| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Learn-Han Lee | + 5325 word(s) | 5325 | 2020-12-02 10:10:56 | | | |
| 2 | Vicky Zhou | Meta information modification | 5325 | 2020-12-09 09:21:49 | | | | |
| 3 | Chan Kok Gan | Meta information modification | 5325 | 2020-12-09 15:20:20 | | |
Video Upload Options
Worldwide cancer incidence and mortality have always been a concern to the community. The cancer mortality rate has generally declined over the years; however, there is still an increased mortality rate in poorer countries that receives considerable attention from healthcare professionals. This suggested the importance of the prompt detection, effective treatment, and prevention strategies. The genus Streptomyces has been documented as a prolific producer of biologically active secondary metabolites. Streptomycetes from mangrove environments attract researchers’ attention due to their ability to synthesize diverse, interesting bioactive metabolites. The present entry highlights research on mangrove-derived streptomycetes and the production of anticancer-related compounds from these microorganisms. Research studies conducted between 2008 and 2019, specifically mentioning the isolation of streptomycetes from mangrove areas and described the successful purification of compound(s) or generation of crude extracts with cytotoxic activity against human cancer cell lines, were compiled in this entry. It is anticipated that there will be an increase in prospects for mangrove-derived streptomycetes as one of the natural resources for the isolation of chemotherapeutic agents.
1. Introduction
In this rapidly changing world, accompanied by an urbanized lifestyle and alteration in dietary habits, our health has inevitably impacted in both positive and negative ways. Unfortunately, cancer remains a global health issue, and it has been recorded as the second leading cause of death following cardiovascular disease [1][2]. There are many speculative causes of cancer that are often related to contemporary unhealthy lifestyles, such as dietary changes towards the consumption of fast food and alcohol, smoking, and the lack of physical activity [3][4][5][6]. The documented global cancer cases were 24.5 million, and the estimated cancer death was 9.6 million in the year 2017[7]. The continuous burden of cancer has been depicted in the report written by Siegel et al. [8]. As of the year 2020, the most common estimated new cases of cancers for Americans based on sex: (a) for men will be prostate, lung, and bronchus, and colorectal cancers; (b) while for women will be breast, lung, and colorectal cancers. Cancer deaths would be at an alarming rate of approximately 1600 deaths per day [8].
Human cancer is a complex process involving cellular and molecular alterations mediated by numerous endogenous and exogenous stimuli [9][10][11][12]. In other words, these stimuli cause the uncontrollable multiplication of cells that become cancerous once they no longer obey normal cell signalling and start to invade other tissues [13][14][15]. Several factors contribute to the progress in reducing cancer-related mortality, such as early detection, effective treatment through chemotherapy, radiation therapy or surgical procedure, and prevention [16][17][18][19]. Among these, the search for effective anticancer drugs is a paramount goal for cancer therapy.
Natural products from various sources, including plants, environments, and microorganisms, have been serving us well in combating cancer. Approximately more than 60% of the present anticancer/antitumor drugs were naturally derived from these sources [20][21][22][23][24]. In general, the phrase “natural product” can be referred to as “primary or secondary metabolites,” biologically active compounds with small molecular weight (less than 3000 Daltons) that are produced by organisms to assist in their survival [25][26][27]. Natural products have the potential to suppress or inhibit cancer progression as well as involve the reversal of progression [16][28]. Natural products could also be an alternative solution to overcome current chemotherapy issues such as multidrug resistance, distressing side effects (e.g., heart failure, diarrhoea, oedema, etc.) due to high toxicity or lack of specificity [29][30][31][32]. Ideally, chemotherapeutic medicines should be highly specific in eliminating only cancer cells by discriminating between the normal cells and cancer cells. However, most of the presently used anticancer treatments tend to destroy cancer and normal cells [33]. Cancer chemoprevention is equally important as an intervention in carcinogenesis. These can be blocking agents that halt neoplastic process or suppressing agents that prevent the development of cancer cells’ malignant phenotype [34][35]. Thus, it is an ongoing effort to search for highly specific and potent chemotherapy/chemopreventive agents from alternative sources such as microorganisms.
2. Microbial Sources: Why the Streptomyces?
Microorganisms have been established as essential resources for natural product drug discovery. The abundance of species can be the root of the enormous supply of structurally diverse natural products. By harvesting these compounds from microbial sources, it will be relatively cost-efficient compared to the chemical synthesis approach [36][37][38]. As a result, many microorganisms have been screened for the production of anticancer compounds/leads. Purportedly, these microbial natural compounds’ anticancer activity could regulate immune function, inhibit cell proliferation, and induce apoptosis [39][40].
In the Bacteria domain, the phylum Actinobacteria is one of the renowned major lineages representing one of the largest taxonomic units with diverse identified species and some of which are well studied model organisms [41][42][43]. This phylum consists of six major classes: Acidimicrobiia, Actinobacteria, Coriobacteriia, Nitriliruptoria, Rubrobacteria, and Thermoleophilia; covering a wide range of Gram-positive bacteria with different characteristics and morphologies [44][45][46]. Actinobacteria have been recognized as primary sources of bioactive natural products as early as in the 1950s, for which approximately half of the secondary metabolites discovered, including enzymes, antibiotics, immunosuppressive, and anti-tumour agents, are produced by actinomycetes [41][47][48][49][50][51]. The well known representative genus of class Actinobacteria is the Streptomyces, which accounts for over 70% of commercially useful antibiotics [48][52][53]. In addition, it is remarkable that 80% of actinobacterial natural products documented hitherto are derived from the genus Streptomyces [47].
Streptomycetes have immense diversity and bioactive potential that overpower many other microbial groups. For instance, streptomycetes are extensively distributed in both terrestrial and marine habitats, which to date, this group of bacteria has more than 900 species identified with a validly published name (https://bacterio.net/). Additionally, an increasing number of studies indicate the existence of streptomycetes in extreme habitats [43][54][55][56]. Streptomycetes produce a high number of spores that are easily dispersed, and thus, it is projected that they have a cosmopolitan distribution [47][57]. Furthermore, the biosynthetic capacity of streptomycetes remains without rival given the fact that streptomycetes have a large genome size (>7 Mbp) [58][59][60][61], and some of them have been previously reported to own more than 20 gene clusters encoding predicted/known biosynthetic pathways [62][63][64]. Hence, this contributed to the unique capability of streptomycetes in the production of various novel and/or useful secondary metabolites with antioxidant, anticancer, antimicrobial, etc. activities which subsequently become the key reason which attracts researchers to explore the bioactive potential of this genus [65][66][67]. Although only limited numbers of the isolated compounds from microbes can be directly utilized as clinically effective medicines in their own right (naturally occurring form), streptomycetes are verified producers of anticancer drugs such as bleomycin (glycopeptides group), dactinomycin (non-ribosomal peptides group), mitomycin C (quinones group), and doxorubicin (anthracyclines group) [68][69]. Furthermore, natural microbial compounds can be optimized/modified and developed into important drug leads [70].
3. Bioprospecting of Streptomyces spp. from Mangrove Environment
Several research studies have adapted the culture-dependent bioprospecting strategy where the microorganisms are initially isolated from neglected or extreme habitats in the journey of unearthing novel natural products from streptomycetes as potential pharmacological agents, such as from mangroves, deserts, and caves [66][71][72][73][74][75]. Generally, this strategy involves utilizing appropriate selective isolation procedures to obtain microorganisms of interest from a neglected habitat, followed by the recognition and dereplication of target microbes and then the selection of representative strains for bioactivity screening purposes. Nonetheless, it should be noted that this strategy relies on the concept that poorly studied habitats can be potential sources for the isolation of novel strains that could produce new and interesting compounds [71][76].
Apart from terrestrial and aquatic environments, streptomycetes’ distribution in underexplored dynamic mangrove environments appears to be relatively extensive, and bioprospecting studies of streptomycetes from mangrove areas are generating fruitful outcomes [47][66][77]. Mangroves are special intertidal ecosystems of tropical and subtropical regions [78][79][80]. Mangrove forests act as highly productive ecosystems providing crucial protections to the coastline in tidal zones, homes to diverse species of marine, freshwater, and terrestrial flora and fauna, as well as ecosystem goods to sustain human wellbeing [48][81][82]. Moreover, this environment contains a rich amount of nutrients due to nutrient cycling between the microbial community and mangrove vegetation; thus, this cycle further promotes the mangrove as a homeland to various microorganisms [83].
There is increasing evidence regarding the utilization of mangrove microorganism resources, which could be due to these microorganisms’ ability to produce a vast array of unusual secondary metabolites driven by inconsistent environmental factors such as a salinity and tidal gradient that is believed to prompt metabolic pathway adaptations in them [48][84][85]. Streptomycetes are successful producers of over 10,000 bioactive compounds. The bioactive potential of mangrove-derived streptomycetes has been of scientific interest since the mangrove ecosystem has become a hot spot for natural product discovery [47][86][87][88]. Research has also proven that mangroves serve as invaluable assets by offering diversified novel taxa. Numerous new species have been first discovered from mangroves and described by researchers around the world. For example, Streptomyces nigra and Streptomyces avicenniae were isolated from mangrove Avicennia marina in Fujian Province, China, and characterized as a novel species by Chen et al. [89] and Xiao et al.[90] respectively; Streptomyces sundarbansensis was first discovered as a novel species from the sediments of mangrove Sundarbans, India, by Arumugam et al. [91]; Streptomyces xiamenensis was described as novel species by Xu et al. [92] and it was isolated from a mangrove forest in Fujian Province, China. Screenings on the bioactivity of secondary metabolites from mangrove-derived streptomycetes have been conducted. The findings revealed that bioactivities such as antibacterial, antifungal, anti-HIV, anticancer, and antioxidant had been demonstrated by these isolates [77][93][94][95][96][97].
4. Discovery of Novel Compounds with Anticancer/Cytotoxic Activity from Mangrove-Derived Streptomyces spp.
The screening for anticancer potentials of mangrove-derived streptomycetes has been an ongoing expedition for over 10 years. The present entry compiled studies carried out specifically on streptomycetes from mangrove areas that demonstrated cytotoxic activity against human cancer cell lines (Table 1). These studies consisted of experiments conducted on pure compounds or crude extracts of diverse Streptomyces spp. which have been isolated from mangrove environments. All studies summarized in Table 1 had utilized the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) assay to analyse the cytotoxic capacity of a compound or extract against different human cancer cells. The MTT assay is based on the concept of living cells capable of converting MTT into formazan crystals, hence, reflecting the activity of mitochondria in living cells. For most cell populations, the total activity of mitochondria is linearly associated with the number of viable cells. The formazan crystals can be solubilized for homogenous measurement whereby formazan concentration reflected in optical density determines the number of viable cells. The MTT assay is the most commonly used method in determining drug cytotoxicity at different concentrations. The advantage of the MTT assay is that it serves to measure viable cells in a high-throughput manner (96-well plates) without requiring the complex counting of cells [98].
