrochemical reactions that are associated with the electrical power generation [13]. Structurally, a PEM fuel cell is comprised of an electrolyte layer at the middle integrated with anode and cathode electrodes over that, along with a current collector layer on top of each side of the cell. The polymer electrolyte membrane is a protonic conductor and electronic insulator sandwiched between a pair of electrodes [14]. The PEM fuel cells are classified into two important types, including low-temperature PEM (LT-PEM) and high-temperature PEM (HT-PEM) fuel cells [15][16]. Direct ethanol fuel cells [17] and direct methanol fuel cells [18] are the subset of PEM fuel cells. Figure 1 summarizes the trend of the total publication records (yearly and cumulative) in “HT-PEM fuel cell” according to the “Web of Science” indexing database till September 2020. This histogram shows gradual growth of the number of publications starting in 2003 and reaches about 1100 papers in 2015, followed by an almost linear decline in the past few years.
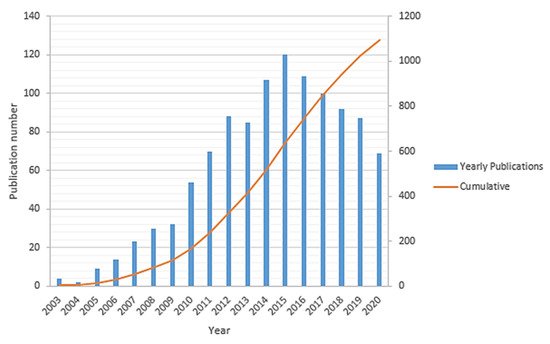
Figure 1. The number of yearly and total publications on HT-PEM fuel cell research field from the WoS indexing database.
VOSviewer application [19][20] (a social network analysis software) was used to qualitatively analyse the network of the published records in the HT-PEM subject within the last couple of decades. The main keywords that have received at least ten occurrences were identified and used to map a connection network model represented in Figure 2. This map reveals the main keywords and categorises them in accordance with their co-occurrence. The size of each node represents the relative importance of a keyword in the literature, and the distance among keywords implies the probability of co-occurrence and relatedness. According to this figure, the most frequent keywords regarding HT-PEMFCs are related to their performance, conductivity, polybenzimidazole (PBI) membranes, acid, composite/nanocomposite membranes, and cell degradation/durability. Many researchers have focused on the performance of cell performance and improving cell conductivity. PBI does not have proton conductivity without acid doping; acid-doped PBI membranes showed the promising electrochemical performance to be used in high-temperature applications [21]. Another practical approach for improving cell performance is the fabrication of polymer composite/nanocomposite membranes which can improve proton conductivity, water uptake/retention, thermal/chemical durability, mechanical strength, etc. [22][23][24].
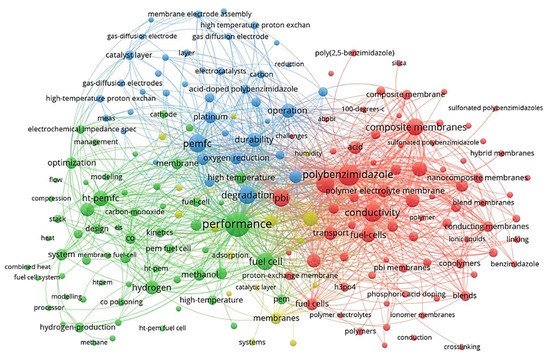
Figure 2. The overview of main keyword clusters in HT-PEM fuel cell research field, representing the main subfield.
2. Desirable Properties of PEMs
The PEM is the heart of the membrane electrode assemblies (MEAs), which are used for the fabrication of the PEM fuel cell stacks [25]. The PEM has a vital role in a fuel cell assembly by performing various functions such as being a carrier path for proton transport from the anode to the cathode side, a dense separation layer to block the mixing of the reactants, and an electric insulation layer between the anode and cathode. Many studies in the past few years have reported the enhancement of various functions of PEMs [26][27][28][29][30]. One of the key objectives for the PEMs’ development is to reduce the cells’ total fabrication cost and improve their electrochemical performance and durability [31][32]. A desirable cost-effective PEM should exhibit an acceptable thermochemical and thermomechanical stability, low permeability to fuel and oxidants, high proton conductivity, high compatibility with the electrodes in an MEA, long durability, and a low electro-osmotic drag coefficient [33][34].
3. Improving Proton Conductivity of the Membranes
3.1. Effects of Dopants and Additives
Several dopants have been used to improve the proton conductivity of PEMs, especially PBI. The proton conductivity of PBI is very low, and it requires the incorporation of dopants, additives, etc. [35]. Full or partial protonation or deprotonation of the polymers happens depending on the concentration and chemical nature of the dopant [36]. PBI is an amphoteric compound and has both proton acceptor and proton donor sites [37]. A wide variety of acids and bases, such as H2SO4 [38], H3PO4 [39], HBr [40], NaOH [41], KOH [42], etc., have been used as dopants. Compared to the low-temperature cells, HT-PEMs require high-boiling dopants which can operate at elevated operational temperatures [43]. Dopant concentration is a key parameter in improving proton conductivity, however, up to a certain amount. Excessive doping may deteriorate the conductivity. For example, acid doping with a high concentration (>11 mol.L−1) decreases the proton conductivity [36].
