
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mattia Bartoli | + 2391 word(s) | 2391 | 2021-01-27 07:44:54 | | | |
| 2 | Catherine Yang | Meta information modification | 2391 | 2021-01-27 11:03:59 | | |
Video Upload Options
Graphene is the most outstanding material among the new nanostructured carbonaceous species discovered and produced. Graphene’s astonishing properties (i.e., electronic conductivity, mechanical robustness, large surface area) have led to a deep change in the material science field.
1. Introduction
In recent years, nanoscale technologies have become the last frontier in material science and pharmaceutical development [1]. A primary role has been played in this by nanostructured carbonaceous materials [2][3] such as carbon nanotubes and graphene (GF) due to their intrinsic properties and easy functionalization [4].
Nowadays, graphene and related materials represent the most advanced frontier in high-performance carbon materials [5] as witnessed by the European Union research council enforcing a strong action named EU Graphene Flagship [6]. This plan aimed to promote basic investigation on graphene and its related derivatives in order to establish the European Community as a world leader in the field [5]. This was consequent to the top properties of this allotropic one-atom-thick planar sheet of carbon tightly packed into a hexagonal cell structure [7]. Graphene and its related materials’ features can be exploited in a wide range of applications to improve the mechanical robustness and electronic properties of composite materials [8][9][10], both plastics [11][12] and metals [13][14], even at a very limited amount However, its price is not negligible, hindering its way through to the market with respect to cheaper solutions [15][16][17][18][19][20][21][22][23]. Due their high cost, graphene and related materials cannot be used in cheap, large-scale production. However, they can be employed in high-added-cost applications such as those represented by frontier medicine [24].
This field has been boosted up by vicious diseases and the increased concern for human healthiness. Pharmaceutical companies and academic institutions have deeply committed themselves to drive to unreached levels newly designed drugs and procedures [25][26]. Despite the wide numbers of available established protocols, new routes [27][28] are explored to develop new and innovative materials for drug delivery [29], regenerative medicine [30], theragnostic treatment [31], and tissue repairing [32].
2. Graphene and Graphene-Related Materials for Biological Applications
In the following section, we report some of the recent advancements in the use of graphene and related materials for drug delivery, tissue engineering, and regenerative medicine.
2.1. Interaction between Graphene and Related Materials and Biological Systems
Graphene-like materials’ viability for biological applications is strongly related to the interactions between graphene and cellular and tissue structures.
Despite the astonishing properties, the use of graphene flakes and rGO as drug carriers without any modification is quite challenging in biological environments due to their high hydrophobicity [33]. The use of GO overcomes this issue due to its high hydrophilicity. This property leads to a cellular uptake through both endocytotic and macro-pinocytotic mechanisms [34][35].
Nonetheless, the use of graphene flakes and rGO in biological environments could be exploited by performing covalent and non-covalent modifications [36]. These less invasive and controlled modifications preserve the high conductivity of graphene flakes and rGO allowing their use in neuronal repairing [37], in vivo cellular imaging [38], and stimuli-mediated drug delivery [39][40].
The extended sp2 system of graphene and rGO represents a strong advantage with respect to other platforms used in the biological environment such as inorganic nanoparticles [41] due to their facile modifications through cycloadditions [42], e.g., 1,3-dipolar cycloadditions [43], nitrene addition [44], and amide condensation [45]. The intrinsic reactivity of graphene and related materials allows to easily build up compound libraries filled with plenty of materials with the basic properties of graphene and tuned biological activity.
2.2. Graphene and Related Materials as Drug Delivery Platforms
The most investigated biological application of graphene and related materials’ still remains drug delivery [46]. The ability of materials to drag and drop chemicals in the human body environment is well exploited by graphene and graphene-related materials [47].
Zhang et al. [48] described the interactions between graphene and proteins elucidating the ability of graphene-based materials to trigger several protein complexes on the cellular membrane to facilitate uptake. This was due to both electron transfer and residual functionalities on graphene-like materials’ surface. Wang et al. [49] deeply explored the relationship between graphene’s surface topological defects and its ability of drug delivery, proving a strong effect of topology. The authors described the folding ability of these materials in several shapes ranging from nanoscrolls to polyhedral ones. This protean ability allows different interactions with different folding agents such as membrane proteins or nucleic acids. A further study by Mohammed et al. [50] theoretically clarified the loading ability of pristine and metal-decorated graphene materials. Among them, hydrogel and foam are the most attracting solutions because drug release could be triggered by both chemical and thermal modifications [51].