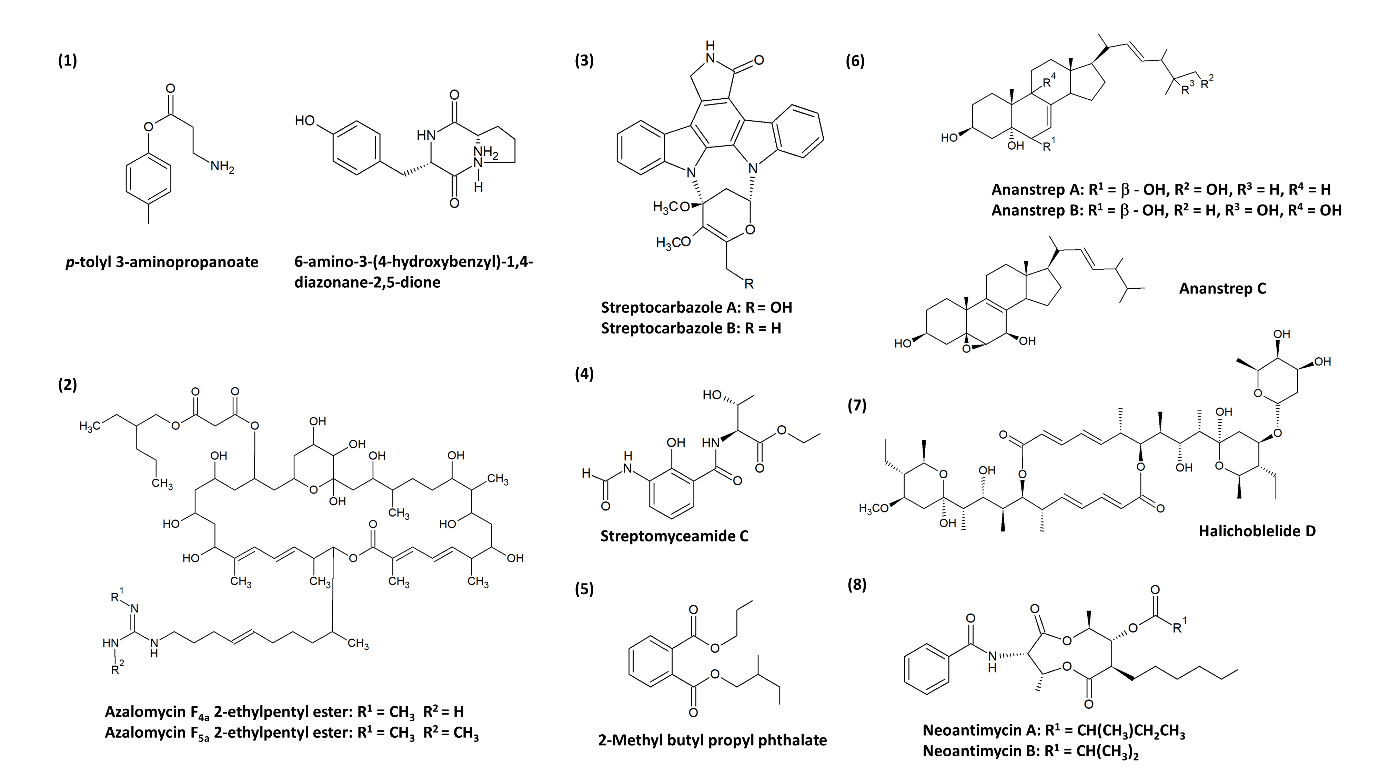
According to the information collected in Table 1, many studies provided compelling evidence that these streptomycetes are good sources for isolating anticancer-related compounds. One example of an earlier study was conducted by Xie et al. [99], where they successfully purified and elucidated five compounds derived from a mangrove Streptomyces strain 124092. Among these compounds, four of them exhibited cytotoxic activity against the human hepatoma SMMC-7721 cell line (Table 1). Additionally, new compounds with anticancer potential have been discovered from mangrove-derived streptomycetes. For instance, Yuan et al. [100] isolated two novel compounds from mangrove Streptomyces sp. 211726: azalomycin F4a 2-ethylpentyl ester and azalomycin F5a 2-ethylpentyl ester, where both compounds exhibited strong cytotoxic activity against the HCT-116 colon cancer cell line with IC50 values as low as 5.64 µg/mL and 2.58 µg/mL, respectively. Furthermore, Fu et al. [101] discovered streptocarbazoles A and B—novel indolocarbazoles isolated from Streptomyces sp. FMA. Streptocarbazole A exhibited impressive cytotoxic effect against human cancer cells including leukaemia HL-60 cells (IC50 = 1.4 µM or 0.67 µg/mL), lung cancer A549 cells (IC50 = 5.0 µM or 2.41 µg/mL), and HeLa cells (IC50 = 34.5µM or 16.61 µg/mL), while the streptocarbazole B exhibited a cytotoxic effect against HeLa cells (IC50 = 22.5µM or 10.47 µg/mL) only. Further analysis revealed that streptocarbazole A (10 µM) caused the cell cycle arrest of HeLa cells in the G2/M phase [101]. Recently, halichoblelide D—a new elaiophylin derivative—was one of the bioactive secondary metabolites isolated from a mangrove strain Streptomyces sp. 219807. It was found that this new compound exerted significant cytotoxicity against HeLa cells (IC50 = 0.3 µM or 0.27 µg/mL and MCF-7 cells (IC50 = 0.33 µM or 0.30 µg/mL) among other known strong cytotoxic compounds that were also isolated, such as 2-methylelaiophylin (IC50 = 0.29 µM or 0.30 µg/mL against both HeLa and MCF-7 cells) and elaiophylin (IC50 = 0.29 µM or 0.30 µg/mL against HeLa cells; IC50 = 0.19 µM or 0.19 µg/mL against MCF-7 cells) [102].
Nevertheless, some studies utilized assays aside from MTT for the evaluation of the cytotoxic activity of the compounds, for examples, the sulforhodamine B (SRB) method, cell counting kit-8 (CCK-8) (Dojindo) assay, and the lactate dehydrogenase (LDH) assay. The SRB method estimates the total cellular protein content, which is based on the ability of SRB dye binding in a pH-dependent and electrostatic manner on protein basic amino acid residues of trichloroacetic acid (TCA)-fixed cells in mild acidic conditions. Weak bases including Tris base enable SRB extraction quantitatively from cells and solubilize SRB for optical density measurement. Therefore, the number of viable cells and cellular protein measured are linearly related [103][104]. For instance, a study conducted by Chen et al. [105] utilized the SRB assay to evaluate the cytotoxic activity of several novel compounds isolated from a mangrove streptomycete. Novel bagremycins C–E, phenol esters of p-hydroxystyrene and p-hydroxybenzoic acid, were produced by Streptomyces sp. Q22, where bagremycin C was active against human glioma cell lines U87MG (IC50 = 2.2 µM), U251 (IC50 = 4.3 µM), and SHG44 (IC50 = 2.4 µM) [105].
The CCK-8 assay is a colorimetric assay utilized to measure the in vitro cell viability for cell cytotoxicity and proliferation experimentation. The assay operates upon the reduction of Dojindo’s water-soluble tetrazolium salt, 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (WST-8) to a yellow formazan dye by dehydrogenase reactions. The amount of formazan dye generated will be equivalent to the number of living cells [106]. The study by Li et al. [107] reported the antiproliferative activity of iakyricidins A–D (produced by Streptomyces iakyrus SCSIO NS104) using the CCK-8 assay. In the study, the most notable compound was iakyricidin A which generated the strongest antiproliferative activity against the human renal carcinoma ACHN cells with IC50 of 0.02 µM, with weak antiproliferative activity against human renal carcinoma 786-O and OS-RC-2 cell lines. However, iakyricidins B–D showed weak to moderate antiproliferative activity against ACHN, 786-O and OS-RC-2 cells. In addition, Yu et al.[108] investigated the antiproliferative and cytotoxic activities of compounds isolated from Streptomyces xiamenensis 318 by using both the CCK-8 assay and lactate dehydrogenase (LDH) assay. The lactate dehydrogenase (LDH) assay is another common method used to determine cytotoxicity by measuring the LDH cytoplasmic enzyme released by damaged cells once the plasma membrane is destructed. This situation occurs when the cell is undergoing a process of cellular damage such as apoptosis and necrosis. The quantification of LDH activity can be done in the conversion process of lactate to pyruvate. This conversion is accompanied with the production of NADH that reduces yellow-coloured tetrazolium salt (INT) into a red-coloured water-soluble formazan-class dye can then be easily quantified. The quantity of this formazan dye directly indicates the content of LDH which is an indicator for cell damage/death [109]. In that study, the compounds, namely ikarugamycin (IC50 = 1.30 µM), capsimycin (IC50 = 3.33 µM), and capsimycin B (IC50 = 3.37 µM) possessed strong antiproliferative activity against pancreatic cancer cells without significantly affecting normal human pancreatic ductal cells [108].
The research on anticancer compounds commonly involves plants as one of the major sources. Plant-derived anticancer compounds such as vincristine of vinca alkaloid family (from Catharanthus roseus) and etoposide (from Podophyllum peltatum) are successful chemotherapy medicines [21][110]. Nevertheless, the expansion in microbial natural products research has led to the revelation of clinically important compounds such as romidepsin (from Chromobacterium violaceum) [111], wortmannin (from Talaromyces wortmannin and Penicillium funiculosum) [112][113], marinomycins (Marinispora spp.) [114], saliniquinones (from Salinispora arenicola) [115] and many more, which exerted potent cytotoxic capabilities. Thus, the current entry attempts to provide a fresh perspective on anticancer compounds from mangrove-derived streptomycetes. There is evidence that several new compounds were successfully recovered from streptomycetes as shown in Figure 1, and this may indicate that the acquisition of streptomycetes from underexplored sources such as mangrove can be an effective strategy to search for new anticancer agents as candidates for drug development [66]. Previous findings have insinuated that mangrove-derived actinomycetes are producers of bioactive compounds of alkaloids, dilactones, macrolides, xiamycins, salinosporamides, indole alkaloids, 2-pyranones, sesquiterpenes, and phenazines [77][88]. According to the studies compiled in this entry, the recovered cytotoxic compounds belonging to dilactones (e.g., antimycins), indole alkaloids (e.g., streptocarbazoles), and macrolides (e.g., azalomycins, elaiophylins) are produced by mangrove-derived streptomycetes, in which the compound families identified are consistent with the earlier findings. Antimycins can be produced by streptomycetes of various origin. For instance, Streptomyces sp. AZ-AR-262 [116] from a terrestrial Egyptian soil sample, Streptomyces sp. K01-0031 [117] from a mountain soil sample and Streptomyces lusitanus [118] of marine source are capable of antimycins production. However, these antimycins were initially known for their antimicrobial activities. The anticancer property of antimycins was later investigated by Hu et al. [119] on lung cancer, breast cancer, and glioblastoma cells, which subsequently demonstrated a promising outcome. Hence, this had established the potential of antimycins as lead compounds for chemotherapeutic medicines. One of the most compelling discoveries was the isolation of ergosterols from mangrove-derived streptomycetes. Ergosterols are conventionally found in fungi, sponges, and corals [120][121][122], until Zhang et al. [123] first described the isolation of cytotoxic ergosterols produced by a Streptomyces strain. This is likely owed to the presence of cholesterol oxidase in streptomycetes which is important for the transformation of sterols. Overall, most of these compounds in Table 1 have unique structures and possible medicinal use, therefore this scenario further accentuates the status of mangrove-derived streptomycetes as astounding natural product producers.
Figure 1. Chemical structures of the novel compounds produced by mangrove-derived streptomycetes. Chemical structures were obtained from the studies conducted by: (1) Xie et al. [99], (2) Yuan et al. [100], (3) Fu et al. [101], (4) Fu et al. [124], (5) Mangamuri et al. [125], (6) Zhang et al. [123], (7) Han et al. [102], and (8) Hu et al. [119].
5. Crude Extracts of Mangrove-Derived Streptomyces spp. with Anticancer/Cytotoxic Activity
As most research focuses on the investigation of bioactivities of pure compounds extracted from streptomycetes, it is also important to acknowledge other studies that performed bioactivity screening on crude extracts of streptomycetes (Table 1). Among these different crude extracts, it can be observed that the crude ethyl acetate extract of Streptomyces sp. ACT01 isolated from the Indian mangrove ecosystem demonstrated potent cytotoxicity against MCF-7 and MDA-MB-231 breast cancer cell lines with low IC50 values of 19.49 µg/mL and 32.79 µg/mL, respectively [126]. Similarly, Tan et al. [127] also revealed that the ethyl acetate fraction of Streptomyces sp. MUM256 isolated from a Malaysia mangrove forest was the most cytotoxic against HCT-116 colon cancer cells (IC50 value of 88.44 µg/mL). It was also reported that this particular fraction of Streptomyces sp. MUM256 crude extract was capable of: (a) reducing the proliferation of HCT-116 cells by causing cell-cycle arrest in the G1 and G2 phases, and (b) inducing mitochondrial-mediated apoptosis in HCT-116 cells.
Additionally, Ser et al. [30] reported the cytotoxic activity showed by the methanolic extract of a novel strain, Streptomyces malaysiense, isolated from a mangrove forest in Malaysia. The extract was active against four types of human cancer cell lines: colon cancer cell lines HCT-116 and HT-29, cervical cancer cell line Ca Ski, and lung cancer cell line A549. The extract exhibited the greatest cytotoxicity against HCT-116 with a cell viability of 48.8% at the highest tested concentration of 400 µg/mL. Notably, the diversity and bioactivities of streptomycetes from mangrove environments in Malaysia were investigated by Law et al. [75]. High-throughput in vitro cytotoxic activity screening was conducted in the study and the results showed that 11 Streptomyces isolates exhibited significant cytotoxicity against several human colon cancer cell lines such as SW480, HT-29, HCT-116, and Caco-2.
In most situations, crude extracts’ cytotoxic activity is often considered a preliminary outcome that may not reflect the true activity. The promising cytotoxicity exhibited by these extracts should not be neglected either. It could be worthwhile to perform additional experiments on these extracts to further evaluate the compounds present responsible for the anticancer-related property. For some cases, the crude extract’s chemical profiling is performed with the application of gas chromatography–mass spectrometry (GC–MS) before conducting any purification of the compound. Such an approach can be observed in several studies that reported the GC–MS detection of various phenolic and pyrrolopyrazine compounds in Streptomyces strains such as Streptomyces pluripotens, Streptomyces malaysiense, Streptomyces colonosanans, Streptomyces sp. MUM256, and MUM265 isolated from Malaysian mangroves [29][30][33][84]. Phenolic compounds and pyrrolopyrazines (cyclic dipeptides) are regarded as compounds capable of exerting anticancer activity and other biological activities such as antioxidant and antimicrobial [128][129][130][131].