Sulfuric acid is an excellent dopant for PEMs; however, its high vapour pressure is a major drawback [36]. Phosphoric acid (PA) has been known to be the most promising additive/dopant for improving the proton conductivity of PBI because of its high thermal stability, high proton conductivity, low vapour pressure, and very low water content [36][44]. Although PA is an excellent proton conductor at high temperatures, which is about 0.8 S.cm−1 at 200 °C, its conductivity decreases after incorporating in a polymer matrix [45]. The main drawbacks associated with PA are membrane degradation and catalyst poisoning due to the harsh operating conditions and unfavourable electrochemical reactions as well as acid leaching. Thereby, the highest performance can be achieved by optimising the acid-doping level (ADL) or other strategies such as covalent crosslinking or making composite PEMs [28]. Depending on the ADL, PA can be classified into two general groups: “bonded acid” and “free acid”. When the acid concentration increases from 2 to 11 M, the H+ hopping between the N-H site and the PO3−4
anions results in proton migration [46]. In this case, the “bonded acid” remains almost constant, but the amount of “free acid” increases and consequently leads to higher proton conductivities. Therefore, the key parameter in determining the proton conductivity of PA-doped membranes is the presence of “free acid” [47]. Mader et al. [48] fabricated PA-doped PBI membranes with high proton conductivity (>10−1 S.cm−1 at above 100 °C) and excellent mechanical strength. The PA loading level was about 22–55 PA/PBI, and the final solid content was about 3.5–4.0 wt.% and IV’s > 1.0 dL.g−1. Li et al. [49] also investigated the effect of PA doping on the performance of HT-PEMs. In order to overcome the deterioration of mechanical properties caused by the “plasticizing effect” of the acid doping, they fabricated a highly acidophilic imidazole-rich crosslinked network with “A2B2-type” structure and an excellent “antiplasticizing” effect. The high reactivity of this structure enabled higher ADL, proton conductivity, mechanical/dimensional stability, and cell performance. Proton conductivity and peak power density of the PBI with a 30% degree of crosslinking were 2.53 × 10−1 S.cm−1 at 200 °C and 533 mW.cm−2 at 160 °C.
Doping the PBI matrix with the alkali cations can also increase the ionic conductivity of the resulting membrane. The ionic conductivity of the alkali-doped PBI membranes depends on the basicity of the resulting compounds. For example, the ionic conductivity of PBI/LiOH and PBI/KOH has been reported to be about 4 × 10−5 S.cm−1 and 6.5 × 10−2 S.cm−1, respectively [50]. Compared to the basic compounds, solid inorganic proton-conducting molecules such as heteropolyacids (HPAs) showed higher proton conductivity. HPAs, such as H3PMo12O40.29H2O (PMo12.30H2O), are highly hygroscopic materials with high proton conductivity (9.1 × 10−2 S.cm−1 for PMo12.30H2O) and excellent thermal stability [51]. High proton conductivity of about 10−2 and 10−1 S.cm−1 at 25 and 100 °C, respectively, has been reported by Zaidi et al. [52] for the HPA-SPEEK composite membranes. Solid acids such as zirconium hydrogen phosphate, phosphotungstic (PWA), and silicotungstic (SiWA) acids have also been investigated for their high proton conductivity. It has been reported that the proton conductivity of heteropolyacids such as PWA and SiWA increases with increasing RH. However, because not all the protons are available at elevated temperatures, their proton conductivity is generally low [35][51].
Inorganic compounds have also been used as dopants in PEMs [53][54]. Park et al. [53] investigated the effect of the Si and Zr cations on the performance of Nafion membranes. They reported that the water uptake of Si-doped membrane was lower than the recast Nafion and commercial Nafion 112, while the water uptake of Zr-doped Nafion reached about 39%. The proton conductivity of the Zr-doped sample at 80 °C and RH = 90% was about 10−1 S.cm−1, which was higher than those of recast Nafion, Nafion 112, and Si-doped membrane (~7 × 10−2 S.cm−1). They also compared the proton conductivity of the membranes at 120 °C and RH = 50% and found that the proton conductivity of the recast Nafion and commercial Nafion 112 was about 1.75 × 10−2 S.cm−1. The proton conductivity of Si-doped, Zr-doped, and (Si/Zr) dual-doped membranes (with Si to Zr ration of 2) was about 2.4 × 10−2, 2.6 × 10−2, and 3 × 10−2 S.cm−1, respectively.
In general, PEMs have low proton conductivity, and in order to improve their cell performance, dopants addition is a practical approach. ADL is a critical issue that has a significant effect on cell performance. Optimized doping can improve proton conductivity; however, excessive doping can deteriorate cell performance as well as its lifetime and mechanical properties.
