Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ellie Rose Mattoon | + 1816 word(s) | 1816 | 2021-06-22 11:14:09 | | | |
| 2 | Ron Wang | Meta information modification | 1816 | 2021-06-25 03:36:12 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mattoon, E.R. Fungal Melanins in Healthcare. Encyclopedia. Available online: https://encyclopedia.pub/entry/11264 (accessed on 08 February 2026).
Mattoon ER. Fungal Melanins in Healthcare. Encyclopedia. Available at: https://encyclopedia.pub/entry/11264. Accessed February 08, 2026.
Mattoon, Ellie Rose. "Fungal Melanins in Healthcare" Encyclopedia, https://encyclopedia.pub/entry/11264 (accessed February 08, 2026).
Mattoon, E.R. (2021, June 24). Fungal Melanins in Healthcare. In Encyclopedia. https://encyclopedia.pub/entry/11264
Mattoon, Ellie Rose. "Fungal Melanins in Healthcare." Encyclopedia. Web. 24 June, 2021.
Copy Citation
Melanin is a complex multifunctional pigment found in all kingdoms of life, including fungi. The complex chemical structure of fungal melanins, yet to be fully elucidated, lends them multiple unique functions ranging from radioprotection and antioxidant activity to heavy metal chelation and organic compound absorption. Given their many biological functions, fungal melanins present many possibilities as natural compounds that could be exploited for human use.
industrial microbiology
melanin
fungi
radioprotection
biotechnology
fungal pigments
1. Introduction
The term melanin refers to a diverse set of dark polymeric pigments found in all kingdoms of life. In fungi, melanin plays a panoply of protective roles against stress (Table 1; Figure 1) [1]. For example, fungal melanin can protect against ionizing radiation, including ultraviolet, X-ray, gamma-ray, and particulate radiation [2][3][4]. In addition, melanin can play a role in thermoregulation and protection against both heat and cold shock [5][6]. For example, melanized Cryptococcus neoformans cells were more likely to survive both heat shock and cold shock compared to non-melanized species [6]. Melanized endophytes in a mutualistic relationship with plants will often help their symbionts thermoregulate by dissipating heat and absorbing ROS [7]. Darkly pigmented yeasts and mushrooms tend to be more common at higher absolute latitudes and colder climates, suggesting that the pigment’s ability to capture radiation energy and dissipate it as heat provides an advantage in generating thermal energy [5][8]. Part of melanin’s role in thermotolerance may be attributable to its ability to react with and neutralize Reactive Oxygen Species (ROS), helping fungal organisms withstand the oxidative stress that often accompanies higher temperatures [9].

Figure 1. Panel of four images of different melanized fungi. Xylaria polymorpha (top left), a mushroom also referred to as ‘Dead Man’s Fingers.’ The stromata of X. polymorpha have an average total length, including rooting bases, of 5 to 8 cm by an average 2 cm diameter [10]. “Black fungi—Xylaria polymorpha (dead man’s fingers)” by ohi007 is licensed under CC BY-NC-SA 2.0; Cladophialosphora bantiana (top right), a pathogenic mold. C. bantiana conidia are approximately 5 to 10 μm in length [11]. “File:Cladophialophora bantiana UAMH10767.jpg” by Medmyco is licensed under CC BY-SA 4.0; Inonotus obliquus (bottom left), also known as the Chaga mushroom. Chaga appears as a sclerotia ranging from 5 to 40 cm in diameter [12]. “Chaga mushroom (Inonotus obliquus)” by Distant Hill Gardens is licensed under CC BY-NC-SA 2.0; Cryptococcus neoformans (bottom right), a pathogenic yeast. Cryptococcal cells range from 5 to 10 µm in diameter [13]. “Cryptococcosis—GMS stain” by Pulmonary Pathology is licensed under CC BY-SA 2.0. Images presented are not to scale.