Ezzati et al. [52] produced a biocompatible graphene foam surface tailored with alanine, cysteine, and glycine for cisplatin drug and release. The authors tested the material with a high load of cisplatin on MCF-7 and HepG2 human cancer cell lines with good results. Additionally, the graphene-based carrier underwent biodegradation inside the body according to the mechanism reported in Figure 1.
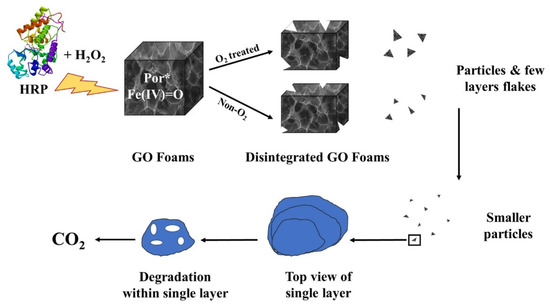
Figure 1. Biodegradation of cisplatin-loaded graphene foams as reported by Ezzati et al. [52].
Graphene and related materials also represent a very promising platform for drug release upon biological stimuli, as reported by Trusek et al. [53] in the case of doxorubicin.
Doxorubicin is a widely diffused anticancer drug acting on the S phase of the cellular metabolic pathway [54]. Several studies reveal the efficacy of graphene-based materials as carriers for doxorubicin as reported by Shen et al. [55]. Contrary to polymer-based formulations, graphene and graphene-like materials could act not merely as delivery systems but also as smart platforms to target and release doxorubicin upon several stimuli on a large time window and to monitor the cell vitality [56]. Furthermore, graphene-like materials could be also coupled with polymers to enhance classical formulation effects. The authors proved the effectiveness of chitosan-tailored graphene materials as carriers through molecular dynamic calculation. Doxorubicin interacts with both the π orbital system of graphene and the polar functionalities of the chitosan. After reaching the target, doxorubicin interacts with protein amine and carboxylic residues losing the chitosan interactions. In this case, the weak interactions with the graphene surface are not sufficient, and it was efficiently released.
This effect was magnified considering that the base of the drug delivery systems is the pH release mechanism [57].
GO-based systems were also used for the delivery of ketamine [58], ibuprofen [59], and medicine for hormone therapy [60]. In all cases, the high control on drug release prevented the sudden increment of drugs, leading to a time-prolonged release.
2.3. Graphene and Related Materials as Tool for Regenerative Medicine and Tissue Repair
The combination of graphene and graphene derivatives with plenty of different matrices has represented a game changing event in the production of biomaterials [61][62]. Fixing or replacing both tissues and organs has been boosted by the graphene-based composites by combining biocompatible scaffolds due to a variety of surface interactions [63].
Tissue engineering is one of the frontiers of medicine, aiming to overcome the drawbacks of the classical transplant procedure through the production of hybrid materials able to perform the same activity as the tissues replaced [64]. Graphene and its related materials have shown very attractive results related to the production of polymeric composites with great mechanical performances together with high biocompatibility for several applications ranging from bone to soft tissue repair [65].
Bahrami et al. [66] combined polyurethane with graphene for the production of conducting-polymer-based-material tissue regeneration. The authors aimed to grow L929 fibroblast and blood vessel endothelial cells on a membrane containing graphene flakes. This was particularly interesting in the case of endothelial cells. Indeed, they could be grown on the inner surface of tubular scaffolds by mimicking the native blood vessel structure [67]. The authors proved the ability of composites to support the attachment, spreading, and proliferation of cell lines. A very close approach was used by Pant et al. [68] for the production of coated stents by using graphene oxide mixed with polyurethane.
As shown in Figure 2, graphene-oxide-based composites create a stable coating on the surface of the stent without any tearing or ablation phenomenon.
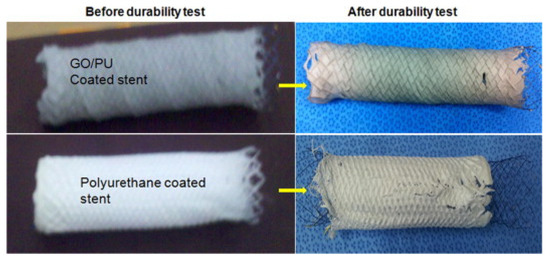
The modification of interphase properties due to the presence of graphene clearly increased the durability of the stent due to the radical-scavenging effect, which preserves the polymeric matrix from degradation promoted by extracellular fluids.
Similar results could be achieved by using titanium-based materials [69], which however show the critical drawback of cellular morphology alteration.
