Biomass can be converted to energy by using thermochemical and biochemical methodsecology; environmental; biomass; pyrolysis. Biochemical methods, including anaerobic digestion and fermentation, constitute the transformation of cellulose and hemicellulose into biofuel at the stages of hydrolysis and fermentation. At present, biochemical methods are cost-intensive, and involve problems with using lignin-rich biomass.
1. Introduction
At present, great importance is attributed to renewable energy, when environmental problems associated with fossil fuels are solved. Different types of biomass, including wood, energy crops, agricultural and forestry waste, algae, etc., are the main available sources of renewable energy. Biomass offers the greatest potential for meeting the energy needs of modern society, both for developed and emerging markets worldwide
[1]. The major advantages of this type of fuel include its variety, wide availability, generation volumes, reasonably fast reproducibility, and numerous alternative conversion technologies (for instance, combustion, gasification, and pyrolysis). Depending on the conversion method, biomass can simultaneously serve as a source of liquid, gaseous, and solid fuel (e.g., liquid motor biofuel, biogas, and solid fuel pellets). The data of the World Bioenergy Association reveals
[2] that the structure of the global bioenergy consumption in 2016 was as follows: 4.9%—liquid biofuel, 91.8%—traditional consumption of biomass considering the present-day types of solid biofuel, 2.2%—biogas, and 1.1%—municipal solid waste (MSW) processing. Producing energy from biomass may significantly promote the commitments under the Kyoto Protocol to reducing greenhouse gases and solving problems pertaining to climate change
[3].
Biomass can be converted to energy by using thermochemical and biochemical methods
[4]. Biochemical methods, including anaerobic digestion and fermentation, constitute the transformation of cellulose and hemicellulose into biofuel at the stages of hydrolysis
[5] and fermentation
[6]. At present, biochemical methods are cost-intensive, and involve problems with using lignin-rich biomass
[6][7]. Moreover, these methods are sporadic in nature. They are characterized by relatively slow speed, and the resulting product is diluted with a great amount of water recirculating in the production process. In the present study, these processes were not considered.
Thermochemical conversion technologies (combustion
[8], gasification
[6] and pyrolysis
[3]) make it possible to convert feedstock to useful energy. Brief characteristics of the above-mentioned processes are presented in
Table 1.
Table 1. Biomass conversion methods.
| Type of Conversion |
Benefits |
Drawbacks |
| Thermochemical Conversion |
| Combustion |
The scale of setups varies from small to industrial ones in the range of 50–3000 MW.
Conversion efficiency is between 20% and 40%.
Biomass can be co-fired with coal. |
Biomass moisture content should be less than 50%.
The process develops at a high temperature (800–1000 °C). |
| Gasification |
The produced gas with a higher heating value of 4–6 MJ/m3 can be burned directly or used (after cleaning) as a fuel for gas engines and turbines.
Syngas production from biomass makes it possible to obtain methanol and hydrogen, each of which can be used as a fuel for transportation. |
The gas with a higher heating value of 4–6 MJ/m3 is not appropriate for pipeline transportation due to its low energy density.
The production of methanol with a higher heating value of 9–11 MJ/m3 requires gasification involving oxygen. |
| Pyrolysis |
Bio-oil can be used in engines and turbines; it also serves as feedstock for oil refineries. |
Low heat stability and high corrosiveness. |
Biomass combustion is used widely for commercial purposes to produce heat and power
[9]. The technology is commercially available, and poses minimal risk for investors. The end product of combustion is thermal energy used for heating and/or electrification. However, the efficiency of energy production from biomass is not high: approximately 20% for small enterprises, and no more than 40% for large and modern power-generating facilities
[9]. Such technologies provide an economic and competitive advantage, provided that waste as initial feedstock is used. Considering that biomass combustion technologies are widespread and well understood
[1][10][11], it is interesting to explore biomass pyrolysis and gasification in this review.