3.2. Effect of Molecular Weight
Asensio et al. [51] reported that molecular weight (MW) has a vital role in improving mechanical strength. In order to prepare a highly conductive membrane, the polymer matrix with a comparably higher molecular weight should be applied. However, such an approach may not have a significant impact on the resulting proton conductivity of PEMs. Berber et al. [55] synthesized polybenzimidazole (ph-PBI) polymers with different MWs and reported that the high MW membrane (119 KDa) exhibited the most increased chemical stability, mechanical strength, and cell performance. In another attempt, Berber [56] fabricated ABPBI membranes with different MWs (20–113 KDa) and investigated the physicochemical properties, acid loading level, dopant retention capabilities, chemical stability, proton conductivity, and IEC of them. They reported that the high MW ABPBI showed the highest acid retention capability because of the chain entanglement, which resulted in trapping more PA molecules. High MW ABPBI showed higher chemical stability (9 wt.% weight loss after seven days in the Fenton reagent at 65 °C). The mechanism of proton conductivity was found to follow the “Grotthuss” mechanism, and the high MW sample possessed the highest proton conductivity among the samples (about 8 × 10−3 S.cm−1 at 140 °C). The IEC value of the high MW ABPBI was found to be increased by order of magnitude compared to the low MW one. Similar observations were reported by other researchers [30][57][58].
3.3. Polymer Composites
An effective approach to improving the proton conductivity of PEMs is to incorporate some additional components. Composite membranes showed promising potential to be used as HT-PEMFCs. Composite materials consist of two or more constituents with different chemical, mechanical, or physical properties [59]. Composite membranes have widely been used in PEMFCs such as PTFE/Nafion [59], metal-oxide-recast/Nafion [60], copper phthalocyanine tetrasulfonic acid tetrasodium salt (CuTSPc)/Nafion [61], calcium titanate/PBI [62], PBI-SiO2 [63], Nafion®/SiO2 [64], polyvinylidene fluoride (PVDF)/Nafion [65], Nafion/polyaniline [66], PBI/graphene oxide [24], etc. Fillers can provide additional chemical resistance, mechanical strength, and proton conductivity. Hydrophilic fillers improve membrane water uptake, and thereby, they can be used in low RH environments [59]. Inorganic fillers have high mechanical strength, thermal stability, and water-absorbing capacity and can be used in low RH and/or at elevated temperatures. The change in H2 crossover and proton conductivity at different operating conditions with/without filler in Nafion is illustrated in Figure 3. As shown in Figure 3a, the channels within the membrane are fully saturated with water and the mechanisms for proton conduction can be either “Grotthus” or diffusion. In this case, open-circuit voltage (OCV) can be reduced due to the molecular H2 passing through the membrane (H2 crossover). At higher temperatures (Figure 3b), water begins evaporation and results in the shrinkage of the channels and decreasing proton conductivity. On the other hand, H2 crossover improves at elevated temperatures. Adding filler to the composite (Figure 3c) can decrease H2 crossover and increase H2 path to migrate from anode to cathode [59].
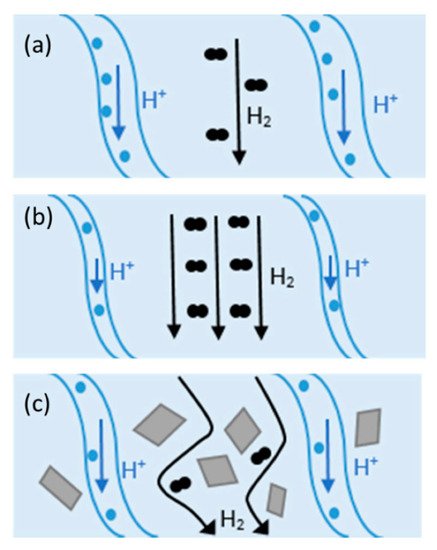
Figure 3. Proton and H2 transport at (a) 80 °C, (b) at elevated temperatures, and (c) in Nafion composite at high temperatures. Reproduced from [59], MDPI: 2020.
Different types of fillers have been used for improving the PEMs performance. Ceria is a filler that can diminish membrane degradation by acting as a regenerative free-radical scavenger [67]. Hydrophilic fillers can absorb a large quantity of water and improve proton conductivity and alleviate the unfavourable effect of high temperatures [60]. Although silica nanoparticles are hydrophobic, it has been reported that they do not have any negative effect on membrane water uptake [68]. Dispersion of sulphonated zirconia in the Nafion matrix can also improve the proton conductivity by functionalizing the Zr with sulphonic groups. Proton conductivity of about 3 × 10−3 S.cm−1 at 120 °C and under anhydrous conditions has been reported for this PEM composite [69]. Although increasing water uptake is favourable, excessive water uptake may negatively affect performance [70]. Graphene oxide is a hydrophilic material with lots of oxygen-containing functional groups and has recently been used as fillers in PEMFCs [71]. It has been reported that graphene oxide (GO) composite membranes can extend the operating temperature because they can retain more water and increase the proton conductivity [24][59].