Table 1. Summary of studies pertaining to the anticancer/cytotoxic activity of mangrove-derived Streptomyces during the period 2008–2019.
|
No. |
Author |
Strain |
Source |
Compound/crude extract |
IC50 (µg/mL) |
|
Pure compound |
|||||
|
1. |
Xie et al. [99] |
Streptomyces sp. 124092 |
Mangrove rhizosphere soil of Heritiera littoralis (China) |
(1) p-tolyl 3-aminopropanoate * (2) 6-amino-3-(4-hydroxybenzyl)-1,4-diazonane-2,5-dione * (3) n-butyl mannoside (4) daidzein |
SMMC-7721 cells: (1) 22.0; (2) 60.0; (3) 13.6; (4) 6.0 |
|
2. |
Yuan et al.[100] |
Streptomyces sp. 211726 |
Mangrove rhizosphere soil of Heritiera globosa (China) |
(1) Azalomycin F4a 2-ethylpentyl ester * (2) Azalomycin F5a 2-ethylpentyl ester * |
HCT-116 cells: (1) 5.64; (2) 2.58 |
|
3. |
Fu et al. [101] |
Streptomyces sp. FMA |
Mangrove soil (China) |
(1) Streptocarbazole A* (2) Streptocarbazole B* |
HL-60 cells: (1) 0.67a A549 cells: (1) 2.41a HeLa cells: (1) 16.61a; (2) 10.47a
|
|
4. |
Cibi and Nair [132] |
Streptomyces parvulus CBJ1 |
Mangrove soil (India) |
Actinomycin D |
A549 cells: 0.52 |
|
5. |
Fu et al. [124] |
Streptomyces antibioticus strain H74-21 |
Sediment from a mangrove site (China) |
Streptomyceamide C *
|
MCF-7 cells: 27.0 |
|
6. |
Mangamuri et al. [125] |
Streptomyces cheonanensis VUK-A |
Sediment from Coringa mangrove ecosystem (India) |
2-Methyl butyl propyl phthalate * |
MCF-7 cells: 1391.7 MDA-MB-231 cells: 278.34 HeLa cells: 27.834 OAW-42 cells: 278.34 |
|
7. |
Zhang et al. [123] |
Streptomyces anandii H41-59 |
Sediment from a mangrove district (China) |
(1) Ananstrep A * (2) Ananstrep B * (3) Ananstrep C * (4) Ergosta-7,22-diene-3β,5α,6β,25-tetraol (5) Ergosta-7,22-diene-3β,5α,6β-triol (6) Ergosta-7,22-diene-3β,5α,6α-triol (7) Ergosta-7,22-diene-3β,5α,6β,9α-tetraol (8) 5α,6α-epoxy-ergosta-8(9),22-diene-3β,7α-diol (9) 5α,6α-epoxy-ergosta-8(14),22-diene-3β,7α-dio (10) Ergosta-8(9),22-diene-3β,5α,6β,7α-tetraol (11) Ergosta-8(14),22-diene-3β,5α,6β,7α-tetraol (12) Ergosta-5,7,22–triene-3β-ol 5β,6β-epoxy-ergosta-8(14),22-diene-3β,7β-diol |
MCF-7 cells: all compounds exhibited > 50; except (3) 18.1, (8) 24.3, (10) 17.3, and (11) 27.4 SF-268 cells: all compounds exhibited > 50; except (3) 13.0, (8) 15.5, (10) 27.8, and (11) 25.1 NCl-H460 cells: all compounds exhibited > 50; except (3) 23.5, (8) 19.8, (10) 23.7, and (11) 23.7
|
|
8. |
Han et al. [102] |
Streptomyces sp. 219807 |
Mangrove soil (China) |
(1) Halichoblelide D* (2) 2-methyl-11,11’-O-dimethylelaiophylin (3) 2-methylelaiophylin (4) Elaiophylin (5) 11-O-methyl-elaiophylin (6) 11,11’-O-dimethylelaiophylin (7) Efomycin G |
HeLa cells: (1) 0.27a; (2) 1.20a; (3) 0.30a; (4) 0.30a; (5) 0.59a; (6) 0.60a; (7) 0.60a MCF-7 cells: (1) 0.30a; (2) 2.26a; (3) 0.30a; (4) 0.19a; (5) 1.00a; (6) 1.01a; (7) 0.80a |
|
9. |
Hu et al. [119] |
Streptomyces antibioticus H12-15 |
Sediment from a mangrove district (China) |
(1) Neoantimycin A * (2) Neoantimycin B * (3) Antimycin A1ab (4) Antimycin A2a (5) Antimycin A9 |
MCF-7 cells: (1) >50; (2) >50; (3) 18.1; (4) 36.4; (5) 26.1 SF-268 cells: (1) 33.6; (2) 41.6; (3) <1.6; (4) <1.6; (5) <1.6 NCl-H460 cells: (1) >50; (2) >50; (3) 21.7; (4) 43.7; (5) 15.5 |
|
Crude extract |
|||||
|
10. |
Ravikumar et al. [126] |
Streptomyces sp. ACT01, ACT02, ACT03, ACT04, and ACT 05 |
Mangrove sediment from Manakkudi mangrove ecosystem (India) |
Crude ethyl acetate extract |
MCF-7 cells: (ACT01) 19.49; (ACT02) 29.94; (ACT03) 75.54; (ACT04) 66.04; (ACT05) 92.64 MDA-MB-231 cells: (ACT01) 32.79; (ACT02) 69.84; (ACT03) 84.09; (ACT04) >100; (ACT05) >100 |
|
11. |
Ser et al. [33] |
Streptomyces pluripotens MUSC 137T |
Mangrove soil (Malaysia) |
Crude methanol extract |
MCF-7 cells: 61.33 HCT-116 cells: 83.72 A549 cells: 147.20 Ca Ski cells: 300.50 HT-29 cells: 300.98 Caco-2, SW480 and DU145 cells: >400 |
|
12. |
Tan et al. [29] |
Streptomyces MUM256 |
Mangrove soil (Malaysia) |
Crude methanol extract |
HCT-116 cells: 292.33 HT-29, Caco-2, SW480, MCF-7, A549, DU 145, and Ca Ski cells: bN.P |
|
13. |
Sanjivkumar et al. [133] |
Streptomyces olivaceus (MSU3) |
Mangrove rhizosphere soil of Rhizophora mucronata (India) |
Crude ethyl acetate extract |
MCF-7 cells: 88.26 HT-29 cells: 104.81 |
|
14. |
Ser et al. [30] |
Streptomyces malaysiense MUSC 136T |
Mangrove soil (Malaysia) |
Crude methanol extract |
HCT-116, HT-29, A549, and Ca Ski cells: bN.P. |
|
15. |
Law et al. [84] |
Streptomyces colonosanans MUSC 93JT |
Mangrove soil (Malaysia) |
Crude methanol extract |
HCT-116, HT-29, Caco-2, and SW480 cells: bN.P. |
|
16. |
Ser et al. [134] |
Streptomyces gilvigriseus MUSC 26T |
Mangrove soil (Malaysia) |
Crude methanol extract |
HCT-116, HT-29, Caco-2, and SW480 cells: bN.P. |
|
17. |
Law et al. [135] |
Streptomyces monashensis MUSC 1JT |
Mangrove soil (Malaysia) |
Crude methanol extract |
HCT-116 and SW480 cells: bN.P. |
|
18 |
Law et al. [75] |
11 strains of Streptomyces sp. |
Mangrove soil (Malaysia) |
Crude methanol extract |
HCT-116, HT-29, Caco-2, and SW480 cells: bN.P. |
|
19 |
Tan et al. [127] |
Streptomyces sp. MUM256 |
Mangrove soil (Malaysia) |
Ethyl acetate extract (further study from Tan et al. [29]) |
HCT-116: 88.44 |
|
20 |
Tan et al. [3] |
Streptomyces sp. MUM 265 |
Mangrove soil (Malaysia) |
Crude methanol extract |
HT29 and Caco-2: bN.P. |
* new compound identified in the study.a IC50 value is converted to µg/mL based on calculation of given data in the study; bN.P = IC50 value is not provided in the study. Note: cytotoxic activity was analysed using the MTT method involving human cancer cell lines (SMMC-7721 = hepatocarcinoma cell line; A549 and NCl-H460 = lung cancer cell lines; HCT-116, HT-29, and SW480 = colon cancer cell lines; HL-60 = leukaemia cell line; MCF-7 and MDA-MB-231 = breast cancer cell lines; SF-268 = glioblastoma cell line; Ca Ski = cervical cancer cell line; DU 145 = prostate cancer cell line; HeLa = cervical cancer cell line; OAW-42 = ovarian cancer cell line).
6. Limitations and Suggestions for Future Research
The research on the anticancer property of mangrove-derived streptomycetes is still in the preliminary stage. Moreover, all relevant studies compiled in Table 1 were conducted on mangrove areas in Asian countries, mainly China, Malaysia, and India. The present information is restricted to these countries, and it is encouraged to explore the anticancer potentials of streptomycetes isolated from mangroves of countries in other regions such as South America, Africa, Australia, and North America [136]. It is also necessary to carry out the in-depth research and exploration of the pure compounds or crude extracts with anticancer activity to provide a better understanding of their potential as chemotherapeutic drugs. For pure compounds, additional in vitro and in vivo analyses are required to investigate the compound’s underlying anticancer mechanisms on the cancer cells (e.g., apoptotic pathway) [77].
Collaborative approaches for interdisciplinary research through innovative techniques for more extensive investigation on both novel strains and bioactive compounds are of significance for discovering new therapeutic agents to improve human health. Bioassay-guided fractionation and purification can be applied to identify the possible pure compound(s) from crude extracts responsible for bioactivity of interest [137]. A crucial step in this process is the separation of extracts into several fractions. This can be done by several chromatography techniques such as high-performance liquid chromatography (HPLC), thin-layer chromatography (TLC), and gel chromatography. The eluted fractions will be tested to search for the bioactivity of interest accordingly. Occasionally, the fraction that is determined to be the most bioactive component of the extract can be further fractionated until the pure active compound is isolated for identification. Finally, the detection and elucidation of the structure of the active compound can be done using a combination of these techniques—mass spectrometry, nuclear magnetic resonance (NMR), or infrared spectroscopy (IR) [138].
It has always been a challenge to cultivate all naturally occurring microbes due to the presence of viable but non-culturable states of bacteria; over 99% of them resist being cultivated in the laboratory. Thus, their chemical richness remain untapped [20]. This could greatly limit the isolation and discovery of potentially useful bacteria. Nonetheless, research involves utilization of recent molecular techniques could enable the detection and identification of non-culturable strains in environmental samples via extraction and sequencing of nucleic acids (ribosomal rRNA or DNA) [20][76][139][140]. An important technique known as “metagenomics” is commonly used for discovering uncultivable microorganisms from different environments. In this technique, microbial DNA will be isolated from a selected environmental sample, and the DNA sequences obtained will be the representation of microorganisms present in the sample, which may contain hundreds or thousands of microbial species per metagenome [141][142]. By taking the study conducted by Kaur et al. [143] as an example, 16S metagenomic approach was applied to investigate the microbial diversity of a Manikaran (India) hot spring. A culture-independent method will be more reliable for the study of bacteria from the extreme environment as their recovery using the traditional culture method may be compromised due to the unique growth conditions adapted by these bacteria in hot springs (e.g., a high temperature of 30–96 ◦C, nutrients level, etc.). This metagenomic study had successfully revealed the diversity of bacteria in the Manikaran hot spring which comprised those belonging to the phyla Actinobacteria, Acidobacteria, Bacteriodetes, Cyanaobacteria, Chloroflexi, Chlorobi, Deinococcus, Proteobacteria, Rhodothermeota, and Verrucomicrobia.