Gasification is considered to be the most efficient biomass-to-fuel conversion method. The process develops at elevated temperatures (650–1200 °C) in the presence of gasification agents (air, oxygen, steam, carbon dioxide), with syngas as the resulting product
[7][12]. Air gasification yields a producer gas with a higher heating value of 4–6 MJ/nm
3 (low-calorific gas). This gas can be burned in boilers and in gas engines or turbines after treatment, yet it is not appropriate for pipeline transportation due to low energy density. Gasification using oxygen yields intermediate heating value gas (10–12 MJ/m
3) suitable for limited pipeline transportation and can be used as syngas to produce power/heat or converted into diesel range hydrocarbons by means of Fischer–Tropsch synthesis, or into dimethyl ether or gasoline range hydrocarbons
[13]. Steam (pyrolytic) gasification yields an intermediate heating value gas with a greater heat of combustion (15–20 MJ/m
3). This is a two-stage process implemented in two fluidized bed reactors. The main benefits of gasification over direct combustion of biomass are minimal emissions of pollutants and high heat efficiency
[14][15]. In addition, integrated gasification combined cycle (IGCC) with carbon capture and storage (CCS) is now widespread
[16][17][18]. CCS in gasification projects is considered a promising technology for cost-effective CO
2 reduction (81–91%). The main advantages of IGCC with CCS IGCC with CCS include: (i) reduction of anthropogenic emissions (SO
2 and NO
x), as compared to combustion in boilers; (ii) reduction of energy losses during separation and capture of CO
2 from synthesis gas; and (iii) production of valuable by-products: sulfur (for example, almost all of the sulfur in fuel can be recovered), nitrogen (from an air separation unit), and CO
2 (from a CO
2 capture unit)
[16][17][18]. At the same time, gasification technologies, especially IGCC with CCS
[16], involve considerable investment, which is much higher than the respective costs when traditional methods of fossil fuel utilization are applied.
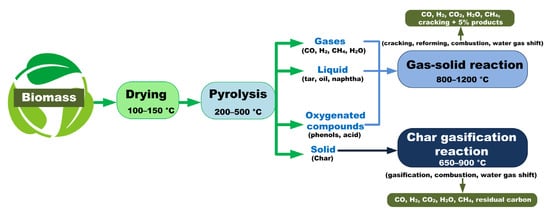
Pyrolysis is endothermic decomposition of feedstock developing under oxygen deficiency. Pyrolysis is the first stage in combustion and gasification; it is followed by complete or partial oxidation of primary products. The end products of biomass pyrolysis are pyrolysis oil (bio–oil), non-condensable gases, and carbon-rich residue (char). The bio–oil yield occurs at temperatures from 350 to 500 °C
[19]. At higher temperatures, the molecules of liquid and solid residue are destroyed to produce smaller molecules that pass to the gas medium. The yield of biomass pyrolysis products can be increased if the following conditions are fulfilled: (i) char—low temperatures and heating rates; (ii) liquid products—average temperatures, high heating rates and short gas residence time; and (iii) gas—high temperatures, low heating rate, and long gas residence time.
Pyrolysis oil can be utilized in diesel engines and power generation units in distributed generation, as well as at large power plants (as an alternative to fuel oil). Shihadeh et al.
[20] showed that when pyrolysis oil is used in internal combustion engines, its efficiency is identical to the thermal efficiency of diesel fuel. However, the ignition delay of pyrolysis oil was longer
[20]. Bio-oils do not yet have a wide industrial application due to existing limitations on the fuel quality, high viscosity, low stability and sustainability, and corrosiveness
[19][21].
Pyrolysis yields from 10 to 35% char. Depending on the composition and physical properties of char, it can be used in different industrial processes: as solid fuel in boilers, activated carbon production, carbon nanotube manufacturing, etc.
[22]. The producer gas resulting from pyrolysis can be converted after treatment into syngas, which can be utilized in engines and turbines, industrial incineration plants, and in methanol production
[23].