Mixed-matrix membranes (MMMs) are a class of membranes comprised of a solid phase uniformly distributed in a polymer matrix. MMMs benefit from the advantages of the polymer membrane (high flexibility and ion exchange capacity) and inorganic constituents (high thermal and mechanical properties, water uptake, and proton conductivity) [72]. Among the inorganic materials, metal-organic frameworks such as Fe, Cr, Al, and Zr have high specific surface areas and offer higher proton conductivity. These MOFs can improve proton conductivity by defect engineering, postsynthetic modification, and impregnation with acidic molecules [73]. Amongst various forms of the applied MOFs, chromium terephthalate such as MIL-101(Cr) (MIL stands for Materials of Institute Lavoisier) with the chemical formula of {Cr3F(H2O)2O(BDC)3.nH2O} (n~25; 1,4-benzenedicarboxylate (BDC) showed higher conductivity than the other types. This material can easily be functionalized with excellent chemical/hydrothermal stability and is strongly resistant against moisture and organic molecules [74]. High proton conductivity of about 4.1 × 10−2 S.c006D−1 at 160 °C and RH = 0% has been reported by Anahidzade et al. [75], who fabricated MIL-101 (Cr) by hydrothermal method followed by functionalizing it via the postgrafting route. However, restricted proton transportation caused by the grain boundary of MOFs resulted in decreasing the proton conductivity [76]. To overcome this issue, MOFs hybridization with other polymers can alleviate the low proton conductivity [77]. UiO-series (UiO stands for the University of Oslo) MOFs are another class of MOFs that has attracted considerable interest because of their high energy conversion rate and low operating temperatures. These materials mainly contain Zr and have excellent chemical and thermal stability due to the highly oxyphilic Zr (IV) atoms [78][79]. In this context, Rao et al. [78] fabricated Nafion/GO@UiO-66-SO3H composites with a high conductivity of about 3.03 × 10−1 S.cm−1 at 90 °C and RH = 95%. They reported that consecutive proton transfer channels were constructed within the PEM composite due to the suitable interconnection of MOF grains and the tethering effect of GO surfaces. It has been reported that UiO-66 possesses a very poor proton conductivity of about 7 × 10−6. However, sulphonated UiO-66 (UiO-66-SO3H) has super protonic conductivity (four orders of magnitude greater than the UiO-66) [80][81][82]. The synergic effect between UiO-66-SO3H and Uio-66-NH2 was found to have a great effect on the proton conductivity of the composite [83]. Other types of MOFs such as zeolite imidazolate frameworks (ZIFs) [84], a chiral 2-D MOF named “MOF 1” [85], which combined protic ionic liquids (PILs) with porous MOFs [86], etc., have also been investigated. Overall, MOFs-modified PEMFCs have shown promising electrochemical performance at high temperatures and anhydrous conditions. MIL class can promote proton conductivity using the abundant hydroxyl groups. The UiO class possesses a high-density spatial structure with strong chemical stability. Selecting the appropriate composite, suitable dispersion condition, and the MOF concentration is the most critical factor affecting the composites’ conductivity and physiochemical/thermal stability.
References
- Song, C.; Lee, S.; Gu, B.; Chang, I.; Cho, G.Y.; Baek, J.D.; Cha, S.W. A Study of Anode-Supported Solid Oxide Fuel Cell Modeling and Optimization Using Neural Network and Multi-Armed Bandit Algorithm. Energies 2020, 13, 1621.
- Wang, Y.; Ruiz Diaz, D.F.; Chen, K.S.; Wang, Z.; Adroher, X.C. Materials, technological status, and fundamentals of PEM fuel cells—A review. Mater. Today 2020, 32, 178–203.
- Vostakola, M.F.; Horri, B.A. Progress in Material Development for Low-Temperature Solid Oxide Fuel Cells: A Review. Energies 2021, 14, 1280.
- Hou, J.; Yang, M.; Wang, D.; Zhang, J. Fundamentals and Challenges of Lithium Ion Batteries at Temperatures between −40 and 60 °C. Adv. Energy Mater. 2020, 10, 1904152.
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445.
- Doetsch, C.; Pohlig, A. The Use of Flow Batteries in Storing Electricity for National Grids; Elsevier Ltd.: Oxford, UK, 2020; ISBN 9780081028865.
- Clemente, A.; Costa-Castelló, R. Redox flow batteries: A literature review oriented to automatic control. Energies 2020, 13, 4514.
- Shri Prakash, B.; Pavitra, R.; Senthil Kumar, S.; Aruna, S.T. Electrolyte bi-layering strategy to improve the performance of an intermediate temperature solid oxide fuel cell: A review. J. Power Sources 2018, 381, 136–155.
- Da Silva, F.S.; de Souza, T.M. Novel materials for solid oxide fuel cell technologies: A literature review. Int. J. Hydrogen Energy 2017, 42, 26020–26036.
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Non-humidified fuel cells using a deep eutectic solvent (DES) as the electrolyte within a polymer electrolyte membrane (PEM): The effect of water and counterions. Phys. Chem. Chem. Phys. 2020, 22, 2917–2929.
- Beydaghi, H.; Bagheri, A.; Salarizadeh, P.; Kashefi, S.; Hooshyari, K.; Amoozadeh, A.; Shamsi, T.; Bonaccorso, F.; Pellegrini, V. Enhancing the Performance of Poly(phthalazinone ether ketone)-Based Membranes Using a New Type of Functionalized TiO2 with Superior Proton Conductivity. Ind. Eng. Chem. Res. 2020, 59, 6589–6599.
- Kumar, A.; Hong, J.-W.; Yun, Y.; Bhardwaj, A.; Song, S.-J. The role of surface lattice defects of CeO2−δ nanoparticles as a scavenging redox catalyst in polymer electrolyte membrane fuel cells. J. Mater. Chem. A 2020, 8, 26023–26034.