Regenerative medicine is another field where graphene-based materials have released their full potential [70][71]. The adherence of a cell on graphene is a critical property as shown by Morçimen et al. [72]. By studying the adherence and proliferation of the SH-SY5Y neuron cell line on graphene foams, authors proved the viability of this approach in more complex procedures.
Feng et al. [73] produced an iron-oxide-based, magnetic, electrospun, short nanofibre-wrapped GO for a guiding cellular behaviour as shown in Figure 3.
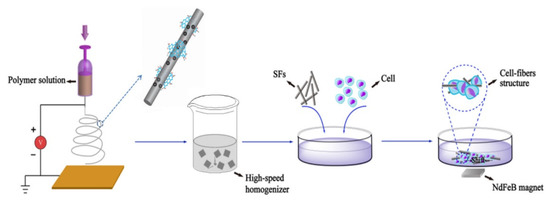
Figure 3. Cellular guidance induced by magnetic GO fibres as described by Feng et al. [73].
As shown in Figure 3, the authors produced short GO magnetic fibres by electrospinning techniques achieving materials with diameters of up to 300 nm and a length of up to 80 μm and a magnetization saturation of up to 50.33 emu/g. These fibres were able to induce an extremely tight adhesion with cells due to the functionalized GO. The observations were particularly promising for tissues manipulation by using external magnetic fields to selectively move specific cellular groups by tailoring the GO surface. Furthermore, the creation of controlled cellular aggregates could be a step forward in specific site repairing.
Agarwal et al. [74] produced a highly elastic and electroconductive graphene/collagen cryogel for spinal cord regeneration. Firstly, the authors tailored the graphene surface with amine residues promoting an efficient cross-linking with collagen showing a conductivity of up to 3.8 ± 0.2 mS/cm and a Young modulus of up to 347 kPa. The authors tested this composite for stem cell transplantation and neural tissue regeneration through the cryogelation approach by using the BM-MSCs cell line. They reported a good stem cell growth and expression enhancement of CD90 and CD73 gene upon electric stimulation of up to 100 mV/mm without the loss of stemness. Additionally, the authors observed an ATP secretion increment that could be helpful to favour neuronal regeneration and immunomodulation. Graphene-based cryogel promoted the neuronal differentiation of BM-MSCs with enhanced expression of MAP-2 kinase and β-tubulin III. In vitro observation proved a high indoleamine 2,3 dioxygenase activity under inflammatory conditions together with the proliferation of macrophages, which could aid in tissue repair.
Techaniyom et al. [74] showed that osteoblast differentiation and gene expression could also be modulated by GO coating of titanium materials due to an upregulation of the expression of the bone matrix protein genes during late-stage osteoblast differentiation.
2.4. From Material to Supramolecular Cluster: Graphene Quantum Dots
Graphene and its related derivatives lack of some properties such as bandgap or optical properties. This reduces the possible applications, and an engineered material could offset these limitations. In recent times, graphene quantum dots (GQDs) have received great attention for their possible unique applications [75] as a solid alternative to classical graphene, GO, and rGO.
Graphene quantum dots consist of graphene sheets, which could be single-sheet or multi-layered. They are nanoparticles with dimensions smaller than 100 nm, commonly under 20 nm, and their shapes are usually circular or elliptical. However, other shapes are produced with different synthetic routes [76].
The synthesis methods to produce GQDs are both top–down and bottom–up as summarized in Figure 4 by using different precursors and synthetic strategies.
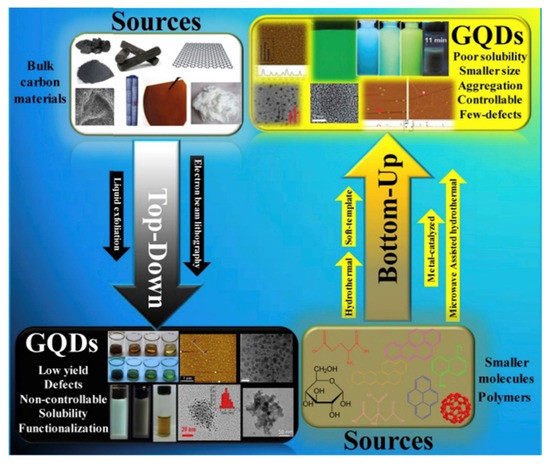
Figure 4. Scheme of graphene quantum dots (GQDs) top–down and bottom–up approaches as reported by [77] (under CC license).
The most common approach is the top–down one that usually consists of physical processes that reduce the dimensions of carbon materials as carbon fibres [78] or coal [79], along with acid oxidation [80] and hydrothermal treatment [81]. Solvothermal route [82], microwave [83], and sonication [84] are also promising alternative approaches.