The presented information is generalized in Table 2 with data about the typical products obtained using different methods of biomass conversion.
Table 2. Typical product yields obtained by biomass conversion.
| Process |
Conditions |
Result |
| Pyrolysis |
Fast |
Moderate temperature (600–800 °C), short residence time particularly vapor (from 10 to 200 °C/s) |
Liquid |
Char |
Gas |
| 75% |
12% |
13% |
| Slow |
Low temperature (300–500 °C), very long residence time (under 1 °C/s) |
30% |
35% |
35% |
| Gasification |
High temperature (650–1200 °C), long residence times (from 1 to 100 °C/s) |
5% |
10% |
85% |
There are several well-established pyrolysis and gasification plants in different parts of the world, the most well-known of them are in Canada, the USA, Finland, and others.
Table 3 [21][24] lists some industrial pyrolysis and gasification units.
Table 3. Worldwide current pyrolysis and gasification operating plants.
| Plant Name |
Location |
Units |
Capacity |
| Pyrolysis |
| Red arrow, WI |
Canada |
Circulating fluidized bed |
1700 kg/h |
| Dyna Motive |
Canada |
Bubbling fluidized bed |
400 kg/h |
| Bio-alternative |
USA |
Fixed bed |
2000 kg/h |
| Battelle |
USA |
Catalytic pyrolysis technology |
1000 kg/h |
| Empyro |
Netherlands |
Flash pyrolysis |
5000 kg/h |
| Bioliq |
Germany |
Fast pyrolysis |
500 kg/h |
| BEST Energy |
Australia |
Bubbling fluidized bed |
300 kg/h |
| Fortum |
Finland |
- |
350 kg/h |
| Unión Fenosa |
Spain |
Bubbling fluidized bed |
200 kg/h |
| IRR manufacturing |
South Africa |
- |
1000 kg/h |
| Gasification |
| Great plains synfuels plant |
USA |
Fixed bed dry bottom |
16,000 t/day |
| Energos Gasification Plant |
Norway |
two-stage thermal treatment process |
78,000 t/year |
| Red Rock Bio |
USA |
TCG Global steam reforming |
136,000 tons/year |
| Shaanxi Weihe Fertilizer Co |
China |
General Electric |
1500 t/day |
| Yunnan Yuntianhua Group Tian’an Chemical Co., Ltd. |
China |
- |
2700 t/day |
2. Mechanisms and Stages of Biomass Pyrolysis and Gasification
Biomass is a system with a rather complex structure. A group of processes, phase transformations, and chemical reactions in a condensed phase and gas medium proceed in biomass when it is heated. Below are equations describing the main processes and transformations, taking into account typical stages of biomass conversion
[6][25][26]: drying, pyrolysis, and gasification (
Figure 1). There are reactors in which most of these processes run simultaneously
[6][25][26][27].
Figure 1. Mechanisms and stages of pyrolysis and gasification.
These processes are intended for producer gas generation. The term “synthesis gas” is quite often used, though it has a rather strict ratio of component concentrations: H2/(2CO + 3CO2) = 1.05. Impurities have been removed from it, and it is used as feedstock for the synthesis of chemical organic compounds currently produced from oil. It is unreasonable to set such requirements for the gas used as fuel. It is also incorrect to refer to biomass-derived producer gas as biogas. Biogas is obtained from biomass using biotechnologies. It consists mainly of methane (CH4). Producer gas contains a small amount of CH4.
The following stages are typical of the process: drying, pyrolysis, gasification, and condensation. These stages can be separated from each other by using intermediate chambers or different heating temperature ranges, and varying the type of medium (inert, reducing, oxidizing).
2.1. Drying
Drying is the first step of fuel preparation
[28]. The moisture content of the initial fuel has a significant effect on gasification. High-moisture fuels are unable to maintain a sustainable combustion front in the layer, due to great energy demands for water evaporation.