Table 1. Examples of the functions of fungal melanin in living fungal organisms.
| Function | Source |
|---|---|
| Photoprotection | [2][3][4] |
| Thermoregulation | [5][6][7][8] |
| Energy Harvesting | [5][14][15][16] |
| Metal Binding | [17][18][19] |
| Chemical Stress Response | [20][21][22][23] |
| Antioxidant | [7][9][24] |
| Anti-Desiccation | [25][24] |
| Virulence | [26][27] |
In addition, melanin can help fungi withstand chemical stressors. In halotolerant black yeast, melanin synthesis inhibitors diminished the yeast’s ability to grow in hypersaline environments [20]. Researchers posited that this could be due to the stabilizing effect that melanin has on the fungal cell wall, which would have permitted a more effective response to osmotic stress [20]. In addition, melanization has also been shown to protect fungal cells from heavy metal stress and hydrolytic enzymes [21][22]. In areas with low water content, melanin is associated with stress response to dry conditions. For example, heavy melanization was associated with survival in microcolonial rock fungi exposed to long periods of desiccation [28]. Some fungal species in spalted woods will melanize in response to periods of low water content, creating black zones in the wood [25]. These numerous protective functions enable melanized fungi to reside in some of civilization’s most extreme environments, from deep-sea vents to the International Space Station [29][30].
Given the numerous functional groups present in melanotic pigments, melanin can bind and interact with many different organic and inorganic molecules [1][17]. One notable example is melanin’s affinity for chelating metal ions, which can be toxic to fungal cells [17][18][19]. Fungal melanin also plays a role in protecting the fungus against the human immune response and is often associated with pathogenicity in Aspergillus fumigatus, Cryptococcus neoformans, and Talaromyces marneffei, among others [26].
Lastly, melanin is correlated with fungal virulence and has been implicated as a possible antifungal target [27]. While fungal melanin’s role in virulence will be limited in this review, please see [26] for a more thorough overview of this topic.
Some of the functions that melanin holds in biology can be utilized for societal benefit. These biological functions of radioprotection, stress response, and substrate binding, among others, often stem from melanin’s unique physical and chemical properties. When properly understood, scientists may be able to use melanin to carry out parallel purposes in the fields of industry, healthcare, and bioremediation.
2. Fungal Melanins
Investigators wishing to work with melanin have several different options for sourcing the material, whether from animals such as cuttlefish, bacteria, fungi, or even synthetic reservoirs. Each of these options has different chemical properties and extraction protocols, conferring various advantages and disadvantages depending on the desired application [31]. For example, there are significant differences between fungal eumelanin and synthetic eumelanin, likely due to the fact that in vivo melanogenesis is vesicle-associated and enzymatically catalyzed, while synthetic melanogenesis often relies on spontaneous autopolymerization in solution [32]. In addition, synthetic melanins can be costly to produce [33]. Melanin from animal and plant sources can be less expensive to produce than the synthetic route, but it can be difficult to purify, as melanin is often associated with other biomolecules. Thus, microbial melanin is often touted as a low-cost and high-yield alternative to synthetic, animal, or plant melanin [33]. More specifically, fungal melanin is attractive due to the well-known protective functions in fungal organisms adapted to survive extreme environments [1].
Fungal organisms can produce different kinds of melanins (Figure 2). Most fungal melanins are allomelanins, which do not contain nitrogen [34]. DHN melanin is a common type of allomelanin derived from the polymerization of 1,8-dihydroxynaphthalene (DHN) [35]. The polyketide pathway that produces allomelanins begins with an endogenously produced molecule of acetyl coA or malonyl coA, which undergoes several reductions and dehydrations to produce 1,8-DHN [36]. The complete literature for scientific studies of fungal melanins dates back to the 1960s [37].
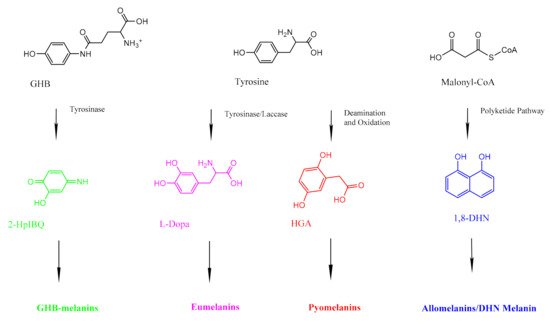
Figure 2. A simplified diagram showing the precursors for the three different kinds of fungal melanin. GHB-melanins (far left, green) are synthesized through a series of reactions with tyrosinase. Eumelanins (center left, pink) are synthesized via the L-Dopa pathway, which uses tyrosine as a precursor. Pyomelanins (center right, red) also use tyrosine as a precursor but are ultimately derived from HGA. Allomelanins (far right, blue) are derived from 1,8-DHN.