- Hooshyari, K.; Javanbakht, M.; Salarizadeh, P.; Bageri, A. Advanced nanocomposite membranes based on sulfonated polyethersulfone: Influence of nanoparticles on PEMFC performance. J. Iran. Chem. Soc. 2019, 16, 1617–1629.
- Jayakumar, A. An Assessment on Polymer Electrolyte Membrane Fuel Cell Stack Components. In Applied Physical Chemistry with Multidisciplinary Approaches; Haghi, A.K., Balköse, D., Thomas, S., Eds.; Apple Academic Press Inc.: Oakville, ON, Canada, 2018; pp. 23–49. ISBN 9781771886062.
- Beydaghi, H.; Javanbakht, M.; Bagheri, A.; Ghafarian-Zahmatkesh, H.; Hooshyari, K. Preparation and Characterization of Electrocatalyst Nanoparticles for Direct Methanol Fuel Cell Applications Using β-D-glucose as Protection Agent. Iran. J. Hydrog. Fuel Cell 2017, 4, 1–11.
- Jannelli, E.; Minutillo, M.; Perna, A. Analyzing microcogeneration systems based on LT-PEMFC and HT-PEMFC by energy balances. Appl. Energy 2013, 108, 82–91.
- Ma, K.B.; Kwak, D.H.; Han, S.B.; Park, H.S.; Kim, D.H.; Won, J.E.; Kwon, S.H.; Kim, M.C.; Moon, S.H.; Park, K.W. Direct Ethanol Fuel Cells with Superior Ethanol-Tolerant Nonprecious Metal Cathode Catalysts for Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2018, 6, 7609–7618.
- Velayutham, P.; Sahu, A.K.; Parthasarathy, S. A Nafion-ceria composite membrane electrolyte for reduced methanol crossover in direct methanol fuel cells. Energies 2017, 10, 259.
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538.
- Van Eck, N.J.; Waltman, L. VOSviewer Manual; Univeristeit Leiden: Leiden, The Netherlands, 2013.
- Zhou, Z.; Zholobko, O.; Wu, X.-F.; Aulich, T.; Thakare, J.; Hurley, J. Polybenzimidazole-Based Polymer Electrolyte Membranes for High-Temperature Fuel Cells: Current Status and Prospects. Energies 2020, 14, 135.
- Moradi, M.; Moheb, A.; Javanbakht, M.; Hooshyari, K. Experimental study and modeling of proton conductivity of phosphoric acid doped PBI-Fe2TiO5 nanocomposite membranes for using in high temperature proton exchange membrane fuel cell (HT-PEMFC). Int. J. Hydrogen Energy 2016, 41, 2896–2910.
- Tian, D.; Gu, T.; Yellamilli, S.N.; Bae, C. Phosphoric acid-doped ion-pair coordinated PEMs with broad relative humidity tolerance. Energies 2020, 13, 1924.
- Üregen, N.; Pehlivanoğlu, K.; Özdemir, Y.; Devrim, Y. Development of polybenzimidazole/graphene oxide composite membranes for high temperature PEM fuel cells. Int. J. Hydrogen Energy 2017, 42, 2636–2647.
- Attaran, A.M. Fabrication and Characterization of Poly Vinyl Alcohol/ Poly Vinyl Pyrrolidone/MnTiO3 Nanocomposite Membranes for PEM Fuel Cells. Iran. J. Energy Environ. 2013, 4, 86–90.
- Salarizadeh, P.; Askari, M.B.; Mohammadi, M.; Hooshyari, K. Electrocatalytic performance of CeO2-decorated rGO as an anode electrocatalyst for the methanol oxidation reaction. J. Phys. Chem. Solids 2020, 142, 109442.
- Li, X.; Wang, P.; Liu, Z.; Peng, J.; Shi, C.; Hu, W.; Jiang, Z.; Liu, B. Arylether-type polybenzimidazoles bearing benzimidazolyl pendants for high-temperature proton exchange membrane fuel cells. J. Power Sources 2018, 393, 99–107.
- Haider, R.; Wen, Y.; Ma, Z.F.; Wilkinson, D.P.; Zhang, L.; Yuan, X.; Song, S.; Zhang, J. High temperature proton exchange membrane fuel cells: Progress in advanced materials and key technologies. Chem. Soc. Rev. 2021, 50, 1138–1187.
- Lee, S.; Seo, K.; Ghorpade, R.V.; Nam, K.H.; Han, H. High temperature anhydrous proton exchange membranes based on chemically-functionalized titanium/polybenzimidazole composites for fuel cells. Mater. Lett. 2020, 263, 127167.
- Berber, M.R.; Nakashima, N. Bipyridine-based polybenzimidazole membranes with outstanding hydrogen fuel cell performance at high temperature and non-humidifying conditions. J. Memb. Sci. 2019, 591, 117354.
- Hosseinabadi, P.; Hooshyari, K.; Javanbakht, M.; Enhessari, M. Synthesis and optimization of nanocomposite membranes based on SPEEK and perovskite nanoparticles for polymer electrolyte membrane fuel cells. New J. Chem. 2019, 43, 16232–16245.
- Salarizadeh, P.; Javanbakht, M.; Pourmahdian, S.; Hazer, M.S.A.; Hooshyari, K.; Askari, M.B. Novel proton exchange membranes based on proton conductive sulfonated PAMPS/PSSA-TiO2 hybrid nanoparticles and sulfonated poly (ether ether ketone) for PEMFC. Int. J. Hydrogen Energy 2019, 44, 3099–3114.