Each type of environmental sample offers a collection of DNA sequences that may encompass gene clusters responsible for natural products’ biosynthesis [144][145]. Genomic studies of streptomycetes could then provide a genetic basis to enhance the understanding of the secondary metabolism and the production of target bioactive metabolites, thus creating an opportunity to discover novel bioactive compounds. The advent of next-generation sequencing (NGS) technology allows the high throughput sequencing of bacteria’s whole genome with improving read lengths. This genomic information can be utilized for biosynthetic gene clusters mining using sophisticated computational software, e.g., antibiotics and Secondary Metabolite Analysis Shell (antiSMASH), to accelerate the annotation of metabolite gene clusters capable of natural product biosynthesis, hence allowing the study of natural product biosynthetic pathway [146][147]. Interestingly, the whole genome sequence of Streptomyces monashensis MUSC 1JT (Table 1) was examined by Ser et al. [148] using antiSMASH analysis and the data showed that there was 59 biosynthetic gene clusters affiliated to the biosynthesis of secondary metabolites. The analysis had detected a gene cluster related to the biosynthesis of desferrioxamine B (83% similarities) in the genome of Streptomyces monashensis MUSC 1JT. Desferrioxamine was used to treat iron overload and has been associated with anticancer properties such as diminishing leukaemia growth and neuroblastoma [149]. The presence of this gene cluster reemphasizes the bioactive value of Streptomyces monashensis MUSC 1JT which possessed antioxidant activity and cytotoxic properties against human colon cancer cells [135][148][149]. Likewise, the whole genome of Streptomyces gilvigriseus MUSC 26T was sequenced and analysed by Ser et al. [67] and thereby disclosed the presence of a biosynthetic gene cluster associated with the production of desferrioxamine B (40% similarities) via antiSMASH, whilst Prediction Informatics for Secondary Metabolomes (PRISM) and Bacteriocin Genome Mining Tool (BAGEL3) detected several biosynthetic gene clusters that related to class I lantipeptide, lasso peptide, and bacteriocin production.
Subsequently, the information obtained from genomic analysis could improve the production of the target compound [150]. This can be achieved by several methods, for example, the overexpression of structural or regulatory genes involved in the biosynthesis of target metabolites, and the activation and expression of silent biosynthetic gene clusters in homologous or heterologous host. A study conducted by Malla et al. [151] reported that the overexpression of regulatory genes dnrl, dnrN, and afsR combination resulted in a 4.3-fold increased production of doxorubicin by Streptomyces peucetius. Typically, the biosynthetic gene clusters involved in the biosynthesis of the metabolite of interest can be identified using various bioinformatics tools. Then, the activation of the target biosynthetic gene clusters can be done through two main strategies [152][153]: (1) homologous expression, which involves eliciting the gene expression in the native host through the manipulation of endogenous transcriptional, translational or metabolic elements by mutation or (bio)chemical stimulants; (2) heterologous expression, which involves the activation of biosynthetic gene clusters in a non-producing ‘clean’ host through direct cloning or gene synthesis. Once the targeted compound is produced, the process is followed by the identification, isolation, and structural elucidation of the corresponding compound [152][154]. The study of biosynthetic pathways can provide a better understanding regarding the mechanisms of production of chemo-preventive lead compound(s) by streptomycetes.
3. Conclusions
Natural products play significant roles in improving human health and wellbeing, including their application in cancer prevention and treatment regimens. The plethora of bioactive secondary metabolites produced by streptomycetes has shown great biopharmaceutical potential. Mangrove environments posed as underexplored habitats have been recently exposed as rich sources of bioactive streptomycetes. The current entry shows that mangrove-derived streptomycetes are promising producers of compounds with anticancer properties, some of which are new compounds such as streptocarbazoles A and B, streptomyceamide C, neoantimycins A, and B. In addition to that, extracts of these mangrove-derived streptomycetes have demonstrated great anticancer potential. Thus, this urges the need to expand the research in this area, which involves several approaches such as the purification and identification of target compounds as well as the genomic studies and analysis of biosynthetic gene clusters of the active strains. There is limited knowledge about the anticancer activity mechanisms of these compounds in cells and living organisms; therefore, it is also important to conduct further investigations to determine the effectiveness of the compounds as potential new drugs or lead compounds for cancer treatment.
References
- Chandrakanth Are; Kelly M. McMasters; Armando Giuliano; Ujwal Yanala; Charles Balch; Benjamin O. Anderson; Russell Berman; Riccardo Audisio; Tibor Kovacs; Dhairyasheel Savant; et al.Rajendra TopraniGamal AmiraIbrahim SallamJeong Heum BaekMoo-Jun BaekDo Joong ParkGregorio Quintero BeuloEnrique Bargallo RochaHector Martinez SaidMuhammad CheemaAbul AlikhanLloyd MackGong ChenClaudio Almeida QuadrosTarcisio ReisHeber Salvador De Castro RibeiroDouglas ZippelAugusto Leon RamirezYasuyuki SetoKazuhiro YoshidaMasaki Mori Global Forum of Cancer Surgeons: Perspectives on Barriers to Surgical Care for Cancer Patients and Potential Solutions. Annals of Surgical Oncology 2019, 26, 1577-1582, 10.1245/s10434-019-07301-2.
- Ghanbar Mahmoodi Chalbatani; Hassan Dana; Feridon Memari; Elahe Gharagozlou; Shirin Ashjaei; Peyman Kheirandish; Vahid Marmari; Habibollah Mahmoudzadeh; Farnaz Mozayani; Ali Reza Maleki; et al.Ehsan SadeghianElham Zainali NiaSeyed Rohollah MiriNeda Zainali NiaOmid RezaeianAnahita EskandaryNarges RazaviMohammad ShirkhodaFatemeh Nouri Rouzbahani Biological function and molecular mechanism of piRNA in cancer. Practical Laboratory Medicine 2019, 13, e00113, 10.1016/j.plabm.2018.e00113.
- Loh Teng-Hern Tan; Kok-Gan Chan; Priyia Pusparajah; Wai Fong Yin; Tahir Mehmood Khan; Learn-Han Lee; Bey Hing Goh; Mangrove derived Streptomyces sp. MUM265 as a potential source of antioxidant and anticolon-cancer agents. BMC Microbiology 2019, 19, 1-16, 10.1186/s12866-019-1409-7.
- Oren Hannun Levine; Kevin Zbuk; Colorectal cancer in adolescents and young adults: Defining a growing threat. Pediatric Blood & Cancer 2019, 66, e27941, 10.1002/pbc.27941.
- Christine B. Ambrosone; Jo L. Freudenheim; Saxon Graham; James R. Marshall; John E. Vena; John R. Brasure; Arthur M. Michalek; Rosemary Laughlin; Takuma Nemoto; Kari A. Gillenwater; et al.Anita M. HarringtonPeter G. Shields Cigarette Smoking, N-Acetyltransferase 2 Genetic Polymorphisms, and Breast Cancer Risk. JAMA 1996, 276, 1494-1501, 10.1001/jama.1996.03540180050032.
- David Limsui; Robert A. Vierkant; Lori S. Tillmans; Alice H. Wang; Daniel J. Weisenberger; Peter W. Laird; Charles F. Lynch; Kristin E. Anderson; Amy J. French; Robert W. Haile; et al.Lisa J. HarnackJohn D. PotterSusan L. SlagerThomas C. SmyrkStephen N. ThibodeauJames R. CerhanPaul J. Limburg Cigarette Smoking and Colorectal Cancer Risk by Molecularly Defined Subtypes. JNCI Journal of the National Cancer Institute 2010, 102, 1012-1022, 10.1093/jnci/djq201.
- Global Burden of Disease Cancer Collaboration; Christina Fitzmaurice; Degu Abate; Naghmeh Abbasi; Hedayat Abbastabar; Foad Abd-Allah; Omar Abdel-Rahman; Ahmed Abdelalim; Amir Abdoli; Ibrahim Abdollahpour; et al.Abdishakur S. M. AbdulleNebiyu Dereje AbebeHaftom Niguse AbrahaLaith Jamal Abu-RaddadAhmed AbualhasanIsaac Akinkunmi AdedejiShailesh M. AdvaniMohsen AfaridehMahdi AfshariMohammad AghaaliDominic AgiusSutapa AgrawalAyat AhmadiElham AhmadianEhsan AhmadpourMuktar Beshir AhmedMohammad Esmaeil AkbariTomi AkinyemijuZiyad Al-AlyAssim M. AlabdulkaderFares AlahdabTahiya AlamGenet Melak AlameneBirhan Tamene T. AlemnewKefyalew Addis AleneCyrus AliniaVahid AlipourSyed Mohamed AljunidFatemeh Allah BakesheiMajid Abdulrahman Hamad AlmadiAmir Almasi-HashianiUbai AlsharifShirina AlsowaidiNelson Alvis-GuzmanErfan AminiSaeed AminiYaw Ampem AmoakoZohreh AnbariNahla Hamed AnberCatalina Liliana AndreiMina AnjomshoaFereshteh AnsariAnsariadi AnsariadiSeth Christopher Yaw AppiahMorteza Arab-ZozaniJalal ArablooZohreh ArefiOlatunde AremuHabtamu Abera AreriAl ArtamanHamid AsayeshEphrem Tsegay AsfawAlebachew Fasil AshagreReza AssadiBahar AtaeiniaHagos Tasew AtalayZerihun AtaroSuleman AtiqueMarcel AusloosLeticia Avila-BurgosEuripide F. G. A. AvokpahoAshish AwasthiNefsu AwokeBeatriz Paulina Ayala QuintanillaMartin Amogre AyanoreHenok Tadesse AyeleEbrahim BabaeeUmar BachaAlaa BadawiMojtaba BagherzadehEleni BagliSenthilkuimar BalakrishnanAbbas BalouchiTill Winfried BärnighausenRobert J. BattistaMasoud BehzadifarMeysam BehzadifarBayu Begashaw BekeleYared Belete BelayYaschilal Muche BelaynehKathleen Kim Sachiko BerfieldAdugnaw BerhaneEduardo BernabeMircea BeuranNickhill BhaktaKrittika BhattacharyyaBelete BiadgoAli BijaniMuhammad Shahdaat Bin SayeedCharles BirungiCatherine BisignanoHelen BitewTone BjørgeArchie BleyerKassawmar Angaw BogaleHunduma Amensisa BojiaAntonio M. BorzìCristina BosettiIbrahim R. Bou-OrmHermann BrennerJerry D. BrewerAndrey Nikolaevich BrikoNikolay Ivanovich BrikoMaria Teresa Bustamante-TeixeiraZahid A. ButtGiulia CarrerasJuan J. CarreroFélix CarvalhoClara CastroFranz CastroFerrán Catalá-LópezEster CerinYazan ChaiahWagaye Fentahun ChanieVijay Kumar ChattuPankaj ChaturvediNeelima Singh ChauhanMohammad ChehraziPeggy Pei-Chia ChiangTesfaye Yitna ChichiabelluOnyema Greg Chido-AmajuoyiOdgerel Chimed-OchirJee-Young J. ChoiDevasahayam J. ChristopherDinh-Toi ChuMaria-Magdalena ConstantinVera M. CostaEmanuele CrocettiChristopher Stephen CroweMaria Paula CuradoSaad M. A. DahlawiGiovanni DamianiAmira Hamed DarwishAhmad DaryaniJosé Das NevesFeleke Mekonnen DemekeAsmamaw Bizuneh DemisBirhanu Wondimeneh DemissieGebre Teklemariam DemozEdgar Denova-GutiérrezAfshin DerakhshaniKalkidan Solomon DeribeRupak DesaiBeruk Berhanu DesalegnMelaku DestaSubhojit DeySamath Dhamminda DharmaratneMeghnath DhimalDaniel DiazMesfin Tadese Tadese DinberuShirin DjalaliniaDavid Teye DokuThomas M. DrakeManisha DubeyEleonora DubljaninEyasu Ejeta DukenHedyeh EbrahimiAndem EffiongAziz EftekhariIman El SayedMaysaa El Sayed ZakiShaimaa I. El-JaafaryZiad El-KhatibDemelash Abewa EleminehHajer ElkoutRichard G. EllenbogenAisha ElsharkawyMohammad Hassan EmamianDaniel Adane EndalewAman Yesuf EndriesBabak EshratiIbtihal FadhilVahid Fallah OmraniMahbobeh FaramarziMahdieh Abbasalizad FarhangiAndrea FarioliFarshad FarzadfarNetsanet FentahunEduarda FernandesGarumma Tolu FeyissaIrina FilipFlorian FischerJames L. FisherLisa M. ForceMasoud ForoutanMarisa FreitasTakeshi FukumotoNeal D. FutranSilvano GallusFortune Gbetoho GankpeReta Tsegaye GayesaTsegaye Tewelde GebrehiwotGebreamlak Gebremedhn GebremeskelGetnet Azeze GedefawBelayneh K. GelawBirhanu GetaSefonias GetachewKebede Embaye GezaeMansour GhafourifardAlireza GhajarAhmad GhashghaeeAsadollah GholamianParamjit Singh GillThemba T. G. GinindzaAlem GirmayMuluken GizawRicardo Santiago GomezSameer Vali GopalaniGiuseppe GoriniBárbara Niegia Garcia GoulartAyman GradaMaximiliano Ribeiro GuerraAndre Luiz Sena GuimaraesPrakash C. GuptaRahul GuptaKishor HadkhaleArvin Haj-MirzaianArya Haj-MirzaianRandah R. HamadehSamer HamidiLolemo Kelbiso HanforeJosep Maria HaroMilad HasankhaniAmir HasanzadehHamid Yimam HassenRoderick J. HaySimon I. HayAndualem HenokNathaniel J. HenryClaudiu HerteliuHagos D. HidruChi Linh HoangMichael K. HolePraveen HoogarNobuyuki HoritaH. Dean HosgoodMostafa HosseiniMehdi HosseinzadehMihaela HostiucSorin HostiucMowafa HousehMohammedaman Mama HussenBogdan IleanuMilena D. IlicKaire InnosSeyed Sina Naghibi IrvaniKufre Robert IsehSheikh Mohammed Shariful IslamFarhad IslamiNader Jafari BalalamiMorteza JafariniaLeila JahangiryMohammad Ali JahaniNader JahanmehrMihajlo JakovljevicSpencer L. JamesMehdi JavanbakhtSudha JayaramanSun Ha JeeEnsiyeh JenabiRavi Prakash JhaJost B Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017. JAMA Oncology 2019, 5, 1749-1768, 10.1001/jamaoncol.2019.2996.