Some basidiomycetous fungi, such as Cryptococcus neoformans, produce eumelanin [38]. Eumelanins are derived from the amino acid tyrosine, and unlike allomelanins, they contain nitrogen [39]. Fungi synthesize eumelanins via the L-3,4-dihydroxyphenylalanine (L-dopa) pathway, which begins by using laccase to oxidize L-dopa into dopaquinone [40]. Eventually, the pathway produces dihydroxyindoles that can polymerize into eumelanins [40]. Cryptococcus neoformans cannot carry out this pathway without an exogenous substrate [40]. Pyomelanins are also derived from tyrosine, but they are produced by fungi such as Aspergillus fumigatus via the tyrosine degradation pathway, which involves the oxidative phosphorylation of homogentisate (HGA) [41].
3. Health
Melanin’s unique properties allow it to have diverse applications in the field of human health, whether in a pharmaceutical, medical device, or antimicrobial. One of the most heavily studied health applications of melanin involves protection from radiation, given that fungal melanin is known for its radioabsorptive properties.
For example, in an experiment where mice were fed black mushrooms Auricularia auricila-judae and soon after irradiated, they tended to exhibit improved survival than the control over the course of 45 days [42]. Researchers postulated that melanin’s ability to dissipate Compton electron energy and scavenge free radicals would shield the mice’s gastrointestinal (GI) tract, preventing cellular apoptosis. When mouse GI tissue was examined 24 h post-irradiation, researchers found fewer apoptotic cells in the tissue of mice that had been fed black mushrooms. In addition, mice fed white mushrooms supplemented with melanin had the same outcome as those who were given melanized mushrooms to begin with, pointing to the role of melanin in the increased survival rates. However, because melanin is insoluble, its protective effects remained mostly limited to the gastrointestinal tract [42]. Melanin extract from Auricularia auricula also showed promise in reducing oxidative stress and enhancing survival in liver cells exposed to high doses of ethanol, providing a theoretical basis for the substance’s ability to treat alcoholic liver disease. The investigators associated their results with the liver cell’s activation of the antioxidase Nrf2 and the inhibition of the cytochrome CYP2E1, which produces ROS as it metabolizes ethanol. Additional investigations would further elucidate the pathway producing this phenomenon [43].
In a separate mice study, those given melanin from the fungus Gliocephalotrichum simplex not only experienced better survival from irradiation, but also showed improvement in spleen parameters, reduced oxidative stress in the liver, and reduced production of inflammatory cytokines [44]. The authors suggested that a key mechanism of melanin’s protective property in the study was its ability to reverse the decrease in phosphorylation of the transducing protein ERK that is commonly seen upon radiation exposure [44]. Radioprotective technologies are needed for the protection of multiple vulnerable demographics. For example, radiation can have a harmful effect on patients receiving it for diagnostic or therapeutic purposes [42]. Cardiac diagnostic procedures alone account for about one-fifth of the radiation exposure per person per year in the US [45]. In addition, some occupations receive high levels of radiation exposure, including healthcare professionals [45] and military personnel [46].
Fungal melanin has also been implicated to have potential use in the functional biointerfaces used for stem cell manipulation [47]. Eumelanins were proposed for this purpose due to their antioxidant and electrical properties. However, eumelanin can be vulnerable to degradation from alkaline or oxidative stress. Manini et al. propose the alternative use of fungal allomelanins, citing the material’s resistance to degradation and smoothness [47]. Such a mycomelanin film was able to encourage the differentiation of stem cells towards an endodermal lineage [47].