- Hooshyari, K.; Javanbakht, M.; Enhessari, M.; Beydaghi, H. Novel PVA/La2Ce2O7 hybrid nanocomposite membranes for application in proton exchange membrane fuel cells. Iran. J. Hydrog. Fuel Cell Iran. J. Hydrog. Fuel Cell IJHFC J. 2014, 2, 105–112.
- Attaran, A.M.; Javanbakht, M.; Hooshyari, K.; Enhessari, M. New proton conducting nanocomposite membranes based on poly vinyl alcohol/poly vinyl pyrrolidone/BaZrO3 for proton exchange membrane fuel cells. Solid State Ionics 2015, 269, 98–105.
- Sun, X.; Simonsen, S.C.; Norby, T.; Chatzitakis, A. Composite membranes for high temperature PEM fuel cells and electrolysers: A critical review. Membranes 2019, 9, 83.
- Jakobsen, M.T.D.; Jensen, J.O.; Cleemann, L.N.; Li, Q. Durability Issues and Status of PBI-Based Fuel Cells. In High Temperature Polymer Electrolyte Membrane Fuel Cells. Approaches, Status, and Perspectives; Li, Q., Aili, D., Hjuler, H.A., Jensen, J.O., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 487–509. ISBN 9783319170824.
- Leykin, A.Y.; Askadskii, A.A.; Vasilev, V.G.; Rusanov, A.L. Dependence of some properties of phosphoric acid doped PBIs on their chemical structure. J. Memb. Sci. 2010, 347, 69–74.
- Kang, Y.; Zou, J.; Sun, Z.; Wang, F.; Zhu, H.; Han, K.; Yang, W.; Song, H.; Meng, Q. Polybenzimidazole containing ether units as electrolyte for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2013, 38, 6494–6502.
- Farrokhi, M.; Abdollahi, M. Enhancing medium/high temperature proton conductivity of poly(benzimidazole)-based proton exchange membrane via blending with poly(vinyl imidazole-co-vinyl phosphonic acid) copolymer: Proton conductivity-copolymer microstructure relationship. Eur. Polym. J. 2020, 131, 109691.
- Bouchet, R.; Siebert, E. Proton conduction in acid doped polybenzimidazole. Solid State Ionics 1999, 118, 287–299.
- Luo, H.; Vaivars, G.; Agboola, B.; Mu, S.; Mathe, M. Anion exchange membrane based on alkali doped poly (2,5-benzimidazole) for fuel cell. Solid State Ionics 2012, 208, 52–55.
- Lin, A.J.; Yan, X.; He, G.; Chen, W.; Zhen, D.; Li, T.; Ma, L.; Wu, X. Thermoplastic interpenetrating polymer networks based on polybenzimidazole and poly (1, 2-dimethy-3-allylimidazolium) for anion exchange membranes. Electrochim. Acta 2017, 257, 9–19.
- Fuel Cells and Hydrogen Production a Volume in the Encyclopedia of Sustainability Science and Technology, 2nd ed.; Lipman, T.E.; Weber, A.Z. (Eds.) Springer: Berkeley, CA, USA, 2019; ISBN 9781493977888.
- Escorihuela, J.; García-Bernabé, A.; Compañ, V. A deep insight into different acidic additives as doping agents for enhancing proton conductivity on polybenzimidazole membranes. Polymers 2020, 12, 1374.
- Schechter, A.; Savinell, R.F. Imidazole and 1-methyl imidazole in phosphoric acid doped polybenzimidazole, electrolyte for fuel cells. Solid State Ionics 2002, 147, 181–187.
- Li, Q.; He, R.; Berg, R.W.; Hjuler, H.A.; Bjerrum, N.J. Water uptake and acid doping of polybenzimidazoles as electrolyte membranes for fuel cells. Solid State Ionics 2004, 168, 177–185.
- He, R.; Li, Q.; Xiao, G.; Bjerrum, N.J. Proton conductivity of phosphoric acid doped polybenzimidazole and its composites with inorganic proton conductors. J. Memb. Sci. 2003, 226, 169–184.
- Mader, J.A.; Benicewicz, B.C. Sulfonated polybenzimidazoles for high temperature PEM fuel cells. Macromolecules 2010, 43, 6706–6715.
- Li, X.; Ma, H.; Wang, P.; Liu, Z.; Peng, J.; Hu, W.; Jiang, Z.; Liu, B.; Guiver, M.D. Highly Conductive and Mechanically Stable Imidazole-Rich Cross-Linked Networks for High-Temperature Proton Exchange Membrane Fuel Cells. Chem. Mater. 2020, 32, 1182–1191.
- Jones, D.J.; Rozière, J. Recent advances in the functionalisation of polybenzimidazole and polyetherketone for fuel cell applications. J. Memb. Sci. 2001, 185, 41–58.
- Asensio, J.A.; Sánchez, E.M.; Romero, P.G. Proton-conducting membranes based on benzimidazole polymers for high-temperature PEM fuel cells. A chemical quest. Chem. Soc. Rev. 2010, 39, 3210–3239.