- Rebecca L. Siegel; Kimberly D. Miller Mph; Ahmedin Jemal; Cancer statistics, 2020. CA: A Cancer Journal for Clinicians 2020, 70, 7-30, 10.3322/caac.21590.
- Lien Ai Pham-Huy; Hua He; Chuong Pham-Huy; Free Radicals, Antioxidants in Disease and Health. International journal of biomedical science : IJBS 2008, 4, 89-96.
- Ruth Clark; Seong-Ho Lee; Anticancer Properties of Capsaicin Against Human Cancer.. Anticancer Research 2016, 36, 837–843.
- Molly A. Thoreson; Albert B. Reynolds; Altered expression of the catenin p120 in human cancer: implications for tumor progression. Differentiation 2002, 70, 583-589, 10.1046/j.1432-0436.2002.700911.x.
- Elanor N. Wainwright; Paola Scaffidi; Epigenetics and Cancer Stem Cells: Unleashing, Hijacking, and Restricting Cellular Plasticity. Trends in Cancer 2017, 3, 372-386, 10.1016/j.trecan.2017.04.004.
- Alvin Silverstein; Virginia Silverstein; Laura Silverstein Nunn. Cancer: Conquering a Deadly Disease: Twenty; Twenty-First Century Books: Minneapolis, MN, USA, 2006; pp. -.
- Andrew G. Clark; Danijela Matic Vignjevic; Modes of cancer cell invasion and the role of the microenvironment. Current Opinion in Cell Biology 2015, 36, 13-22, 10.1016/j.ceb.2015.06.004.
- Qinghua Cui; Yun Ma; Maria Jaramillo; Hamza Bari; Arif Awan; Song Yang; Simo Zhang; Lixue Liu; Meng Lu; Maureen O'Connor‐McCourt; et al.Enrico O PurisimaEdwin Wang A map of human cancer signaling. Molecular Systems Biology 2007, 3, 152, 10.1038/msb4100200.
- Rajandeep Kaur; Karan Kapoor; Harpreet Kaur; Plants as a source of anticancer agents.. Journal of Natural Product and Plant Resources 2011, 1, 119–124.
- Peter E Huber; Marc Bischof; Jürgen Jenne; Sabine Heiland; Peter Peschke; Rainer Saffrich; Hermann-Josef Gröne; Jürgen Debus; Kenneth E. Lipson; Amir Abdollahi; et al. Trimodal Cancer Treatment: Beneficial Effects of Combined Antiangiogenesis, Radiation, and Chemotherapy. Cancer Research 2005, 65, 3643-3655, 10.1158/0008-5472.can-04-1668.
- Steven J. Katz; Paula M. Lantz; Nancy K. Janz; Angela Fagerlin; Kendra Schwartz; Lihua Liu; Dennis Deapen; Barbara Salem; Indu Lakhani; Monica Morrow; et al. Patient Involvement in Surgery Treatment Decisions for Breast Cancer. Journal of Clinical Oncology 2005, 23, 5526-5533, 10.1200/jco.2005.06.217.
- Larissa K.F. Temple; Lillian Hsieh; W. Douglas Wong; Leonard Saltz; Deborah Schrag; Use of Surgery Among Elderly Patients With Stage IV Colorectal Cancer. Journal of Clinical Oncology 2004, 22, 3475-3484, 10.1200/jco.2004.10.218.
- Gordon M. Cragg; D. J. Newman; Nature: a vital source of leads for anticancer drug development. Phytochemistry Reviews 2009, 8, 313-331, 10.1007/s11101-009-9123-y.
- A B Da Rocha; Rafael M Lopes; Gilberto Schwartsmann; Natural products in anticancer therapy. Current Opinion in Pharmacology 2001, 1, 364-369, 10.1016/s1471-4892(01)00063-7.
- T Luke Simmons; Eric Andrianasolo; Kerry McPhail; Patricia Flatt; William H Gerwick; Marine natural products as anticancer drugs.. Molecular Cancer Therapeutics 2005, 4, 333-42.
- M Gordaliza; Natural products as leads to anticancer drugs. Clinical and Translational Oncology 2007, 9, 767-776, 10.1007/s12094-007-0138-9.
- Stefania Nobili; Donatella Lippi; Ewa Witort; Martino Donnini; Letizia Bausi; Enrico Mini; Sergio Capaccioli; Natural compounds for cancer treatment and prevention. Pharmacological Research 2009, 59, 365-378, 10.1016/j.phrs.2009.01.017.
- A. Douglas Kinghorn; Young-Won Chin; Steven M. Swanson; Discovery of natural product anticancer agents from biodiverse organisms. Current opinion in drug discovery & development 2009, 12, 189-96.
- Nwokeji Paul Anulika; Enodiana Osamiabe Ignatius; Ezenweani Sunday Raymond; Osaro-Itota Osasere; Akatah Hilda Abiola; The chemistry of natural product: Plant secondary metabolites. International Journal of Technology Enhancements and Emerging Engineering Research 2016, 4, 1-8.
- Lixin Zhang; Rong An; Jinping Wang; Nuo Sun; Si Zhang; Jiangchun Hu; Jun Kuai; Exploring novel bioactive compounds from marine microbes. Current Opinion in Microbiology 2005, 8, 276-281, 10.1016/j.mib.2005.04.008.
- Kandan Aravindaram; Ning-Sun Yang; Anti-Inflammatory Plant Natural Products for Cancer Therapy. Planta Medica 2010, 76, 1103-1117, 10.1055/s-0030-1249859.
- Loh Teng-Hern Tan; Hooi-Leng Ser; Wai-Fong Yin; Kok-Gan Chan; Learn-Han Lee; Bey Hing Goh; Investigation of Antioxidative and Anticancer Potentials of Streptomyces sp. MUM256 Isolated from Malaysia Mangrove Soil. Frontiers in Microbiology 2015, 6, 1316, 10.3389/fmicb.2015.01316.
- Hooi-Leng Ser; Uma Devi Palanisamy; Wai-Fong Yin; Kok-Gan Chan; Bey-Hing Goh; Learn-Han Lee; Streptomyces malaysiense sp. nov.: A novel Malaysian mangrove soil actinobacterium with antioxidative activity and cytotoxic potential against human cancer cell lines. Scientific Reports 2016, 6, 24247, 10.1038/srep24247.
- Frank Kroschinsky; on behalf of the Intensive Care in Hematological and Oncological Patients (iCHOP) Collaborative Group; Friedrich Stölzel; Simone Von Bonin; Gernot Beutel; Matthias Kochanek; Michael Kiehl; Peter Schellongowski; New drugs, new toxicities: severe side effects of modern targeted and immunotherapy of cancer and their management. Critical Care 2017, 21, 1-11, 10.1186/s13054-017-1678-1.
- Thomas M. Suter; Michael S. Ewer; Cancer drugs and the heart: importance and management. European Heart Journal 2012, 34, 1102-1111, 10.1093/eurheartj/ehs181.
- Hooi-Leng Ser; Nurul-Syakima Ab Mutalib; Wai-Fong Yin; Kok-Gan Chan; Bey-Hing Goh; Learn-Han Lee; Evaluation of Antioxidative and Cytotoxic Activities of Streptomyces pluripotens MUSC 137 Isolated from Mangrove Soil in Malaysia. Frontiers in Microbiology 2015, 6, 1398, 10.3389/fmicb.2015.01398.
- C. Gyllenhaal; D D Soejarto; G. Tan; Biodiversity as a Source of Anticancer Drugs. Current Drug Targets 2006, 7, 265-277, 10.2174/138945006776054942.
- Young-Joon Surh; Cancer chemoprevention with dietary phytochemicals. Nature Reviews Cancer 2003, 3, 768-780, 10.1038/nrc1189.
- Peng Li; Xiang Li; Rathi Saravanan; Chang Ming Li; Susanna Su Jan Leong; Antimicrobial macromolecules: synthesis methods and future applications. RSC Advances 2012, 2, 4031-4044, 10.1039/c2ra01297a.
- Nurul-Syakima Ab Mutalib; Sunny Hei Wong; Hooi-Leng Ser; Acharaporn Duangjai; Jodi Woan-Fei Law; Shanti Ratnakomala; Loh Teng-Hern Tan; Vengadesh Letchumanan; Bioprospecting of Microbes for Valuable Compounds to Mankind. Progress In Microbes & Molecular Biology 2020, 3, a0000088, 10.36877/pmmb.a0000088.
- János Bérdy; Bioactive Microbial Metabolites. The Journal of Antibiotics 2004, 58, 1-26, 10.1038/ja.2005.1.
- Anwar Rayan; Jamal Raiyn; Mizied Falah; Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLOS ONE 2017, 12, e0187925, 10.1371/journal.pone.0187925.
- Nancy Martínez-Montiel; Nora Hilda Rosas-Murrieta; Mónica Martínez-Montiel; Mayra Patricia Gaspariano-Cholula; Rebeca D. Martínez-Contreras; Microbial and Natural Metabolites That Inhibit Splicing: A Powerful Alternative for Cancer Treatment. BioMed Research International 2016, 2016, 1-14, 10.1155/2016/3681094.
- Marco Ventura; Carlos Canchaya; Andreas Tauch; Govind Chandra; Gerald F. Fitzgerald; Keith F. Chater; Douwe Van Sinderen; Genomics of Actinobacteria: Tracing the Evolutionary History of an Ancient Phylum. Microbiology and Molecular Biology Reviews 2007, 71, 495-548, 10.1128/mmbr.00005-07.
- Cindy J. Castelle; Jillian F. Banfield; Major New Microbial Groups Expand Diversity and Alter our Understanding of the Tree of Life. Cell 2018, 172, 1181-1197, 10.1016/j.cell.2018.02.016.
- Jodi Woan-Fei Law; Priyia Pusparajah; Nurul-Syakima Ab Mutalib; Sunny Hei Wong; Bey-Hing Goh; Learn-Han Lee; A Review on Mangrove Actinobacterial Diversity: The Roles of Streptomyces and Novel Species Discovery. Progress In Microbes & Molecular Biology 2019, 2, a0000024, 10.36877/pmmb.a0000024.
- Jodi Woan-Fei Law; Vengadesh Letchumanan; Loh Teng-Hern Tan; Hooi-Leng Ser; Bey-Hing Goh; Learn-Han Lee; The Rising of “Modern Actinobacteria” Era. Progress In Microbes & Molecular Biology 2020, 3, a0000064, 10.36877/pmmb.a0000064.
- Essaid Ait Barka; Parul Vatsa; Lisa Sanchez; Nathalie Gaveau-Vaillant; Cedric Jacquard; Hans-Peter Klenk; Christophe Clément; Yder Ouhdouch; Gilles P. Van Wezel; Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiology and Molecular Biology Reviews 2015, 80, 1-43, 10.1128/mmbr.00019-15.
- Nimaichand Salam; Jian-Yu Jiao; Xiao-Tong Zhang; Wen-Jun Li; Update on the classification of higher ranks in the phylum Actinobacteria. International Journal of Systematic and Evolutionary Microbiology 2020, 70, 1331-1355, 10.1099/ijsem.0.003920.
- Selvakumar Dharmaraj; Marine Streptomyces as a novel source of bioactive substances. World Journal of Microbiology and Biotechnology 2010, 26, 2123-2139, 10.1007/s11274-010-0415-6.