A top–down physical process is the acid exfoliation, followed by the oxidation of the nano-obtained product. Li et al. [85] suggested an electrochemical synthesis route to produce a variety of GQDs, although the products differ in size, emission colours, and other intrinsic properties. Hydrothermal synthesis can be done just on precise precursors, such as GO, with a functionalization with oxidizing agents to introduce functional groups on the product surface [86].
The bottom–up method provides for the generation of aromatic molecules as precursors and can lead to high yield. Li et al. [87] developed a protocol to fabricate GQDs through direct pyrolysis on citric acid.
The synthesis routes mentioned above lead to a differentiation of the GQDs produced and their different physicochemical properties which depend on procedure, precursors, defects, and functional groups [88][89]. The GQDs’ properties of interest are absorbance, photoluminescence (PL), electrochemical photoluminescence among the optical ones and of course their biocompatibility; all these properties make them great candidates for bioimaging and theragnostic applications.
The absorption characteristics of GQDs are shown in the short-wavelength region because of C=C bonds and around 270–390 nm they show peaks for the C=O bonds. The passivation of the surface with a functional group can alter the disposition of the peaks; it strongly depends on the type of dot.
The principal and most fascinating property of the carbon dots is their photoluminescence. The unclear photoluminescence mechanism is one of the impediments to understand completely the physical mechanism behind these promising materials. The tuneable property of photoluminescence is size-dependent since it is related to quantum confinement. Moreover, other factors such as shape, defect, and doping also influence the effect. In detail, Zhu et al. [90] reported that PL is related to the quantum confinement effect of the electrons in sp2 carbons [91]. Another structure-dependent characteristic of GQDs is the electron transfer [92]. Biocompatibility and almost absent toxicity make them eligible for in vivo applications. In vitro studies have been made by Shang et al. [93], and they studied the GQDs’ effects on the cellular life finding out how the nanomaterial did not affect either the viability or the reproduction capacity. In vivo studies have been made on mice by exposing them to different nanomaterials. The results showed no abnormalities in organs or habits and brought to awareness their excellent biocompatibility, raising interest in the possible bio-applications.
The possible bio-applications of GQDs are cellular imaging [94], real-time in vivo biosensing [95], and drug delivery [96].
The main advantages of GQDs upon traditional imaging solution is related to the extremely high biocompatibility together with facile tailoring properties. Furthermore, the metabolic excretion after the procedure promotes a rapid removal of toxic formulation elements, e.g., heavy metals such as gadolinium or europium used in imaging procedures [97].
References
- Emerich, D.F.; Thanos, C.G. Nanotechnology and medicine. Expert Opin. Biol. Ther. 2003, 3, 655–663.
- Lu, S.-N.; Xie, N.; Feng, L.-C.; Zhong, J. Applications of nanostructured carbon materials in constructions: The state of the art. J. Nanomater. 2015, 2015, 1–10.
- Candelaria, S.L.; Shao, Y.; Zhou, W.; Li, X.; Xiao, J.; Zhang, J.-G.; Wang, Y.; Liu, J.; Li, J.; Cao, G. Nanostructured carbon for energy storage and conversion. Nano Energy 2012, 1, 195–220.
- Xu, Y.J.; Weinberg, G.; Liu, X.; Timpe, O.; Schlögl, R.; Su, D.S. Nanoarchitecturing of activated carbon: Facile strategy for chemical functionalization of the surface of activated carbon. Adv. Funct. Mater. 2008, 18, 3613–3619.
- Wei, D.; Kivioja, J. Graphene for energy solutions and its industrialization. Nanoscale 2013, 5, 10108–10126.
- Graphene Flagship. Available online: https://graphene-flagship.eu/ (accessed on 17 December 2020).
- Yang, G.; Li, L.; Lee, W.B.; Ng, M.C. Structure of graphene and its disorders: A review. Sci. Technol. Adv. Mater. 2018, 19, 613–648.
- Ji, X.; Xu, Y.; Zhang, W.; Cui, L.; Liu, J. Review of functionalization, structure and properties of graphene/polymer composite fibers. Compos. Part A Appl. Sci. Manuf. 2016, 87, 29–45.
- Giorcelli, M.; Bartoli, M. Carbon Nanostructures for Actuators: An Overview of Recent Developments. Actuators 2019, 8, 46.
- Lavagna, L.; Massella, D.; Priola, E.; Pavese, M. Relationship between oxygen content of graphene and mechanical properties of cement-based composites. Cem. Concr. Compos. 2021, 115, 103851.