By choosing the correct thermal mode in the reactor, wet fuel can be gasified under the conditions of steam-air blowing without adding any external steam. According to thermodynamic calculations, high content of moisture reduces the efficiency of the process, but increases the content of hydrogen in the producer gas. Water in the solid fuel can be physically or chemically bound. Since coal, biomass, peat, and other solid fuels are porous, their drying proceeds in the same way.
At the initial stage of drying, the content of moisture decreases almost linearly with time. This region is referred to as the period of the constant drying rate. During this period, the drying rate is determined by the external mass exchange of the surface with the surrounding gas medium. Unbound moisture is the first to evaporate, followed by inherent moisture evaporating in a quasi-steady mode. As soon as moisture content becomes critical, the drying rate starts decreasing. The period of the falling drying rate begins. At the same time, the rate of moisture diffusion inside the particle becomes lower than that of the external mass exchange. Thus, experimental data about the coefficient of moisture diffusion in the material makes it possible to calculate the rate and duration of fuel drying.
2.2. Pyrolysis
Thermal decomposition (pyrolysis) of biomass (which is usually implemented in industrial plants at temperatures exceeding 550 °C) is a complex of transformations resulting in gaseous products and a solid residue
[29]. Pyrolysis proceeds under oxidizer deficiency. When biomass is heated, the proportions of gas, liquid, and semicoke produced depend on the pyrolysis mode and type of the system used. Three main components of biomass participating in pyrolysis are distinguished
[30]: cellulose, hemicellulose, and lignin. Hemicellulose decomposes at a temperature from 250 to 400 °C and generates 20% semicoke when heated to 720 °C; higher temperatures (from 310 to 430 °C) are required for cellulose to decompose with 8% semicoke produced; lignin decomposes at 300–530 °C with the production of approximately 55% semicoke
[30]. At lower temperatures, hydrocarbons depolymerize to produce smaller particles. Dehydration occurs at about 300 °C, with the production of unsaturated polymers and semicoke. A further temperature growth leads to extensive rupture of C–C and C–H with the production of oxygenates C
2–4 and products: CO, CO
2, H
2, and CH
4 [13].
2.3. Gasification of Carbonaceous Residue
Biomass gasification is a method of thermochemical conversion which includes converting chemical structures of biomass at elevated temperatures (>700 °C) in the presence of a gasifying agent (air/O
2/steam/CO
2 or a combination of these). Biomass is gasified in order to convert feedstock with a low calorific value into gaseous products with an average calorific value
[31]. In addition to H
2, CO, CO
2, and CH
4, untreated syngas also contains tars, lighter hydrocarbons, N
2, and sulfur compounds, as well as traces of chloride. These decrease the gas quality. Among all these syngas components, H
2 and CO are the most essential. Pyrolysis and gasification are interdependent processes. The gasification of the carbonaceous residue of biomass after pyrolysis is the process of interaction of carbon in a solid state with gaseous pyrolysis products CO, H
2, and CO
2. It proceeds in the following way:
-
C + CO
2 → 2CO (absorbed heat, i.e., endothermic effect −14.6∙10
6 J/kg)
[32],
-
C + H
2O → CO + H
2 (absorbed heat, i.e., endothermic effect −10.9∙10
6 J/kg)
[32],
-
C + 2H
2 → CH
4 (proceeds only at temperatures above 500 °C with heat released, i.e., exothermic effect +8∙10
6 J/kg)
[29]. These reactions develop on the surface and in the pores of biomass particles.
Thus, the main purpose of biomass gasification is the production of gas, while maximizing H2 concentrations and minimizing the tar content.
2.4. Pyrolysis and Gasification
The processes, phase transformations and chemical reactions described in the three previous sub-sections can be consecutive or parallel. The pyrolysis and gasification of biomass particles can be controlled by varying the initial concentrations of H2O and CO2 in the gas medium.