- Learn-Han Lee; Nurullhudda Zainal; Adzzie-Shazleen Azman; Shu-Kee Eng; Bey-Hing Goh; Wai-Fong Yin; Nurul-Syakima Ab Mutalib; Kok-Gan Chan; Diversity and Antimicrobial Activities of Actinobacteria Isolated from Tropical Mangrove Sediments in Malaysia. The Scientific World Journal 2014, 2014, 1-14, 10.1155/2014/698178.
- Learn-Han Lee; Nurullhudda Zainal; Adzzie-Shazleen Azman; Shu-Kee Eng; Nurul-Syakima Ab Mutalib; Wai-Fong Yin; Kok-Gan Chan; Streptomyces pluripotens sp. nov., a bacteriocin-producing streptomycete that inhibits meticillin-resistant Staphylococcus aureus. International Journal of Systematic and Evolutionary Microbiology 2014, 64, 3297-3306, 10.1099/ijs.0.065045-0.
- Loh Teng-Hern Tan; Kok-Gan Chan; Tahir Mehmood Khan; Sarah Ibrahim Bukhari; Surasak Saokaew; Acharaporn Duangjai; Priyia Pusparajah; Learn-Han Lee; Bey-Hing Goh; Streptomyces sp. MUM212 as a Source of Antioxidants with Radical Scavenging and Metal Chelating Properties. Frontiers in Pharmacology 2017, 8, 276, 10.3389/fphar.2017.00276.
- Hefa Mangzira Kemung; Loh Teng-Hern Tan; Tahir Mehmood Khan; Kok-Gan Chan; Priyia Pusparajah; Bey-Hing Goh; Learn-Han Lee; Streptomyces as a Prominent Resource of Future Anti-MRSA Drugs. Frontiers in Microbiology 2018, 9, 2221, 10.3389/fmicb.2018.02221.
- Sheila Marie Pimentel-Elardo; Svitlana Kozytska; Tim S. Bugni; Chris M. Ireland; Heidrun Moll; Ute Hentschel; Anti-Parasitic Compounds from Streptomyces sp. Strains Isolated from Mediterranean Sponges. Marine Drugs 2010, 8, 373-380, 10.3390/md8020373.
- Hooi-Leng Eser; Jodi Woan-Fei Law; Nathorn Echaiyakunapruk; Sabrina Anne Jacob; Uma Devi Epalanisamy; Kok Gan Echan; Bey Hing Goh; Learn-Han Lee; Fermentation Conditions that Affect Clavulanic Acid Production in Streptomyces clavuligerus: A Systematic Review. Frontiers in Microbiology 2016, 7, 522, 10.3389/fmicb.2016.00522.
- Rakesh Santhanam; Xiaoying Rong; Ying Huang; Barbara A. Andrews; Juan A. Asenjo; Michael Goodfellow; Streptomyces bullii sp. nov., isolated from a hyper-arid Atacama Desert soil. Antonie van Leeuwenhoek 2012, 103, 367-373, 10.1007/s10482-012-9816-x.
- Learn-Han Lee; Yoke Kqueen Cheah; Shiran Mohd Sidik; Nurul-Syakima Ab Mutalib; Yi-Li Tang; Hai-Peng Lin; Kui Hong; Molecular characterization of Antarctic actinobacteria and screening for antimicrobial metabolite production. World Journal of Microbiology and Biotechnology 2012, 28, 2125-2137, 10.1007/s11274-012-1018-1.
- Demet Tatar; Kiymet Guven; Cathrin Spröer; Hans-Peter Klenk; Nevzat Sahin; Streptomyces iconiensis sp. nov. and Streptomyces smyrnaeus sp. nov., two halotolerant actinomycetes isolated from a salt lake and saltern. International Journal of Systematic and Evolutionary Microbiology 2014, 64, 3126-3133, 10.1099/ijs.0.062216-0.
- Klas Flärdh; Mark J. Buttner; Streptomyces morphogenetics: dissecting differentiation in a filamentous bacterium. Nature Reviews Genetics 2009, 7, 36-49, 10.1038/nrmicro1968.
- Hooi-Leng Ser; Wen-Si Tan; Nurul-Syakima Ab Mutalib; Huey-Jia Cheng; Wai-Fong Yin; Kok-Gan Chan; Learn-Han Lee; Genome sequence of Streptomyces pluripotens MUSC 135T exhibiting antibacterial and antioxidant activity. Marine Genomics 2015, 24, 281-283, 10.1016/j.margen.2015.09.010.
- Hooi-Leng Ser; Kok-Gan Chan; Wen-Si Tan; Wai-Fong Yin; Bey-Hing Goh; Nurul-Syakima Ab Mutalib; Learn-Han Lee; Complete genome of mangrove-derived anti-MRSA streptomycete, Streptomyces pluripotens MUSC 135T. Progress In Microbes & Molecular Biology 2018, 1, a0000004, 10.36877/pmmb.a0000004.
- Hooi-Leng Ser; Wen-Si Tan; Wai-Fong Yin; Kok-Gan Chan; Nurul Syakima Ab Mutalib; Learn-Han Lee; Whole genome sequence of Streptomyces humi strain MUSC 119T isolated from intertidal soil. Progress in Drug Discovery & Biomedical Science 2019, 2, a0000020, 10.36877/pddbs.0000020.
- Hooi-Leng Ser; Jodi Woan-Fei Law; Wen-Si Tan; Wai-Fong Yin; Kok-Gan Chan; Whole genome sequence of Streptomyces colonosanans strain MUSC 93JT isolated from mangrove forest in Malaysia. Progress In Microbes & Molecular Biology 2020, 3, a0000061, 10.36877/pmmb.a0000061.
- Yukinori Tanaka; Ken Kasahara; Yutaka Hirose; Kiriko Murakami; Rie Kugimiya; Kozo Ochi; Activation and Products of the Cryptic Secondary Metabolite Biosynthetic Gene Clusters by Rifampin Resistance (rpoB) Mutations in Actinomycetes. Journal of Bacteriology 2013, 195, 2959-2970, 10.1128/jb.00147-13.
- Stephen D. Bentley; Keith F Chater; A.-M. Cerdeño-Tárraga; Gregory L Challis; Nicholas R Thomson; K. D. James; D. E. Harris; Michael A Quail; H M Kieser; David P Harper; et al.A G BatemanStephen BrownGovind ChandraCarton W ChenMichael T CollinsAlexander D CroninAndrew G FraserArlette GobleJ. HidalgoT. HornsbySamuel J HowarthC.-H. HuangTobias KieserLauren E LarkeLeon O MurphyK. OliverShawn P OneilEster RabbinowitschMarieadele RajandreamK M D RutherfordS M RutterKathy SeegerDiane G O SaundersStephen J SharpR. SquaresK N R TaylorT. WarrenAndreas WietzorrekJ. WoodwardB. G. BarrellJohn A ParkhillDavid A Hopwood Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 2002, 417, 141-147, 10.1038/417141a.
- Haruo Ikeda; Jun Ishikawa; Akiharu Hanamoto; Mayumi Shinose; Hisashi Kikuchi; Tadayoshi Shiba; Yoshiyuki Sakaki; Masahira Hattori; Satoshi Ōmura; Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nature Biotechnology 2003, 21, 526-531, 10.1038/nbt820.
- Jodi Woan-Fei Law; Hooi-Leng Ser; Tahir M. Khan; Lay-Hong Chuah; Priyia Pusparajah; Kok-Gan Chan; Bey-Hing Goh; Learn-Han Lee; The Potential of Streptomyces as Biocontrol Agents against the Rice Blast Fungus, Magnaporthe oryzae (Pyricularia oryzae). Frontiers in Microbiology 2017, 8, 3, 10.3389/fmicb.2017.00003.
- Learn-Han Lee; Kok-Gan Chan; Jem Stach; Elizabeth M. H. Wellington; Bey-Hing Goh; Editorial: The Search for Biological Active Agent(s) From Actinobacteria. Frontiers in Microbiology 2018, 9, 824, 10.3389/fmicb.2018.00824.
- Hooi-Leng Ser; Wen-Si Tan; Nurul-Syakima Ab Mutalib; Wai-Fong Yin; Kok-Gan Chan; Bey-Hing Goh; Learn-Han Lee; Genome sequence of Streptomyces gilvigriseus MUSC 26 T isolated from mangrove forest. Brazilian Journal of Microbiology 2018, 49, 207-209, 10.1016/j.bjm.2017.04.012.
- Arnold L. Demain; Preeti Vaishnav; Natural products for cancer chemotherapy. Microbial Biotechnology 2010, 4, 687-699, 10.1111/j.1751-7915.2010.00221.x.
- Biplob Bhattacharya; Sreya Mukherjee; Cancer Therapy Using Antibiotics. Journal of Cancer Therapy 2015, 6, 849-858, 10.4236/jct.2015.610093.
- Zhiyan Xiao; Susan L. Morris-Natschke; Kuo-Hsiung Lee; Strategies for the Optimization of Natural Leads to Anticancer Drugs or Drug Candidates. Medicinal Research Reviews 2015, 36, 32-91, 10.1002/med.21377.
- Michael Goodfellow; Hans-Peter Fiedler; A guide to successful bioprospecting: informed by actinobacterial systematics. Antonie van Leeuwenhoek 2010, 98, 119-142, 10.1007/s10482-010-9460-2.
- Chinyere K. Okoro; Roselyn Brown; Amanda L. Jones; Barbara A. Andrews; Juan A. Asenjo; Michael Goodfellow; Alan T. Bull; Diversity of culturable actinomycetes in hyper-arid soils of the Atacama Desert, Chile. Antonie van Leeuwenhoek 2008, 95, 121-133, 10.1007/s10482-008-9295-2.
- Marta Maciejewska; Igor Stelmach Pessi; Anthony Arguelles-Arias; Pauline Noirfalise; Géraldine Luis; Marc Ongena; Hazel Barton; Monique Carnol; Sébastien Rigali; Streptomyces lunaelactis sp. nov., a novel ferroverdin A-producing Streptomyces species isolated from a moonmilk speleothem. Antonie van Leeuwenhoek 2014, 107, 519-531, 10.1007/s10482-014-0348-4.
- Hooi-Leng Ser; Nurul-Syakima Ab Mutalib; Wai-Fong Yin; Bey-Hing Goh; Learn-Han Lee; Kok-Gan Chan; Genome sequence of Streptomyces antioxidans MUSC 164T isolated from mangrove forest. Progress In Microbes & Molecular Biology 2018, 1, a0000001, 10.36877/pmmb.a0000001.
- Jodi Woan-Fei Law; Kok-Gan Chan; Ya-Wen He; Tahir Mehmood Khan; Nurul-Syakima Ab Mutalib; Bey Hing Goh; Learn-Han Lee; Diversity of Streptomyces spp. from mangrove forest of Sarawak (Malaysia) and screening of their antioxidant and cytotoxic activities. Scientific Reports 2019, 9, 1-15, 10.1038/s41598-019-51622-x.
- Jodi Woan-Fei Law; Kei-Xian Tan; Sunny Hei Wong; Nurul-Syakima Ab Mutalib; Learn-Han Lee; Taxonomic and Characterization Methods of Streptomyces: A Review. Progress In Microbes & Molecular Biology 2018, 1, a0000009, 10.36877/pmmb.a0000009.
- Hooi-Leng Ser; Loh Teng-Hern Tan; Jodi Woan-Fei Law; Kok-Gan Chan; Acharaporn Duangjai; Surasak Saokaew; Priyia Pusparajah; Nurul-Syakima Ab Mutalib; Tahir Mehmood Khan; Bey Hing Goh; et al.Learn-Han Lee Focused Review: Cytotoxic and Antioxidant Potentials of Mangrove-Derived Streptomyces. Frontiers in Microbiology 2017, 8, 2065-2065, 10.3389/fmicb.2017.02065.
- Karuppiah Valliappan; Wei Sun; Zhiyong Li; Marine actinobacteria associated with marine organisms and their potentials in producing pharmaceutical natural products. Applied Microbiology and Biotechnology 2014, 98, 7365-7377, 10.1007/s00253-014-5954-6.
- Hooi-Leng Eser; Uma Devi Epalanisamy; Wai-Fong Eyin; Sri Nurestri Eabd Malek; Kok-Gan Echan; Bey-Hing Egoh; Learn-Han Lee; Presence of antioxidative agent, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- in newly isolated Streptomyces mangrovisoli sp. nov.. Frontiers in Microbiology 2015, 6, 854, 10.3389/fmicb.2015.00854.
- Hooi-Leng Ser; Nurullhudda Zainal; Uma Devi Palanisamy; Bey-Hing Goh; Wai-Fong Yin; Kok-Gan Chan; Learn-Han Lee; Streptomyces gilvigriseus sp. nov., a novel actinobacterium isolated from mangrove forest soil. Antonie van Leeuwenhoek 2015, 107, 1369-1378, 10.1007/s10482-015-0431-5.