- Lavagna, L.; Marchisio, S.; Merlo, A.; Nisticò, R.; Pavese, M. Polyvinyl butyral-based composites with carbon nanotubes: Efficient dispersion as a key to high mechanical properties. Polym. Compos. 2020.
- Kumar, A.; Sharma, K.; Dixit, A.R. A review of the mechanical and thermal properties of graphene and its hybrid polymer nanocomposites for structural applications. J. Mater. Sci. 2019, 54, 5992–6026.
- Dadkhah, M.; Saboori, A.; Fino, P. An overview of the recent developments in metal matrix nanocomposites reinforced by graphene. Materials 2019, 12, 2823.
- Hidalgo-Manrique, P.; Lei, X.; Xu, R.; Zhou, M.; Kinloch, I.A.; Young, R.J. Copper/graphene composites: A review. J. Mater. Sci. 2019, 54, 12236–12289.
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A. A Review of Non-Soil Biochar Applications. Materials 2020, 13, 261.
- Strongone, V.; Bartoli, M.; Jagdale, P.; Arrigo, R.; Tagliaferro, A.; Malucelli, G. Preparation and Characterization of UV-LED Curable Acrylic Films Containing Biochar and/or Multiwalled Carbon Nanotubes: Effect of the Filler Loading on the Rheological, Thermal and Optical Properties. Polymers 2020, 12, 796.
- Savi, P.; Yasir, M.; Bartoli, M.; Giorcelli, M.; Longo, M. Electrical and Microwave Characterization of Thermal Annealed Sewage Sludge Derived Biochar Composites. Appl. Sci. 2020, 10, 1334.
- Noori, A.; Bartoli, M.; Frache, A.; Piatti, E.; Giorcelli, M.; Tagliaferro, A. Development of Pressure-Responsive PolyPropylene and Biochar-Based Materials. Micromachines 2020, 11, 339.
- Barbalini, M.; Bartoli, M.; Tagliaferro, A.; Malucelli, G. Phytic Acid and Biochar: An Effective All Bio-Sourced Flame Retardant Formulation for Cotton Fabrics. Polymers 2020, 12, 811.
- Arrigo, R.; Bartoli, M.; Malucelli, G. Poly (lactic Acid)–Biochar Biocomposites: Effect of Processing and Filler Content on Rheological, Thermal, and Mechanical Properties. Polymers 2020, 12, 892.
- Giorcelli, M.; Bartoli, M. Development of Coffee Biochar Filler for the Production of Electrical Conductive Reinforced Plastic. Polymers 2019, 11, 1916.
- Bartoli, M.; Giorcelli, M.; Rosso, C.; Rovere, M.; Jagdale, P.; Tagliaferro, A. Influence of Commercial Biochar Fillers on Brittleness/Ductility of Epoxy Resin Composites. Appl. Sci. 2019, 9, 3109.
- Arrigo, R.; Jagdale, P.; Bartoli, M.; Tagliaferro, A.; Malucelli, G. Structure–Property Relationships in Polyethylene-Based Composites Filled with Biochar Derived from Waste Coffee Grounds. Polymers 2019, 11, 1336.
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostruct. Chem. 2018, 8, 123–137.
- Leachman, S.A.; Merlino, G. Medicine: The final frontier in cancer diagnosis. Nature 2017, 542, 36–38.
- Wender, P.A.; Miller, B.L. Synthesis at the molecular frontier. Nature 2009, 460, 197–201.
- Bartoli, M.; Jagdale, P.; Tagliaferro, A. A Short Review on Biomedical Applications of Nanostructured Bismuth Oxide and Related Nanomaterials. Materials 2020, 13, 5234.
- Ivorra, M.; Paya, M.; Villar, A. A review of natural products and plants as potential antidiabetic drugs. J. Ethnopharmacol. 1989, 27, 243–275.
- Rosen, H.; Abribat, T. The rise and rise of drug delivery. Nat. Rev. Drug Discov. 2005, 4, 381–385.
- DeWitt, N. Regenerative medicine. Nature 2008, 453, 301.
- Zarrintaj, P.; Mostafapoor, F.; Milan, P.B.; Saeb, M.R. Theranostic platforms proposed for cancerous stem cells: A review. Curr. Stem Cell Res. Ther. 2019, 14, 137–145.
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470.
- Aminov, R.I. The role of antibiotics and antibiotic resistance in nature. Environ. Microbiol. 2009, 11, 2970–2988.
- Mintmire, J.W.; Dunlap, B.I.; White, C.T. Are fullerene tubules metallic? Phys. Rev. Lett. 1992, 68, 631.