The main reactions of such interactions are as follows
[29][32][33][34][35]:
The explanations in line with the concepts
[29][32][33][34][35] are presented below. Most of the oxygen (pure oxygen or oxygen of the air), supplied to the gas generator, is spent on the reactions (1)–(3). This releases thermal energy required for the drying of the solid residue, destruction of chemical bonds, and temperature increase in the gasification zone, as well as for the reactions (4)–(9). The reactions (4,5) are the main gasification reactions. They are endothermic and proceed in a high-temperature and low-pressure zone. The reaction (6) is a primary reaction during the combustion of carbon (endothermic). It is much slower than that of combustion (1) under the same temperatures. The reaction (7) describes the interaction of carbon with hydrogen to produce methane. The rate of this reaction is not high, except under the conditions of high pressure. The interaction (8) is very important for hydrogen synthesis. A temperature increase (over 600 °C) facilitates the reaction (9) towards methane generation. It is quite slow under relatively low temperatures. The reaction (10) is quite neutral in terms of heat release.
To use the gas obtained from biomass pyrolysis and gasification as an energy-efficient (with a high calorific value) and environmentally friendly (with a low content of SOx and NOx) fuel, factors influencing its composition should be carefully analyzed. The following sections focus on these parameters.
3. Biomass Types Used for Pyrolysis and Gasification
The following categories of biomass are distinguished in the context of pyrolysis and gasification: (i) primary wood waste, such as chips, sawdust, and tree branches; (ii) energy crops grown for the use in the energy sector, such as rapeseed, jatropha, miscanthus, and sugar cane; (iii) agricultural waste, such as sugar cane bagasse, nut shell (coconut, sunflower), corn husk, wheat straw, oil production waste (olive, rapeseed and sunflower waste), and palm seeds; and (iv) municipal solid waste, animal waste, and food waste. Table 4 presents data on types of biomass used for pyrolysis and gasification in different regions of the world.
Table 4. Characteristics of components (type of biomass) used in gasification and pyrolysis.
| Component |
Country |
Ultimate Analysis (wt %) |
Proximate Analysis (wt %) |
Ref. |
| C |
H |
O |
N |
S |
Moisture |
Volatile
Matter |
Fixed Carbon |
Ash |
Heat of
Combustion
(MJ/kg) |
| Woody biomass |
| Beech wood |
Germany |
44.1 |
6.3 |
49.4 |
0.2 |
0 |
4.7 |
87.6 |
8 |
0.8 |
19.5 |
[36] |
| Wood pellet |
UK |
52.3 |
6.8 |
40.7 |
0.16 |
- |
6.7 |
84.3 |
15.7 |
0.8 |
20.8 |
[37] |
| Soft wood |
Ukraine |
45.34 ± 0.13 |
5.86 ± 0.04 |
42.45 ± 0.04 |
0.58 ± 0.11 |
0.17 ± 0.07 |