- Patrik Rönnbäck; Beatrice Crona; Lisa Ingwall; The return of ecosystem goods and services in replanted mangrove forests: perspectives from local communities in Kenya. Environmental Conservation 2007, 34, 313-324, 10.1017/s0376892907004225.
- Elizabeth C. Ashton; Donald J. MacIntosh; Preliminary assessment of the plant diversity and community ecology of the Sematan mangrove forest, Sarawak, Malaysia. Forest Ecology and Management 2002, 166, 111-129, 10.1016/s0378-1127(01)00673-9.
- Kalpana Sahoo; Nabin Kumar Dhal; Potential microbial diversity in mangrove ecosystems: A review. Indian Journal of Marine Sciences 2009, 32, 249-256.
- Jodi Woan-Fei Law; Hooi-Leng Ser; Acharaporn Duangjai; Surasak Saokaew; Sarah I. Bukhari; Tahir M. Khan; Nurul-Syakima Ab Mutalib; Kok-Gan Chan; Bey Hing Goh; Learn-Han Lee; et al. Streptomyces colonosanans sp. nov., A Novel Actinobacterium Isolated from Malaysia Mangrove Soil Exhibiting Antioxidative Activity and Cytotoxic Potential against Human Colon Cancer Cell Lines. Frontiers in Microbiology 2017, 8, 877, 10.3389/fmicb.2017.00877.
- Hooi-Leng Ser; Loh Teng-Hern Tan; Uma D. Palanisamy; Sri N. Abd Malek; Wai-Fong Yin; Kok-Gan Chan; Bey Hing Goh; Learn-Han Lee; Streptomyces antioxidans sp. nov., a Novel Mangrove Soil Actinobacterium with Antioxidative and Neuroprotective Potentials. Frontiers in Microbiology 2016, 7, 899, 10.3389/fmicb.2016.00899.
- Annaliesa S. Anderson; E M Wellington; The taxonomy of Streptomyces and related genera.. International Journal of Systematic and Evolutionary Microbiology 2001, 51, 797-814, 10.1099/00207713-51-3-797.
- Kui Hong; An-Hui Gao; Qing-Yi Xie; Hao Gao Gao; Ling Zhuang; Hai-Peng Lin; Hai-Ping Yu; Jia Li; Xin-Sheng Yao; Michael Goodfellow; et al.Jisheng Ruan Actinomycetes for Marine Drug Discovery Isolated from Mangrove Soils and Plants in China. Marine Drugs 2009, 7, 24-44, 10.3390/md7010024.
- Dong-Bo Xu; Wan-Wan Ye; Ying Han; Zixin Deng; Kui Hong; Natural Products from Mangrove Actinomycetes. Marine Drugs 2014, 12, 2590-2613, 10.3390/md12052590.
- Can Chen; Yanghui Ye; Ruijun Wang; Yinglao Zhang; Chen Wu; Sanjit C. Debnath; Zhongjun Ma; Jidong Wang; Min Wu; Streptomyces nigra sp. nov. Is a Novel Actinobacterium Isolated From Mangrove Soil and Exerts a Potent Antitumor Activity in Vitro. Frontiers in Microbiology 2018, 9, 1587, 10.3389/fmicb.2018.01587.
- Jing Xiao; Yin Wang; Yingxue Luo; Shu-Jie Xie; Ji-Sheng Ruan; Luo Yingxue; Streptomyces avicenniae sp. nov., a novel actinomycete isolated from the rhizosphere of the mangrove plant Avicennia mariana. International Journal of Systematic and Evolutionary Microbiology 2009, 59, 2624-2628, 10.1099/ijs.0.009357-0.
- Meyyappan Arumugam; Anindita Mitra; Arnab Pramanik; Malay Saha; Ratan Gachhui; Joydeep Mukherjee; Streptomyces sundarbansensis sp. nov., an actinomycete that produces 2-allyloxyphenol. International Journal of Systematic and Evolutionary Microbiology 2011, 61, 2664-2669, 10.1099/ijs.0.028258-0.
- Jing Xu; Yin Wang; Shu-Jie Xie; Jing Xiao; Ji-Sheng Ruan; Streptomyces xiamenensis sp. nov., isolated from mangrove sediment. International Journal of Systematic and Evolutionary Microbiology 2009, 59, 472-476, 10.1099/ijs.0.000497-0.
- Ling Ding; Jan Münch; Helmar Goerls; Armin Maier; H.H Fiebig; Wen-Han Lin; Christian Hertweck; Xiamycin, a pentacyclic indolosesquiterpene with selective anti-HIV activity from a bacterial mangrove endophyte. Bioorganic & Medicinal Chemistry Letters 2010, 20, 6685-6687, 10.1016/j.bmcl.2010.09.010.
- Hefa Mangzira Kemung; Loh Teng-Hern Tan; Kok-Gan Chan; Hooi-Leng Ser; Jodi Woan-Fei Law; Learn-Han Lee; Bey-Hing Goh; Investigating the antioxidant potential of Streptomyces sp. MUSC 11 from mangrove soil in Malaysia. Progress in Drug Discovery & Biomedical Science 2019, 2, a0000033, 10.36877/pddbs.a0000033.
- Hefa Mangzira Kemung; Loh Teng-Hern Tan; Kok-Gan Chan; Hooi-Leng Ser; Jodi Woan-Fei Law; Learn-Han Lee; Bey Hing Goh; Antioxidant Activities of Streptomyces sp. strain MUSC 14 from Mangrove Forest Soil in Malaysia.. BioMed Research International 2020, 2020, 6402607-12, 10.1155/2020/6402607.
- Hefa Mangzira Kemung; Loh Teng-Hern Tan; Kok-Gan Chan; Hooi-Leng Ser; Jodi Woan-Fei Law; Bey Hing Goh; Streptomyces sp. strain MUSC 5 from mangrove forest in Malaysia: Identification, antioxidant potential and chemical profiling of its methanolic extract. Progress In Microbes & Molecular Biology 2020, 3, a0000087, 10.36877/pmmb.a0000087.
- Hefa Mangzira Kemung; Loh Teng-Hern Tan; Kok-Gan Chan; Hooi-Leng Ser; Jodi Woan-Fei Law; Learn-Han Lee; Bey Hing Goh; Streptomyces sp. Strain MUSC 125 from Mangrove Soil in Malaysia with Anti-MRSA, Anti-Biofilm and Antioxidant Activities. Molecules 2020, 25, 3545, 10.3390/molecules25153545.
- Johan Van Meerloo; Gertjan J. L. Kaspers; J. Cloos; Cell Sensitivity Assays: The MTT Assay. Springer Protocols Handbooks 2011, 731, 237-245, 10.1007/978-1-61779-080-5_20.
- Xiu-Chao Xie; Wen-Li Mei; Yan-Bo Zeng; Hai-Peng Lin; Ling Zhuang; Hao-Fu Dai; Kui Hong; Cytotoxic constituents from marine actinomycete Streptomyces sp. 124092. Chemical Journal of Chinese Universities 2008, 29, 2183–2186.
- Ganjun Yuan; Haipeng Lin; Cheng Wang; Kui Hong; Yue Liu; Jia Li; 1 H and 13 C assignments of two new macrocyclic lactones isolated from Streptomyces sp. 211726 and revised assignments of Azalomycins F3a , F4a and F5a. Magnetic Resonance in Chemistry 2010, 49, 30-37, 10.1002/mrc.2697.
- Peng Fu; Chunli Yang; Yi Wang; Peipei Liu; Yiming Ma; Lei Xu; Mingbo Su; Kui Hong; Weiming Zhu; Streptocarbazoles A and B, Two Novel Indolocarbazoles from the Marine-Derived Actinomycete Strain Streptomyces sp. FMA. Organic Letters 2012, 14, 2422-2425, 10.1021/ol3008638.
- Ying Han; Erli Tian; Dong-Bo Xu; Min Ma; Zixin Deng; Kui Hong; Halichoblelide D, a New Elaiophylin Derivative with Potent Cytotoxic Activity from Mangrove-Derived Streptomyces sp. 219807. Molecules 2016, 21, 970, 10.3390/molecules21080970.
- Wieland Voigt; Sulforhodamine B Assay and Chemosensitivity. Chemosensitivity 2005, 110, 039-048, 10.1385/1-59259-869-2:039.
- Esteban A. Orellana; Andrea L. Kasinski; Sulforhodamine B (SRB) Assay in Cell Culture to Investigate Cell Proliferation. BIO-PROTOCOL 2016, 6, e1984-e1984, 10.21769/bioprotoc.1984.
- Lei Chen; Weiyun Chai; Wenling Wang; Tengfei Song; Xiao-Yuan Lian; Zhizhen Zhang; Cytotoxic Bagremycins from Mangrove-Derived Streptomyces sp. Q22. Journal of Natural Products 2017, 80, 1450-1456, 10.1021/acs.jnatprod.6b01136.
- Sang Beom Han; Young Joo Shin; Joon Young Hyon; Won Ryang Wee; Cytotoxicity of Voriconazole on Cultured Human Corneal Endothelial Cells. Antimicrobial Agents and Chemotherapy 2011, 55, 4519-4523, 10.1128/aac.00569-11.
- Kunlong Li; Zhi Liang; Weihao Chen; Xiaowei Luo; Wei Fang; Shengrong Liao; Xiuping Lin; Bin Yang; Junfeng Wang; Lan Tang; et al.Yonghong LiuXuefeng Zhou Iakyricidins A–D, Antiproliferative Piericidin Analogues Bearing a Carbonyl Group or Cyclic Skeleton from Streptomyces iakyrus SCSIO NS104. The Journal of Organic Chemistry 2019, 84, 12626-12631, 10.1021/acs.joc.9b01270.
- He-Lin Yu; Shu-Heng Jiang; Xu-Liang Bu; Jia-Hua Wang; Jing-Yi Weng; Xiao-Mei Yang; Kun-Yan He; Zhi-Gang Zhang; Ping Ao; Jun Xu; et al.Min-Juan Xu Structural diversity of anti-pancreatic cancer capsimycins identified in mangrove-derived Streptomyces xiamenensis 318 and post-modification via a novel cytochrome P450 monooxygenase. Scientific Reports 2017, 7, 40689, 10.1038/srep40689.
- Priti Kumar; Arvindhan Nagarajan; Pradeep D. Uchil; Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harbor Protocols 2018, 2018, 2018, 10.1101/pdb.prot095497.
- E L Baldwin; E. L. Baldwin And N. Osheroff; Etoposide, Topoisomerase II and Cancer. Current Medicinal Chemistry-Anti-Cancer Agents 2005, 5, 363-372, 10.2174/1568011054222364.
- Lesley-Ann Giddings; David J. Newman; Microbial natural products: molecular blueprints for antitumor drugs. Journal of Industrial Microbiology & Biotechnology 2013, 40, 1181-1210, 10.1007/s10295-013-1331-1.
- Vineeta Singh; Vandana Praveen; Divya Tripathi; Shafiul Haque; Pallavi Somvanshi; S. B. Katti; C. K. M. Tripathi; Isolation, characterization and antifungal docking studies of wortmannin isolated from Penicillium radicum. Scientific Reports 2015, 5, 11948, 10.1038/srep11948.
- Arnd Steffen Boehle; Roland Kurdow; Lars Boenicke; Bodo Schniewind; Fred Faendrich; Peter Dohrmann; Holger Kalthoff; Wortmannin inhibits growth of human non-small-cell lung cancer in vitro and in vivo. Langenbeck's Archives of Surgery 2002, 387, 234-239, 10.1007/s00423-002-0314-x.
- Hak Cheol Kwon; Christopher A. Kauffman; Paul R. Jensen; William Fenical; Marinomycins A−D, Antitumor-Antibiotics of a New Structure Class from a Marine Actinomycete of the Recently Discovered Genus “Marinispora”. Journal of the American Chemical Society 2006, 128, 1622-1632, 10.1021/ja0558948.
- Brian T. Murphy; Tadigoppula Narender; Christopher A. Kauffman; Matthew Woolery; Paul R. Jensen; William Fenical; Saliniquinones A - F, New Members of the Highly Cytotoxic Anthraquinone-γ-Pyrones from the Marine Actinomycete Salinispora arenicola. Australian Journal of Chemistry 2010, 63, 929-934, 10.1071/ch10068.
- Houssam M. Atta; Maged S. Ahmad.; Antimycin-a antibiotic biosynthesis produced by streptomyces sp. Az-ar-262: Taxonomy, fermentation, purification and biological activities. Australian Journal of Basic and Applied Sciences 2009, 3, 126–135.