- Yan, J.-A.; Ruan, W.; Chou, M. Electron-phonon interactions for optical-phonon modes in few-layer graphene: First-principles calculations. Phys. Rev. B 2009, 79, 115443.
- Dresselhaus, M.; Jorio, A.; Saito, R. Characterizing graphene, graphite, and carbon nanotubes by Raman spectroscopy. Annu. Rev. Condens. Matter Phys. 2010, 1, 89–108.
- Rhee, K.Y. Electronic and Thermal Properties of Graphene. Nanomaterials 2020, 10, 926.
- Lee, H.C.; Liu, W.-W.; Chai, S.-P.; Mohamed, A.R.; Lai, C.W.; Khe, C.-S.; Voon, C.; Hashim, U.; Hidayah, N. Synthesis of single-layer graphene: A review of recent development. Procedia Chem. 2016, 19, 916–921.
- Narita, A.; Wang, X.-Y.; Feng, X.; Müllen, K. New advances in nanographene chemistry. Chem. Soc. Rev. 2015, 44, 6616–6643.
- Sun, Z.; Fang, S.; Hu, Y.H. 3D Graphene Materials: From Understanding to Design and Synthesis Control. Chem. Rev. 2020.
- Brisebois, P.; Siaj, M. Harvesting graphene oxide–years 1859 to 2019: A review of its structure, synthesis, properties and exfoliation. J. Mater. Chem. C 2020, 8, 1517–1547.
- Brodie, B.C. XIII. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259.
- Staudenmaier, L. Verfahren zur darstellung der graphitsäure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487.
- William, S.; Hummers, J.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339.
- Poh, H.L.; Šaněk, F.; Ambrosi, A.; Zhao, G.; Sofer, Z.; Pumera, M. Graphenes prepared by Staudenmaier, Hofmann and Hummers methods with consequent thermal exfoliation exhibit very different electrochemical properties. Nanoscale 2012, 4, 3515–3522.
- Araújo, M.P.; Soares, O.; Fernandes, A.; Pereira, M.; Freire, C. Tuning the surface chemistry of graphene flakes: New strategies for selective oxidation. RSC Adv. 2017, 7, 14290–14301.
- Szabó, T.; Berkesi, O.; Forgó, P.; Josepovits, K.; Sanakis, Y.; Petridis, D.; Dékány, I. Evolution of surface functional groups in a series of progressively oxidized graphite oxides. Chem. Mater. 2006, 18, 2740–2749.
- Lavagna, L.; Meligrana, G.; Gerbaldi, C.; Tagliaferro, A.; Bartoli, M. Graphene and Lithium-Based Battery Electrodes: A Review of Recent Literature. Energies 2020, 13, 4867.
- Lerf, A.; He, H.; Riedl, T.; Forster, M.; Klinowski, J. 13C and 1H MAS NMR studies of graphite oxide and its chemically modified derivatives. Solid State Ion. 1997, 101, 857–862.
- Thakur, S.; Karak, N. Alternative methods and nature-based reagents for the reduction of graphene oxide: A review. Carbon 2015, 94, 224–242.
- Guex, L.G.; Sacchi, B.; Peuvot, K.F.; Andersson, R.L.; Pourrahimi, A.M.; Ström, V.; Farris, S.; Olsson, R.T. Experimental review: Chemical reduction of graphene oxide (GO) to reduced graphene oxide (rGO) by aqueous chemistry. Nanoscale 2017, 9, 9562–9571.
- Liu, S.; Tian, J.; Wang, L.; Sun, X. A method for the production of reduced graphene oxide using benzylamine as a reducing and stabilizing agent and its subsequent decoration with Ag nanoparticles for enzymeless hydrogen peroxide detection. Carbon 2011, 49, 3158–3164.
- Lee, X.J.; Hiew, B.Y.Z.; Lai, K.C.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Rigby, S. Review on graphene and its derivatives: Synthesis methods and potential industrial implementation. J. Taiwan Inst. Chem. Eng. 2019, 98, 163–180.
- Muñoz, R.; Gómez-Aleixandre, C. Review of CVD synthesis of graphene. Chem. Vap. Depos. 2013, 19, 297–322.
- Khurana, G.; Misra, P.; Katiyar, R.S. Multilevel resistive memory switching in graphene sandwiched organic polymer heterostructure. Carbon 2014, 76, 341–347.
- Li, W.; Li, D.; Fu, Q.; Pan, C. Conductive enhancement of copper/graphene composites based on high-quality graphene. RSC Adv. 2015, 5, 80428–80433.