- Zaidi, S.M.J.; Mikhailenko, S.D.; Robertson, G.P.; Guiver, M.D.; Kaliaguine, S. Proton conducting composite membranes from polyether ether ketone and heteropolyacids for fuel cell applications. J. Memb. Sci. 2000, 173, 17–34.
- Park, K.T.; Jung, U.H.; Choi, D.W.; Chun, K.; Lee, H.M.; Kim, S.H. ZrO2–SiO2/Nafion® composite membrane for polymer electrolyte membrane fuel cells operation at high temperature and low humidity. J. Power Sources 2008, 177, 247–253.
- Hooshyari, K.; Khanamiri, S.N.; Salarizadeh, P.; Beydaghi, H. Nanocomposite Membranes with High Fuel Cell Performance Based on Sulfonated Poly (1,4-phenylene ether ether sulfone) and Ytterbium/Yttrium Doped-Perovskite Nanoparticles. J. Electrochem. Soc. 2019, 166, F976–F989.
- Berber, M.R.; Nakashima, N. Tailoring Different Molecular Weight Phenylene-Polybenzimidazole Membranes with Remarkable Oxidative Stability and Conductive Properties for Higherature Polymer Electrolyte Fuel Cells. ACS Appl. Mater. Interfaces 2019, 11, 46269–46277.
- Berber, M.R. Molecular Weight Impact of Poly(2,5-Benzimidazole) Polymer on Film Conductivity, Ion Exchange Capacity, Acid Retention Capability, and Oxidative Stability. Front. Energy Res. 2020, 8, 1–11.
- Yang, J.S.; Cleemann, L.N.; Steenberg, T.; Terkelsen, C.; Li, Q.F.; Jensen, J.O.; Hjuler, H.A.; Bjerrum, N.J.; He, R.H. High molecular weight polybenzimidazole membranes for high temperature PEMFC. Fuel Cells 2014, 14, 7–15.
- Schaffer, J.V.; Lupatini, K.N.; Machado, B.; Silva, E.S.; Ferracin, R.J.; Alves, H.J. Parameters effect on proton conductivity to obtain chitosan membranes for use as electrolytes in PEMFC. Int. J. Energy Res. 2018, 42, 1381–1385.
- Gagliardi, G.G.; Ibrahim, A.; Borello, D.; El-Kharouf, A. Composite polymers development and application for polymer electrolyte membrane technologies—A review. Molecules 2020, 25, 1712.
- Adjemian, K.T.; Dominey, R.; Krishnan, L.; Ota, H.; Majsztrik, P.; Zhang, T.; Mann, J.; Kirby, B.; Gatto, L.; Velo-Simpson, M.; et al. Function and Characterization of Metal Oxide−Nafion Composite Membranes for Elevated-Temperature H2/O2 PEM Fuel Cells. Chem. Mater. 2006, 18, 2238–2248.
- Wei, Y.; Qian, T.; Liu, J.; Guo, X.; Gong, Q.; Liu, Z.; Tian, B.; Qiao, J. Novel composite Nafion membranes modified with copper phthalocyanine tetrasulfonic acid tetrasodium salt for fuel cell application. J. Mater. 2019, 5, 252–257.
- Muthuraja, P.; Prakash, S.; Shanmugam, V.M.; Radhakrsihnan, S.; Manisankar, P. Novel perovskite structured calcium titanate-PBI composite membranes for high-temperature PEM fuel cells: Synthesis and characterizations. Int. J. Hydrogen Energy 2018, 43, 4763–4772.
- Ioana-Maria, N.; Aurora, J.; Victoria, C.; Cristian, B. Advanced polymeric materials based on PBI/SiO2 composite with high-performances designated for PEM-fuel cells. In Proceedings of the 2017 Electric Vehicles International Conference, Bucharest, Romania, 5–6 October 2017; pp. 1–6.
- Li, X.; Ke, C.; Qu, S.; Li, J.; Shao, Z.; Yi, B. High Temperature PEM Fuel Cells Based on Nafion®/SiO2 Composite Membrane, Energy Storage in the Emerging Era of Smart Grids. In Energy Storage in the Emerging Era of Smart Grids; Carbone, R., Ed.; IntechOpen: Reggio Calabria, Italy, 2011; pp. 279–298. ISBN 978-953-307-269-2.
- He, Y.; Wang, D.; Li, Q.; Huang, L.; Bao, H. Composite Polymer Electrolyte Membranes based on Nafion and Modified PVDF Electrospun Nanofiber Mats. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2020, 35, 677–681.
- Yang, J.; Shen, P.K.; Varcoe, J.; Wei, Z. Nafion/polyaniline composite membranes specifically designed to allow proton exchange membrane fuel cells operation at low humidity. J. Power Sources 2009, 189, 1016–1019.
- Trogadas, P.; Parrondo, J.; Ramani, V. Degradation mitigation in polymer electrolyte membranes using cerium oxide as a regenerative free-radical scavenger. Electrochem. Solid-State Lett. 2008, 11, 113–116.
- Di Noto, V.; Boaretto, N.; Negro, E.; Pace, G. New inorganic-organic proton conducting membranes based on Nafion and hydrophobic fluoroalkylated silica nanoparticles. J. Power Sources 2010, 195, 7734–7742.