4. Bioremediation
Melanized fungi and fungal melanin have both been proposed as mechanisms for removing various toxins from a polluted environment. Fungi can degrade volatile organic compounds (VOCs), and the ability of melanotic fungi to survive acidic or dry conditions makes them great candidates for VOC absorption [48]. In western countries where individuals spend much of their time indoors, VOCs emitted from construction materials, appliances, and cleaning chemicals have been implicated as a cause of sick building syndrome (SBS) by the United States Environmental Protection Agency (EPA) [49]. Researchers found that melanized fungal cultures placed in an indoor air model were able to reduce the VOC content by more than 96% after 48 h [50]. However, researchers noted that which melanotic fungal species were used was an important factor for the feasibility of using fungi to eliminate VOCs in indoor spaces [50]. For example, some species produced putative volatile metabolites in low concentrations [50]. In addition, fungal species prone to rapid growth and/or sporulation may become prone to aerosolization, presenting challenges both as an allergen and as a pathogen to immunocompromised individuals [50]. The researchers ultimately concluded that slow-growing melanotic fungi may be optimal for future applications [50].
References
- Cordero, R.J.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 2017, 31, 99–112.
- Wang, Y.; Casadevall, A. Decreased susceptibility of melanized Cryptococcus neoformans to UV light. Appl. Environ. Microbiol. 1994, 60, 3864–3866.
- Khajo, A.; Bryan, R.A.; Friedman, M.; Burger, R.M.; Levitsky, Y.; Casadevall, A.; Magliozzo, R.S.; Dadachova, E. Protection of melanized Cryptococcus neoformans from lethal dose gamma irradiation involves changes in melanin’s chemical structure and paramagnetism. PLoS ONE 2011, 6, e25092.
- Pacelli, C.; Bryan, R.A.; Onofri, S.; Selbmann, L.; Shuryak, I.; Dadachova, E. Melanin is effective in protecting fast and slow growing fungi from various types of ionizing radiation. Environ. Microbiol. 2017, 19, 1612–1624.
- Cordero, R.J.B.; Robert, V.; Cardinali, G.; Arinze, E.S.; Thon, S.M.; Casadevall, A. Impact of yeast pigmentation on heat capture and latitudinal distribution. Curr. Biol. 2018, 28, 2657–2664.e3.
- Rosas, Á.L.; Casadevall, A. Melanization affects susceptibility of Cryptococcus neoformans to heat and cold1. FEMS Microbiol. Lett. 2006, 153, 265–272.
- Redman, R.S.; Sheehan, K.B.; Stout, R.G.; Rodriguez, R.J.; Henson, J.M. Thermotolerance generated by plant/fungal symbiosis. Science 2002, 298, 1581.
- Krah, F.-S.; Büntgen, U.; Schaefer, H.; Müller, J.; Andrew, C.; Boddy, L.; Diez, J.; Egli, S.; Freckleton, R.; Gange, A.C.; et al. European mushroom assemblages are darker in cold climates. Nat. Commun. 2019, 10, 2890.
- Jacobson, E.S.; Tinnell, S.B. Antioxidant function of fungal melanin. J. Bacteriol. 1993, 175, 7102–7104.
- Rogers, J.D.; Callan, B.E. Xylaria polymorpha and its allies in continental united states. Mycologia 1986, 78, 391–400.
- Emmons, C.W.; Binford, C.H.; Utz, J.; Kwon-Chung, K. Medical Mycology. In Philadelphia: Lea & Febiger, 3rd ed.; 1977; Available online: (accessed on 17 June 2021).
- Zheng, W.; Miao, K.; Liu, Y.; Zhao, Y.; Zhang, M.; Pan, S.; Dai, Y. Chemical diversity of biologically active metabolites in the sclerotia of Inonotus obliquus and submerged culture strategies for up-regulating their production. Appl. Microbiol. Biotechnol. 2010, 87, 1237–1254.
- Okagaki, L.H.; Strain, A.K.; Nielsen, J.N.; Charlier, C.; Baltes, N.J.; Chrétien, F.; Heitman, J.; Dromer, F.; Nielsen, K. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog 2010, 17, e1000953.
- Dadachova, E.; Bryan, R.A.; Huang, X.; Moadel, T.; Schweitzer, A.D.; Aisen, P.; Nosanchuk, J.D.; Casadevall, A. Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS ONE 2007, 2, e457.