- Kazuro Shiomi; Kenji Hatae; Hiroko Hatano; Atsuko Matsumoto; Yoko Takahashi; Cheng-Lin Jiang; Hiroshi Tomoda; Susumu Kobayashi; Haruo Tanaka; Satoshi Ōmura; et al. A New Antibiotic, Antimycin A9, Produced by Streptomyces sp. K01-0031. The Journal of Antibiotics 2005, 58, 74-78, 10.1038/ja.2005.10.
- Zhuang Han; Ying Xu; Oliver McConnell; Lingli Liu; Yong-Xin Li; Shuhua Qi; Xiangzhong Huang; P.-Y. Qian; Two Antimycin A Analogues from Marine-Derived Actinomycete Streptomyces lusitanus. Marine Drugs 2012, 10, 668-676, 10.3390/md10030668.
- Chen Hu; Shi-Wen Zhou; Fang Chen; Xin-Heng Zheng; Hui-Fan Sheng; Birun Lin; Guang-Xiong Zhou; Neoantimycins A and B, Two Unusual Benzamido Nine-Membered Dilactones from Marine-Derived Streptomyces antibioticus H12-15. Molecules 2017, 22, 557, 10.3390/molecules22040557.
- Shaodan Chen; Tianqiao Yong; Yifang Zhang; Jiyan Su; Chunwei Jiao; Yizhen Xie; Anti-tumor and Anti-angiogenic Ergosterols from Ganoderma lucidum. Frontiers in Chemistry 2017, 5, 85-85, 10.3389/fchem.2017.00085.
- Fazuo Wang; Yuchun Fang; Min Zhang; Aiqun Lin; Tianjiao Zhu; QianQun Gu; Weiming Zhu; Six new ergosterols from the marine-derived fungus Rhizopus sp.. Steroids 2008, 73, 19-26, 10.1016/j.steroids.2007.08.008.
- Ali A. H. El-Gamal; Shang-Kwei Wang; Chang-Feng Dai; Chang-Yih Duh; New Nardosinanes and 19-Oxygenated Ergosterols from the Soft CoralNephtheaarmataCollected in Taiwan. Journal of Natural Products 2004, 67, 1455-1458, 10.1021/np0400858.
- Yang-Mei Zhang; Hong-Yu Li; Chen Hu; Hui-Fan Sheng; Ying Zhang; Birun Lin; Guang-Xiong Zhou; Ergosterols from the Culture Broth of Marine Streptomyces anandii H41-59. Marine Drugs 2016, 14, 84, 10.3390/md14050084.
- Shuna Fu; Fan Wang; Hongyu Li; Yixuan Bao; Yu Yang; Huifang Shen; Birun Lin; Guang-Xiong Zhou; Secondary metabolites from marine-derived Streptomyces antibioticus strain H74-21. Natural Product Research 2016, 30, 2460-2467, 10.1080/14786419.2016.1201668.
- Ushakiranmayi Mangamuri; Muvva Vijayalakshmi; Sudhakar Poda; Krishna Naragani; Rajesh Kumar Munaganti; Bhujangarao Chitturi; Venkateswarlu Yenamandra; Bioactive metabolites produced by Streptomyces Cheonanensis VUK-A from Coringa mangrove sediments: isolation, structure elucidation and bioactivity. 3 Biotech 2016, 6, 63, 10.1007/s13205-016-0398-6.
- Ravikumar Sundaram; M Fredimoses; M Gnanadesigan; Anticancer property of sediment actinomycetes against MCF–7 and MDA–MB–231 cell lines. Asian Pacific Journal of Tropical Biomedicine 2012, 2, 92-96, 10.1016/s2221-1691(11)60199-8.
- Loh Teng-Hern Tan; Chim-Kei Chan; Priyia Pusparajah; Tahir Mehmood Khan; Hooi-Leng Ser; Learn-Han Lee; Bey Hing Goh; Streptomyces sp. MUM256: A Source for Apoptosis Inducing and Cell Cycle-Arresting Bioactive Compounds against Colon Cancer Cells. Cancers 2019, 11, 1742, 10.3390/cancers11111742.
- S. Narendhran; P. Rajiv; P. Vanathi; Rajeshwari Sivaraj.; Spectroscopic analysis of bioactive compounds from streptomyces cavouresis ku-v39: Evaluation of antioxidant and cytotoxicity activity. International Journal of Pharmacy and Pharmaceutical Sciences 2014, 6, 319–322.
- Sai Lakshman Mithun.V; C.S.V.Ramachandra Rao; Isolation and molecular characterization of anti-cancerous compound producing marine bacteria by using 16s rrna sequencing and gc-ms techniques. International Journal of Modern Engineering Research 2012, 2, 4510–4515.
- Mohan Gopi; Nagarajan Balachandran Dhayanithi; Karuppaiah Nanthini Devi; Thipramalai Thankappan Ajith Kumar; Marine natural product, Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro- (C7H10N2O2) of antioxidant properties from Bacillus species at Lakshadweep archipelago. Journal of Coastal Life Medicine 2014, 2, 636–641, 10.12980/jclm.2.201414j40.
- Seth Clint Brauns; Pieter Milne; Ryno Naudé; Maryna Van De Venter; Selected cyclic dipeptides inhibit cancer cell growth and induce apoptosis in HT-29 colon cancer cells.. Anticancer Research 2004, 24, 1713–1720.
- R. Cibi; A. Jayakumaran Nair; Purification of Actinomycin D from Streptomyces parvulus Isolated from Mangrove Ecosystem of Kerala, India. International Journal of Current Microbiology and Applied Sciences 2016, 5, 461-467, 10.20546/ijcmas.2016.507.049.
- M. Sanjivkumar; D. Ramesh Babu; A.M. Suganya; Silambarasan Tamil Selvan; Ramasamy Balagurunathan; G. Immanuel; Investigation on pharmacological activities of secondary metabolite extracted from a mangrove associated actinobacterium Streptomyces olivaceus (MSU3). Biocatalysis and Agricultural Biotechnology 2016, 6, 82-90, 10.1016/j.bcab.2016.03.001.
- Hooi-Leng Ser; Wai-Fong Yin; Kok-Gan Chan; Tahir Mehmood Khan; Bey-Hing Goh; Learn-Han Lee; Antioxidant and cytotoxic potentials of Streptomyces gilvigriseus MUSC 26T isolated from mangrove soil in Malaysia. Progress In Microbes & Molecular Biology 2018, 1, a0000002, 10.36877/pmmb.a0000002.
- Jodi Woan-Fei Law; Hooi-Leng Ser; Nurul-Syakima Ab Mutalib; Surasak Saokaew; Acharaporn Duangjai; Tahir Mehmood Khan; Kok-Gan Chan; Bey Hing Goh; Learn-Han Lee; Streptomyces monashensis sp. nov., a novel mangrove soil actinobacterium from East Malaysia with antioxidative potential. Scientific Reports 2019, 9, 1-18, 10.1038/s41598-019-39592-6.
- Mark Spalding; Mami Kainuma; Lorna Collins. World Atlas of Mangroves; Earthscan Routledge: New York, NY, USA, 2010; pp. -.
- Agnes M. Rimando; Maria Olofsdotter; Franck E. Dayan; Stephen O. Duke; Searching for Rice Allelochemicals. Agronomy Journal 2001, 93, 16-20, 10.2134/agronj2001.93116x.
- Michael G. Weller; A Unifying Review of Bioassay-Guided Fractionation, Effect-Directed Analysis and Related Techniques. Sensors 2012, 12, 9181-9209, 10.3390/s120709181.
- Patrick D Schloss; Jo Handelsman; Biotechnological prospects from metagenomics. Current Opinion in Biotechnology 2003, 14, 303-310, 10.1016/s0958-1669(03)00067-3.
- Jo Handelsman; Metagenomics: Application of Genomics to Uncultured Microorganisms. Microbiology and Molecular Biology Reviews 2004, 68, 669-685, 10.1128/mmbr.68.4.669-685.2004.
- Agnieszka Felczykowska; Sylwia Kamila Bloch; Bożena Nejman-Faleńczyk; Sylwia Barańska; Metagenomic approach in the investigation of new bioactive compounds in the marine environment.. Acta Biochimica Polonica 2012, 59, 501-5, 10.18388/abp.2012_2084.
- Philip Hugenholtz; Gene W. Tyson; Metagenomics. Nature 2008, 455, 481-483, 10.1038/455481a.
- Ramanpreet Kaur; Changanamkandath Rajesh; Rohit Sharma; Jaspreet Kaur Boparai; Pushpender Kumar Sharma; Metagenomic investigation of bacterial diversity of hot spring soil from Manikaran, Himachal Pradesh, India. Ecological Genetics and Genomics 2018, 6, 16-21, 10.1016/j.egg.2017.11.003.
- Gui-Yang-Sheng Wang; Edmund Graziani; Barbara Waters; Wubin Pan; Xiang Li; Joe McDermott; Guido Meurer; Geeta Saxena; Raymond J. Andersen; Julian Davies; et al. Novel Natural Products from Soil DNA Libraries in a Streptomycete Host. Organic Letters 2000, 2, 2401-2404, 10.1021/ol005860z.
- Karin Kleigrewe; Jehad Almaliti; Isaac Yuheng Tian; Robin B. Kinnel; Anton Korobeynikov; Emily A. Monroe; Brendan M. Duggan; Vincenzo Di Marzo; David H. Sherman; Pieter C. Dorrestein; et al.Lena GerwickWilliam H. Gerwick Combining Mass Spectrometric Metabolic Profiling with Genomic Analysis: A Powerful Approach for Discovering Natural Products from Cyanobacteria. Journal of Natural Products 2015, 78, 1671-1682, 10.1021/acs.jnatprod.5b00301.
- Camelia Herdini; Sofia Mubarika; Bambang Hariwijanto; Nastiti Wijayanti; Akira Hosoyama; Atsushi Yamazoe; Hideaki Nojiri; Jaka Widada; SECONDARY BIOACTIVE METABOLITE GENE CLUSTERS IDENTIFICATION OF ANTICANDIDA-PRODUCING Streptomyces Sp. GMR22 ISOLATED FROM WANAGAMA FOREST AS REVEALED BY GENOME MINING APPROACH. INDONESIAN JOURNAL OF PHARMACY 2017, 28, 26, 10.14499/indonesianjpharm28iss1pp26.
- Juan-Pablo Gomez-Escribano; Silke Alt; Mervyn J Bibb; Next Generation Sequencing of Actinobacteria for the Discovery of Novel Natural Products. Marine Drugs 2016, 14, 78, 10.3390/md14040078.
- Hooi-Leng Ser; Jodi Woan-Fei Law; Wen-Si Tan; Wai-Fong Yin; Kok-Gan Chan; Learn-Han Lee; Genome sequence of bioactive streptomycete isolated from mangrove forest in East Malaysia, Streptomyces monashensis MUSC 1JT. Progress in Drug Discovery & Biomedical Science 2019, 2, -, 10.36877/pddbs.a0000045.
- Robert Musiol; Maciej Serda; Jaroslaw Polanski; Prodrugs in photodynamic anticancer therapy. Current Pharmaceutical Design 2011, 17, 3548-3559, 10.2174/138161211798194549.
- Ju Yeon Song; Young Ji Yoo; Si-Kyu Lim; Sun Ho Cha; Ji-Eun Kim; Jung-Hye Roe; Jihyun F. Kim; Yeo Joon Yoon; Complete genome sequence of Streptomyces venezuelae ATCC 15439, a promising cell factory for production of secondary metabolites. Journal of Biotechnology 2016, 219, 57-58, 10.1016/j.jbiotec.2015.12.028.
- Sailesh Malla; Narayan Prasad Niraula; Kwangkyoung Liou; Jae Kyung Sohng; Improvement in doxorubicin productivity by overexpression of regulatory genes in Streptomyces peucetius. Research in Microbiology 2010, 161, 109-117, 10.1016/j.resmic.2009.12.003.
- Brian O. Bachmann; Steven G. Van Lanen; Richard H. Baltz; Microbial genome mining for accelerated natural products discovery: is a renaissance in the making?. Journal of Industrial Microbiology & Biotechnology 2013, 41, 175-184, 10.1007/s10295-013-1389-9.
- Hala A. Iqbal; Lila Low-Beinart; Joseph U. Obiajulu; Sean F. Brady; Natural Product Discovery through Improved Functional Metagenomics in Streptomyces. Journal of the American Chemical Society 2016, 138, 9341-9344, 10.1021/jacs.6b02921.
- Hengqian Ren; Bin Wang; Huimin Zhao; Breaking the silence: new strategies for discovering novel natural products. Current Opinion in Biotechnology 2017, 48, 21-27, 10.1016/j.copbio.2017.02.008.