- Giffin, G.A.; Piga, M.; Lavina, S.; Navarra, M.A.; D’Epifanio, A.; Scrosati, B.; Noto, V. Di Characterization of sulfated-zirconia/Nafion® composite membranes for proton exchange membrane fuel cells. J. Power Sources 2012, 198, 66–75.
- Saccà, A.; Gatto, I.; Carbone, A.; Pedicini, R.; Passalacqua, E. ZrO2-Nafion composite membranes for polymer electrolyte fuel cells (PEFCs) at intermediate temperature. J. Power Sources 2006, 163, 47–51.
- He, D.; Tang, H.; Kou, Z.; Pan, M.; Sun, X.; Zhang, J.; Mu, S. Engineered Graphene Materials: Synthesis and Applications for Polymer Electrolyte Membrane Fuel Cells. Adv. Mater. 2017, 29, 1601741.
- Bakangura, E.; Wu, L.; Ge, L.; Yang, Z.; Xu, T. Mixed matrix proton exchange membranes for fuel cells: State of the art and perspectives. Prog. Polym. Sci. 2016, 57, 103–152.
- Escorihuela, J.; Narducci, R.; Compañ, V.; Costantino, F. Proton Conductivity of Composite Polyelectrolyte Membranes with Metal-Organic Frameworks for Fuel Cell Applications. Adv. Mater. Interfaces 2019, 6, 1801146.
- Bhattacharjee, S.; Chen, C.; Ahn, W.S. Chromium terephthalate metal-organic framework MIL-101: Synthesis, functionalization, and applications for adsorption and catalysis. RSC Adv. 2014, 4, 52500–52525.
- Anahidzade, N.; Abdolmaleki, A.; Dinari, M.; Firouz Tadavani, K.; Zhiani, M. Metal-organic framework anchored sulfonated poly(ether sulfone) as a high temperature proton exchange membrane for fuel cells. J. Memb. Sci. 2018, 565, 281–292.
- Liu, Q.; Li, Z.; Wang, D.; Li, Z.; Peng, X.; Liu, C.; Zheng, P. Metal Organic Frameworks Modified Proton Exchange Membranes for Fuel Cells. Front. Chem. 2020, 8, 694.
- Kitao, T.; Zhang, Y.; Kitagawa, S.; Wang, B.; Uemura, T. Hybridization of MOFs and polymers. Chem. Soc. Rev. 2017, 46, 3108–3133.
- Rao, Z.; Feng, K.; Tang, B.; Wu, P. Construction of well interconnected metal-organic framework structure for effectively promoting proton conductivity of proton exchange membrane. J. Memb. Sci. 2017, 533, 160–170.
- Donnadio, A.; Narducci, R.; Casciola, M.; Marmottini, F.; D’Amato, R.; Jazestani, M.; Chiniforoshan, H.; Costantino, F. Mixed Membrane Matrices Based on Nafion/UiO-66/SO3H-UiO-66 Nano-MOFs: Revealing the Effect of Crystal Size, Sulfonation, and Filler Loading on the Mechanical and Conductivity Properties. ACS Appl. Mater. Interfaces 2017, 9, 42239–42246.
- Yang, Q.; Wiersum, A.D.; Llewellyn, P.L.; Guillerm, V.; Serre, C.; Maurin, G. Functionalizing porous zirconium terephthalate UiO-66(Zr) for natural gas upgrading: A computational exploration. Chem. Commun. 2011, 47, 9603–9605.
- DeStefano, M.R.; Islamoglu, T.; Garibay, S.J.; Hupp, J.T.; Farha, O.K. Room Temperature Synthesis of UiO-66 and Thermal Modulation of Densities of Defect Sites. Chem. Mater. 2017, 29, 1357–1361.
- Patel, H.A.; Mansor, N.; Gadipelli, S.; Brett, D.J.L.; Guo, Z. Superacidity in Nafion/MOF Hybrid Membranes Retains Water at Low Humidity to Enhance Proton Conduction for Fuel Cells. ACS Appl. Mater. Interfaces 2016, 8, 30687–30691.
- Rao, Z.; Tang, B.; Wu, P. Proton Conductivity of Proton Exchange Membrane Synergistically Promoted by Different Functionalized Metal-Organic Frameworks. ACS Appl. Mater. Interfaces 2017, 9, 22597–22603.
- Liu, Q.; Li, Y.; Zheng, L.; Shang, J.; Liu, X.; Yu, R.; Shui, J. Sequential Synthesis and Active-Site Coordination Principle of Precious Metal Single-Atom Catalysts for Oxygen Reduction Reaction and PEM Fuel Cells. Adv. Energy Mater. 2020, 10, 2000689.
- Liang, X.; Zhang, F.; Feng, W.; Zou, X.; Zhao, C.; Na, H.; Liu, C.; Sun, F.; Zhu, G. From metal-organic framework (MOF) to MOF-polymer composite membrane: Enhancement of low-humidity proton conductivity. Chem. Sci. 2013, 4, 983–992.
- Horike, S.; Umeyama, D.; Inukai, M.; Itakura, T.; Kitagawa, S. Coordination-network-based ionic plastic crystal for anhydrous proton conductivity. J. Am. Chem. Soc. 2012, 134, 7612–7615.
More
Information
Subjects:
Chemistry, Applied
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
09 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