- Choi, H.; Kim, H.; Hwang, S.; Choi, W.; Jeon, M. Dye-sensitized solar cells using graphene-based carbon nano composite as counter electrode. Sol. Energy Mater. Sol. Cells 2011, 95, 323–325.
- Gao, Y.; Jing, H.W.; Chen, S.J.; Du, M.R.; Chen, W.Q.; Duan, W.H. Influence of ultrasonication on the dispersion and enhancing effect of graphene oxide–carbon nanotube hybrid nanoreinforcement in cementitious composite. Compos. Part B Eng. 2019, 164, 45–53.
- Liu, Y.; Zhang, B.; Xu, Q.; Hou, Y.; Seyedin, S.; Qin, S.; Wallace, G.G.; Beirne, S.; Razal, J.M.; Chen, J. Development of graphene oxide/polyaniline inks for high performance flexible microsupercapacitors via extrusion printing. Adv. Funct. Mater. 2018, 28, 1706592.
- Kumar, S.N.; Keshavamurthy, R.; Haseebuddin, M.; Koppad, P.G. Mechanical properties of aluminium-graphene composite synthesized by powder metallurgy and hot extrusion. Trans. Indian Inst. Met. 2017, 70, 605–613.
- Melanitis, N.; Tetlow, P.L.; Galiotis, C. Characterization of PAN-based carbon fibres with laser Raman spectroscopy. J. Mater. Sci. 1996, 31, 851–860.
- Koenig, F. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 5.
- Shukla, S.; Saxena, S. Spectroscopic investigation of confinement effects on optical properties of graphene oxide. Appl. Phys. Lett. 2011, 98, 073104.
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549–552.
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 1048–1058.
- Torrengo, S.; Canteri, R.; Dell’Anna, R.; Minati, L.; Pasquarelli, A.; Speranza, G. XPS and ToF-SIMS investigation of nanocrystalline diamond oxidized surfaces. Appl. Surf. Sci. 2013, 276, 101–111.
- Zhang, Y.; Tamijani, A.A.; Taylor, M.E.; Zhi, B.; Haynes, C.L.; Mason, S.E.; Hamers, R.J. Molecular Surface Functionalization of Carbon Materials via Radical-Induced Grafting of Terminal Alkenes. J. Am. Chem. Soc. 2019, 141, 8277–8288.
- Rathnayake, R.M.N.M.; Wijayasinghe, H.W.M.A.C.; Pitawala, H.M.T.G.A.; Yoshimura, M.; Huang, H.-H. Synthesis of graphene oxide and reduced graphene oxide by needle platy natural vein graphite. Appl. Surf. Sci. 2017, 393, 309–315.
- Malinský, P.; Macková, A.; Mikšová, R.; Kováčiková, H.; Cutroneo, M.; Luxa, J.; Bouša, D.; Štrochová, B.; Sofer, Z. Graphene oxide layers modified by light energetic ions. Phys. Chem. Chem. Phys. 2017, 19, 10282–10291.
- Cooper, A.J.; Wilson, N.R.; Kinloch, I.A.; Dryfe, R.A.W. Single stage electrochemical exfoliation method for the production of few-layer graphene via intercalation of tetraalkylammonium cations. Carbon 2014, 66, 340–350.
- Zhang, H.-B.; Zheng, W.-G.; Yan, Q.; Yang, Y.; Wang, J.-W.; Lu, Z.-H.; Ji, G.-Y.; Yu, Z.-Z. Electrically conductive polyethylene terephthalate/graphene nanocomposites prepared by melt compounding. Polymer 2010, 51, 1191–1196.
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105.
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388.
- Guo, K.W.; Tam, H.-Y. TEM Morphology of Carbon Nanotubes (CNTs) and its Effect on the Life of Micropunch. Transm. Electron Microsc. Theory Appl. 2015.
- Liu, Z.; Suenaga, K.; Harris, P.J.; Iijima, S. Open and closed edges of graphene layers. Phys. Rev. Lett. 2009, 102, 015501.
- Coats, A.; Redfern, J. Thermogravimetric analysis. A review. Analyst 1963, 88, 906–924.
- Lavagna, L.; Musso, S.; Pavese, M. A facile method to oxidize carbon nanotubes in controlled flow of oxygen at 350 °C. Mater. Lett. 2021, 283, 128816.
- Leenaerts, O.; Partoens, B.; Peeters, F. Water on graphene: Hydrophobicity and dipole moment using density functional theory. Phys. Rev. B 2009, 79, 235440.
- Mullick Chowdhury, S.; Lalwani, G.; Zhang, K.; Yang, J.Y.; Neville, K.; Sitharaman, B. Cell specific cytotoxicity and uptake of graphene nanoribbons. Biomaterials 2013, 34, 283–293.