5.15 |
- |
- |
5.60 ± 0.38 |
18.23 ± 0.13 |
[38] |
| Woody biomass |
Sweden |
51.3 |
6.2 |
42 |
0.1 |
0.021 |
4.3 |
83.8 |
- |
0.3 |
19.36 |
[39] |
| Pine |
Russia |
47.88 |
6.34 |
45.69 |
0.09 |
0 |
- |
72.5 |
27.0 |
0.5 |
- |
[40] |
| Pine sawdust |
India |
50.3 |
6 |
42.99 |
0.69 |
- |
6.09 ± 0.3 |
78.03 ± 0.2 |
12.16 ± 0.1 |
2.07 ± 0.03 |
18.44 ± 09 |
[41] |
| Sal sawdust |
India |
49.83 |
6.01 |
43.56 |
0.58 |
- |
8.88 ± 0.2 |
76.03 ± 0.1 |
14.09 ± 0.2 |
1.14 ± 0.01 |
18.20 ± 09 |
[41] |
| Pine wood chips |
Canada |
48.3 |
5.8 |
45.4 |
0.5 |
- |
4.5 |
78.4 |
- |
2.6 |
16.1 |
[42] |
| Sawdust |
Ecuador |
46.1 |
6.3 |
46.7 |
0.3 |
- |
7.4 |
- |
- |
0.6 |
- |
[43] |
| Pine sawdust |
India |
53.5 |
6.93 |
32.55 |
3.33 |
0.66 |
7.85 ± 0.05 |
77.27 ± 0.65 |
12.20 ± 0.15 |
2.78 ± 0.12 |
18.55 ± 0.43 |
[44] |
| Root of mango tree |
Australia |
45.56 |
6.44 |
47.24 |
0.56 |
0.20 |
5.73 |
67.87 |
22.49 |
3.91 |
18.52 |
[45] |
| Eucalyptus urophylla |
Brazil |
45.03 |
4.78 |
38.46 |
0.11 |
- |
11.37 |
75.34 |
13.04 |
0.27 |
17.16 |
[46] |
| Herbaceous and agricultural biomass |
| Miscanthus |
Australia |
50.73 |
7.08 |
41.95 |
0.14 |
0.10 |
10.67 |
65.65 |
18.34 |
5.34 |
17.00 |
[45] |
| Palm empty fruit bunches |
UAE |
44.7 |
5.97 |
49.05 |
0.74 |
0.18 |
8.73 |
67.51 |
17.47 |
6.28 |
17.2 |
[47] |
| Palm leaves |
UAE |
40.76 |
5.55 |
52.14 |
1.32 |
0.24 |
12.03 |
58.17 |
15.41 |
14.4 |
18.9 |
[47] |
| Palm leave stems |
UAE |
42.67 |
5.83 |
50.78 |
0.58 |
0.15 |
11.65 |
68.84 |
10 |
9.51 |
16.5 |
[47] |
| Corn stalks |
Ukraine |
36.38 ± 1.36 |
5.40 ± 0.13 |
44.08 ± 0.38 |
1.68 ± 0.01 |
0.16 ± 0.02 |
8.13 |
- |
- |
12.30 ± 0.87 |
14.24 ± 0.46 |
[38] |
| Jerusalem artichoke stalks |
China |
45.36 |
6.11 |
47.26 |
0.75 |
0.52 |
15.76 |
67.4 |
13.5 |
3.34 |
15.69 |
[48] |
| Cane |
China |
42.78 |
5.17 |
50.51 |
1.33 |
0.21 |
5.89 |
72.12 |
13.52 |
8.47 |
16.16 |
[48] |
| Gulmohar seed |
India |
51.3 |
6 |
40.56 |
2.58 |
- |
7.09 ± 0.05 |
75.56 ± 0.5 |
15.80 ± 0.2 |
2.07 ± 0.12 |
19.65 ± 0.11 |
[44] |
| Corncob |
China |
46.6 |
5.8 |
47.0 |
0.4 |
0.2 |
- |
86.9 |
11.8 |
1.3 |
- |
[49] |
| Corn cob |
India |
44.2 |
5.9 |
44.2 |
0.54 |
0.08 |
10.2 |
80 |
4.2 |
5.7 |
15.5 |
[41] |
| Palm kernel shell |
UK |
50.11 |
6.24 |
42.16 |
1.50 |
0 |
6.70 |
67.52 |
22.13 |
3.65 |
- |
[50] |
| Olive waste |
UK |
52.8 |
6.5 |
39.1 |
1.6 |
- |
5.9 |
80.1 |
19.9 |
7.6 |
20.1 |
[37] |
| Palm kernel cake |
China |
49.04 |
5.93 |
34.10 |
2.46 |
0.29 |
2.88 |
75.83 |
15.99 |
5.30 |
- |
[51] |
| Jatropha seeds cake |
China |
45.3 |
6.2 |
43.8 |
4.5 |
0.2 |
- |
73.5 |
18.2 |
7.3 |
- |
[49] |
| Bagasse |
China |
46.4 |
6.7 |
45.8 |
0.7 |
0.4 |
- |
87.4 |
9.7 |
2.9 |
- |
[49] |
| Sugarcane |
India |
43.2 |
6.2 |
43.2 |
0.4 |
0.8 |
10 |
76 |
9.6 |
4.4 |
17.2 |
[41] |