- Robertson, K.L.; Mostaghim, A.; Cuomo, C.A.; Soto, C.M.; Lebedev, N.; Bailey, R.F.; Wang, Z. Adaptation of the black yeast Wangiella dermatitidis to ionizing radiation: Molecular and cellular mechanisms. PLoS ONE 2012, 7, e48674.
- Pinkert, S.; Zeuss, D. Thermal Biology: Melanin-Based Energy Harvesting across the Tree of Life. Curr. Biol. 2018, 28, R887–R889.
- Buszman, E.; Pilawa, B.; Zdybel, M.; Wilczyński, S.; Gondzik, A.; Witoszyńska, T.; Wilczok, T. EPR examination of Zn2+ and Cu2+ binding by pigmented soil fungi Cladosporium cladosporioides. Sci. Total Environ. 2006, 363, 195–205.
- Hong, L.; Simon, J.D. Current understanding of the binding sites, capacity, affinity, and biological significance of metals in melanin. J. Phys. Chem. B 2007, 111, 7938–7947.
- Fogarty, R.V.; Tobin, J.M. Fungal melanins and their interactions with metals. Enzym. Microb. Technol. 1996, 19, 311–317.
- Kejžar, A.; Gobec, S.; Plemenitaš, A.; Lenassi, M. Melanin is crucial for growth of the black yeast Hortaea werneckii in its natural hypersaline environment. Fungal Biol 2013, 117, 368–379.
- García-Rivera, J.; Casadevall, A. Melanization of Cryptococcus neoformans reduces its susceptibility to the antimicrobial effects of silver nitrate. Med. Mycol. 2001, 39, 353–357.
- Bloomfield, B.J.; Alexander, M. Melanins and resistance of fungi to lysis. J. Bacteriol. 1967, 93, 1276–1280.
- Larsson, B.S. Interaction between chemicals and melanin. Pigment. Cell Melanoma Res. 1993, 6, 127–133.
- Fernandez, C.W.; Koide, R.T. The function of melanin in the ectomycorrhizal fungus Cenococcum geophilum under water stress. Fungal Ecol. 2013, 6, 479–486.
- Tudor, D.; Robinson, S.C.; Cooper, P.A. The influence of moisture content variation on fungal pigment formation in spalted wood. AMB Express 2012, 2, 69.
- Smith, D.F.Q.; Casadevall, A. The role of melanin in fungal pathogenesis for animal hosts. Curr. Top. Microbiol. Immunol. 2019, 422, 1–30.
- Nosanchuk, J.D.; Ovalle, R.; Casadevall, A. Glyphosate inhibits melanization of Cryptococcus neoformans and prolongs survival of mice after systemic infection. J. Infect. Dis. 2001, 183, 1093–1099.
- Gorbushina, A.A.; Kotlova, E.R.; Sherstneva, O.A. Cellular responses of microcolonial rock fungi to long-term desiccation and subsequent rehydration. Stud. Mycol. 2008, 61, 91–97.
- Le Calvez, T.; Burgaud, G.; Mahé, S.; Barbier, G.; Vandenkoornhuyse, P. Fungal diversity in deep-sea hydrothermal ecosystems. Appl. Environ. Microbiol. 2009, 75, 6415–6421.
- Novikova, N.; De Boever, P.; Poddubko, S.; Deshevaya, E.; Polikarpov, N.; Rakova, N.; Coninx, I.; Mergeay, M. Survey of environmental biocontamination on board the International Space Station. Res. Microbiol. 2006, 157, 5–12.
- Pralea, I.-E.; Moldovan, R.-C.; Petrache, A.-M.; Ilieș, M.; Hegheș, S.-C.; Ielciu, I.; Nicoară, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From extraction to advanced analytical methods: The challenges of melanin analysis. Int. J. Mol. Sci. 2019, 20, 3943.
- Camacho, E.; Vij, R.; Chrissian, C.; Prados-Rosales, R.; Gil, D.; O’Meally, R.N.; Cordero, R.J.B.; Cole, R.N.; McCaffery, J.M.; Stark, R.E.; et al. The structural unit of melanin in the cell wall of the fungal pathogen Cryptococcus neoformans. J. Biol. Chem. 2019, 294, 10471–10489.