- Mu, Q.; Su, G.; Li, L.; Gilbertson, B.O.; Yu, L.H.; Zhang, Q.; Sun, Y.-P.; Yan, B. Size-Dependent Cell Uptake of Protein-Coated Graphene Oxide Nanosheets. ACS Appl. Mater. Interfaces 2012, 4, 2259–2266.
- Zhang, Q.; Wu, Z.; Li, N.; Pu, Y.; Wang, B.; Zhang, T.; Tao, J. Advanced review of graphene-based nanomaterials in drug delivery systems: Synthesis, modification, toxicity and application. Mater. Sci. Eng. C 2017, 77, 1363–1375.
- Cherian, R.; Ashtami, J.; Mohanan, P. Effect of surface modified reduced graphene oxide nanoparticles on cerebellar granule neurons. J. Drug Deliv. Sci. Technol. 2020, 58, 101706.
- Luo, R.; Zhou, H.; Dang, W.; Long, Y.; Tong, C.; Xie, Q.; Daniyal, M.; Liu, B.; Wang, W. A DNAzyme-rGO coupled fluorescence assay for T4PNK activity in vitro and intracellular imaging. Sens. Actuators B Chem. 2020, 310, 127884.
- Yang, K.; Feng, L.; Liu, Z. Stimuli responsive drug delivery systems based on nano-graphene for cancer therapy. Adv. Drug Deliv. Rev. 2016, 105, 228–241.
- Depan, D.; Shah, J.; Misra, R. Controlled release of drug from folate-decorated and graphene mediated drug delivery system: Synthesis, loading efficiency, and drug release response. Mater. Sci. Eng. C 2011, 31, 1305–1312.
- Murakami, T.; Tsuchida, K. Recent advances in inorganic nanoparticle-based drug delivery systems. Mini Rev. Med. Chem. 2008, 8, 175.
- Suggs, K.; Reuven, D.; Wang, X.-Q. Electronic properties of cycloaddition-functionalized graphene. J. Phys. Chem. C 2011, 115, 3313–3317.
- Quintana, M.; Spyrou, K.; Grzelczak, M.; Browne, W.R.; Rudolf, P.; Prato, M. Functionalization of graphene via 1, 3-dipolar cycloaddition. ACS Nano 2010, 4, 3527–3533.
- Strom, T.A.; Dillon, E.P.; Hamilton, C.E.; Barron, A.R. Nitrene addition to exfoliated graphene: A one-step route to highly functionalized graphene. Chem. Commun. 2010, 46, 4097–4099.
- Castillo, A.E.R.; VanáTendeloo, G. Selective organic functionalization of graphene bulk or graphene edges. Chem. Commun. 2011, 47, 9330–9332.
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2.
- Zhang, Y.; Wu, C.; Guo, S.; Zhang, J. Interactions of graphene and graphene oxide with proteins and peptides. Nanotechnol. Rev. 2013, 2, 27–45.
- Wang, Y.; Wang, C. Self-assembly of graphene sheets actuated by surface topological defects: Toward the fabrication of novel nanostructures and drug delivery devices. Appl. Surf. Sci. 2020, 505, 144008.
- Mohammed, M.H.; Hanoon, F.H. Theoretical prediction of delivery and adsorption of various anticancer drugs into pristine and metal-doped graphene nanosheet. Chin. J. Phys. 2020, 68, 578–595.
- Mauri, E.; Salvati, A.; Cataldo, A.; Mozetic, P.; Basoli, F.; Abbruzzese, F.; Trombetta, M.; Bellucci, S.; Rainer, A. Graphene-laden hydrogels: A strategy for thermally triggered drug delivery. Mater. Sci. Eng. C 2021, 118, 111353.
- Ezzati, N.; Mahjoub, A.R.; Shokrollahi, S.; Amiri, A.; Abolhosseini Shahrnoy, A. Novel Biocompatible Amino Acids-Functionalized Three-dimensional Graphene Foams: As the Attractive and Promising Cisplatin Carriers for Sustained Release Goals. Int. J. Pharm. 2020, 589, 119857.
- Abdel-Bary, A.S.; Tolan, D.A.; Nassar, M.Y.; Taketsugu, T.; El-Nahas, A.M. Chitosan, magnetite, silicon dioxide, and graphene oxide nanocomposites: Synthesis, characterization, efficiency as cisplatin drug delivery, and DFT calculations. Int. J. Biol. Macromol. 2020, 154, 621–633.