| Sugarcane |
UK |
44.34 |
5.92 |
49.17 |
0.57 |
0 |
5.33 |
83.39 |
7.79 |
3.49 |
- |
[50] |
| Sugarcane |
Brazil |
43.79 |
5.16 |
38.90 |
0.29 |
- |
7.32 |
74.86 |
13.27 |
4.55 |
17.81 |
[46] |
| Sugarcane trash |
Brazil |
44.7 |
5.8 |
48.97 |
0.45 |
0.08 |
9.92 |
81.55 |
6.90 |
11.57 |
17.74 (16.50) |
[52] |
| Cherry pulp |
Turkey |
50.80 |
6.79 |
39.66 |
2.67 |
- |
6.42 |
72.02 |
19.70 |
1.86 |
19.82 |
[53] |
| Straw |
| Wheat straw |
Ukraine |
39.90 ± 0.15 |
5.75 ± 0.02 |
41.97 ± 0.07 |
0.65 ± 0.08 |
0.13 ± 0.05 |
6.84 |
- |
- |
11.59 ± 0.76 |
16.12 ± 0.19 |
[37] |
| Wheat straw |
UK |
40.58 |
4.84 |
53.84 |
0.74 |
0 |
5.19 |
64.24 |
15.60 |
14.97 |
- |
[50] |
| Wheat straw |
China |
45.94 |
5.83 |
39.08 |
0.56 |
0.45 |
2.50 |
72.36 |
18.00 |
5.64 |
- |
[51] |
| Rice straw |
China |
42.66 |
5.68 |
37.37 |
1.03 |
0.44 |
1.51 |
69.09 |
18.09 |
11.31 |
- |
[51] |
| Cotton stalk |
India |
46.8 |
6.4 |
46.8 |
0.3 |
0.2 |
8.9 |
71 |
16.6 |
3.5 |
19.2 |
[41] |
| Cotton stalk |
UK |
43.10 |
6.24 |
49.07 |
1.59 |
0 |
7.33 |
69.54 |
19.47 |
3.67 |
- |
[50] |
| Rice Husk |
UK |
37.60 |
5.26 |
55.45 |
1.69 |
0 |
8.02 |
61.43 |
12.53 |
18.02 |
- |
[50] |
| Nut husk and shells |
| Sunflower husks |
Ukraine |
45.82 ± 0.08 |
6.32 ± 0.02 |
38.31 ± 0.08 |
2.61 ± 0.05 |
0.14 ± 0.02 |
6.1 |
- |
- |
6.81 ± 0.51 |
19.31 ± 0.13 |
[38] |
| Areca nut husk |
India |
48.8 |
5.79 |
43.45 |
1.95 |
0.1 |
7.43 ± 0.1 |
74.05 ± 0.2 |
15.55 ± 0.3 |
2.48 ± 0.05 |
18.21 ± 09 |
[41] |
| Peanut shell |
China |
49.7 |
5.8 |
43.7 |
0.6 |
0.1 |
- |
84.1 |
14.5 |
1.4 |
- |
[49] |
| Palm kernel shell |
Malaysia |
48.82 |
5.68 |
45.08 |
0.42 |
- |
13.65 |
75.32 |
20.81 |
3.87 |
14.88 (14.75) |
[54] |
| Walnut shells |
Ukraine |
43.41 ± 0.17 |
5.66 ± 0.06 |
48.44 ± 0.08 |
1.98 ± 0.06 |
0.11 ± 0.03 |
4.1 |
- |
- |
0.41 ± 0.11 |
16.79 ± 0.08 |
[38] |
| Coconut shell |
UK |
48.32 |
5.26 |
46.14 |
0.29 |
0 |
7.16 |
68.58 |
22.00 |
2.26 |
- |
[50] |
| Other |
| Cellulose |
UK |
41.61 |
5.63 |
52.64 |
0.11 |
0 |
4.74 |
84.16 |
9.85 |
1.25 |
- |
[50] |
| Natural rubber |
Malaysia |
83.63 |
11.97 |
2.71 |
1.58 |
0.12 |
1.71 |
89.98 |
4.71 |
3.60 |
45 |
[55] |
| Spent coffee grounds |
China |
55.98 |
6.73 |
31.07 |
2.0 |
0.31 |
2.66 |
80.44 |
15.65 |
1.25 |
- |
[51] |
| Brewer’s spent grain |
Brazil |
42.2 |
7.2 |
37.6 |
3.6 |
1.1 |
3.97 |
83.3 |
9.51 |
3.22 |
21.6 |
[56] |
| Microalgae |
China |
52.07 |
7.15 |
21.65 |
8.57 |
0.62 |
- |
72.37 |
22.16 |
5.46 |
24.19 |
[57] |
| Microalgae |
China |
49.6 |
7.0 |
25.4 |
8.2 |
0.5 |
10 |
81 |
16 |
9 |
- |
[57] |
| Microalgae |
Russia |
61.3 |
6.4 |
22.5 |
8.8 |
1.1 |
3.0 |
- |
- |
5.1 |
25.04 |
[58] |
There are certain conditions to be met when choosing the type of biomass for pyrolysis and gasification, which provide maximum efficiency of the processes. Based on data