- Martínez, L.M.; Martinez, A.; Gosset, G. Production of melanins with recombinant microorganisms. Front. Bioeng. Biotechnol. 2019, 7, 285.
- Britton, G. The Biochemistry of Natural Pigments; Cambridge University Press: Cambridge, UK, 1983.
- Tsai, H.F.; Wheeler, M.H.; Chang, Y.C.; Kwon-Chung, K.J. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 1999, 181, 6469–6477.
- Bell, A.A.; Wheeler, M.H. Biosynthesis and functions of fungal melanins. Annu. Rev. Phytopathol. 1986, 24, 411–451.
- Langfelder, K.; Streibel, M.; Jahn, B.; Haase, G.; Brakhage, A.A. Biosynthesis of fungal melanins and their importance for human pathogenic fungi. Fungal Genet. Biol. 2003, 38, 143–158.
- Williamson, P.R. Biochemical and molecular characterization of the diphenol oxidase of Cryptococcus neoformans: Identification as a laccase. J. Bacteriol. 1994, 176, 656–664.
- Millington, K.R. Improving the whiteness and photostability of wool. In Advances in Wool Technology; Woodhead Publishing Limit: Cambridge, UK, 2009; pp. 217–247.
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940.
- Heinekamp, T.; Thywißen, A.; Macheleidt, J.; Keller, S.; Valiante, V.; Brakhage, A.A. Aspergillus fumigatus melanins: Interference with the host endocytosis pathway and impact on virulence. Front. Microbiol. 2012, 3, 440.
- Revskaya, E.; Chu, P.; Howell, R.C.; Schweitzer, A.D.; Bryan, R.A.; Harris, M.; Gerfen, G.; Jiang, Z.; Jandl, T.; Kim, K.; et al. Compton scattering by internal shields based on melanin-containing mushrooms provides protection of gastrointestinal tract from ionizing radiation. Cancer Biother. Radiopharm. 2012, 27, 570–576.
- Hou, R.; Liu, X.; Wu, X.; Zheng, M.; Fu, J. Therapeutic effect of natural melanin from edible fungus Auricularia auricula on alcohol-induced liver damage in vitro and in vivo. Food Sci. Hum. Wellness 2021, 10, 514–522.
- Kunwar, A.; Adhikary, B.; Jayakumar, S.; Barik, A.; Chattopadhyay, S.; Raghukumar, S.; Priyadarsini, K.I. Melanin, a promising radioprotector: Mechanisms of actions in a mice model. Toxicol. Appl. Pharmacol. 2012, 264, 202–211.
- Massalha, S.; Almufleh, A.; Small, G.; Marvin, B.; Keidar, Z.; Israel, O.; Kennedy, J.A. Strategies for minimizing occupational radiation exposure in cardiac imaging. Curr. Cardiol. Rep. 2019, 21, 71.
- Blake, P.K.; Komp, G.R. Radiation exposure of U.S. military individuals. Health Phys. 2014, 106, 272–278.
- Cavallini, C.; Vitiello, G.; Adinolfi, B.; Silvestri, B.; Armanetti, P.; Manini, P.; Pezzella, A.; d Ischia, M.; Luciani, G.; Menichetti, L. Melanin and Melanin-Like Hybrid Materials in Regenerative Medicine. Nanomaterials 2020, 10, 1518.
- Blasi, B.; Poyntner, C.; Rudavsky, T.; Prenafeta-Boldú, F.X.; Hoog, S.D.; Tafer, H.; Sterflinger, K. Pathogenic yet environmentally friendly? black fungal candidates for bioremediation of pollutants. Geomicrobiol. J. 2016, 33, 308–317.
- United States Environmental Protection Agency. Indoor Air Facts No. 4 (Revised) Sick Building Syndrome; United States Environmental Protection Agency: Washington, DC, USA, 1991.
- Prenafeta-Boldú, F.X.; Roca, N.; Villatoro, C.; Vera, L.; de Hoog, G.S. Prospective application of melanized fungi for the biofiltration of indoor air in closed bioregenerative systems. J. Hazard. Mater. 2019, 361, 1–9.
More
Information
Subjects:
Microbiology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
25 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