[6][26][59], the list of factors determining the choice of biomass type has been prepared (
Table 5).
Table 5. Factors in choosing biomass type for pyrolysis and gasification.
| Biomass Properties |
Factors |
Favorable Conditions for Pyrolysis and Gasification |
| Moisture content |
There are two kinds of biomass moisture: inherent (the content of moisture in biomass not affected by the weather conditions), and external (the content of moisture in biomass considering the weather conditions). |
A high content of moisture enhances pyrolysis and gasification. |
| Heating value |
There are higher and lower heating values.
The higher heating value is the maximum amount of energy potentially derived from this biomass source. It includes the content of energy released during the fuel combustion in the air, as well as latent heat in the water steam.
The lower heating value is the minimum amount of energy released from biomass conversion. |
The higher the heating value, the more rapid is pyrolysis and gasification. |
| Proportion of bound carbon and volatiles |
The content of volatiles in the solid fuel, part of the fuel that is released as gas when it is heated.
The content of bound carbon is a mass remaining after the release of volatiles, excluding the content of ash and moisture.
The content of volatiles and bound carbon account for the rate of ignition and then gasification or oxidation.
The elemental analysis, including the values of O, H, C, N, and S, indicates that a higher percentage of oxygen as compared with carbon reduces the heat of combustion of the fuel due to lower energy. |
A high content of volatiles and carbon combined with a low content of oxygen. |
| Content of ash |
The chemical degradation of a biomass-derived fuel as a result of thermochemical or biochemical processes produces a solid residue which is actually ash.
The content of ash in biomass affects both the cost of processing and the total cost of biomass energy conversion. Depending on the ash content, the available energy of the fuel proportionally decreases. After combustion, ash can form slag, a liquid phase (at a high temperature), which impairs the performance of the facility and increases operating costs. |
Low ash content. |
| Content of alkali metals |
Alkali metals (Na, K, Mg, P, and Ca) in biomass lead to the formation of a sticky mobile liquid phase (slag) which may obstruct the flue gas path. |
Lower content of alkali metals. |
| Cellulose/lignin ratio |
Cellulose decomposes at lower temperatures than lignin does. Therefore, the total conversion of the carbon-containing plant matter in the form of cellulose is higher than that of plants with a higher proportion of lignin. |
High content of cellulose and low content of lignin. |