
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yoshio Sumida | + 2567 word(s) | 2567 | 2020-08-12 06:07:02 | | | |
| 2 | Catherine Yang | -2 word(s) | 2565 | 2020-08-19 10:54:07 | | |
Video Upload Options
Nonalcoholic fatty liver disease (NAFLD) is becoming the leading cause of hepatocellular carcinoma (HCC), liver-related mortality, and liver transplantation. There is sufficient epidemiological cohort data to recommend the surveillance of patients with NAFLD based upon the incidence of HCC. The American Gastroenterology Association (AGA) expert review published in 2020 recommends that NAFLD patients with cirrhosis or advanced fibrosis estimated by non-invasive tests (NITs) consider HCC surveillance. NITs include the fibrosis-4 (FIB-4) index, the enhanced liver fibrosis (ELF) test, FibroScan, and MR elastography. The recommended surveillance modality is abdominal ultrasound (US), which is cost effective and noninvasive with good sensitivity. However, US is limited in obese patients and those with NAFLD. In NAFLD patients with a high likelihood of having an inadequate US, or if an US is attempted but inadequate, CT or MRI may be utilized. The GALAD score, consisting of age, gender, AFP, the lens culinaris-agglutinin-reactive fraction of AFP (AFP-L3), and the protein induced by the absence of vitamin K or antagonist-II (PIVKA-II), can help identify a high risk of HCC in NAFLD patients. Innovative parameters, including a Mac-2 binding protein glycated isomer, type IV collagen 7S, free apoptosis inhibitor of the macrophage, and a combination of single nucleoside polymorphisms, are expected to be established. Considering the large size of the NAFLD population, optimal screening tests must meet several criteria, including high sensitivity, cost effectiveness, and availability.
1. Introduction
The control of viral hepatitis (hepatitis B virus (HBV) and hepatitis C virus (HCV)) has now become possible, and the so-called non-HBV non-HCV hepatocellular carcinoma (NBNC-HCC) has become 1/3 of the total number of HCC cases in Japan [1]. The main background of NBNC-HCC is fatty liver disease (FLD), which is caused by alcohol consumption and/or lifestyle-related factors [1][2]]. In the past, low-drinking FLD was called nonalcoholic fatty liver disease (NAFLD). It has been proposed to change this name to metabolic dysfunction associated fatty liver disease (MAFLD) [3]. NAFLD comprises a broad spectrum of syndromes, ranging from simple steatosis and nonalcoholic steatohepatitis (NASH) to fibrosis, cirrhosis, and HCC [4]. Some NAFLD patients with the progression of fibrosis experience liver disease-related mortality (HCC, liver failure, or esophageal varices hemorrhaging) or require liver transplantation [5]. NAFLD affects about 25% of adults [6][7], but about 25% (6.7%–59%) transition to NASH [8], 25% of whom develop cirrhosis. Since 25% of cancers occur over 10 years [9] (Figure 1, 25% rule), it is estimated that only 1 or 2 out of every 100 NAFLD cases develop HCC (Figure 1) [10][11]. Although it is clear that NAFLD portends a lower risk for HCC than HBV or HCV, the high prevalence of NAFLD in the population underlies the importance of NAFLD in the development of HCC [12]. However, poor surveillance is a constant problem for patients with NAFLD. According to cohort studies from Italy and the United States, many patients with NAFLD-related HCC are not diagnosed through regular surveillance (compared to patients with HCV-related HCC), resulting in a more advanced HCC burden at diagnosis [13][14]. This review outlines the most efficient types of surveillance for HCC in NAFLD.
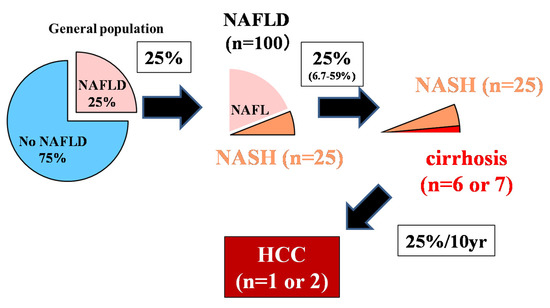
Figure 1. The 25% rule in nonalcoholic fatty liver disease (NAFLD) [10].
Although 25% of adults have NAFLD, about 25% will progress to NASH in their lifetime, and 25% will progress from NASH to liver cirrhosis. The incident rate of HCC 10 years after liver cirrhosis is about 25%. Out of 100 NAFLD patients, it is rare for 1–2 people with NAFLD to develop HCC. The authors have copyright of this figure [10].
2. Novel Indicators for Predicting Incident HCC Risk
Methods for assessing the risk of hepatocarcinogenesis itself, rather than advanced fibrosis, have also been studied (Figure 2). The FIB-4 index and NFS are also useful for predicting cancer risk [15]. In a national multicenter study led by JSG-NAFLD, Kawaguchi et al. reported that a favorable prognostic factor in NASH-HCC is serum albumin of 4.0 g/dL or more; further, the early detection of HCC can provide an indication of curative treatments, such as surgery and radiofrequency ablation therapy [16]. This suggests the importance of diagnosing HCC at an early stage when the hepatic reserve is maintained. Most NASH-HCC patients do not undergo regular surveillance. Consequently, their tumor sizes are large at the time of diagnosis, resulting in a poor prognosis [17][18].
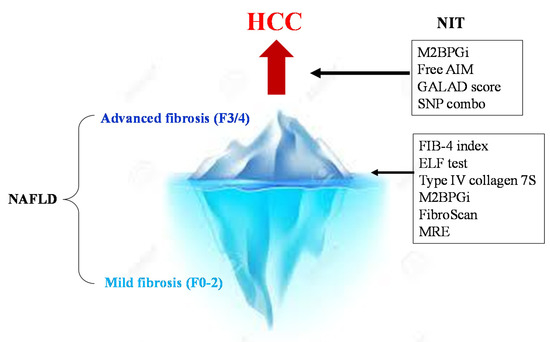
Figure 2. NITs for the surveillance of severe fibrosis and HCC in NAFLD. First, the NIT is used to determine advanced fibrosis (F3/4) from NAFLD. In cases of advanced fibrosis, regular image examinations are performed to detect HCC early. Then, strict surveillance is conducted among patients with a high GALAD score (>−0.63), high M2bpGi cases (>1.26), and PNPLA3 GG homozygous cases.
2.1. Mac2 Protein Glycosylated Isomer
Mac-2-binding protein (M2BP) is a secretory glycoprotein that contains seven N-glycans per monomer [19]. In the serum, 10–16 monomers of M2BP form a doughnut-shaped polymer that presents 70–112 N-glycans. Alterations in M2BP occur during the progression of liver disease and fibrosis due to changes in N-glycosylation (i.e., sialylation or the extension of polylactosamine). However, the underlying mechanism is unclear. As a robust lectin that binds the GalNAc residue of N-glycans and O-glycans and the clustered LacNAc structure, Wisteria floribunda agglutinin (WFA) can recognize the altered N-glycans of M2BP specifically. Thus, this specific glycoprotein was described as WFA+-M2BP and renamed to M2BPGi after the commercialization of the diagnostic reagent. Level of WFA+ -M2BP in the sera were measured by a HISCL™ M2BPGi™ assay kit using an automated immunoanalyzer (HISCL™-800; Sysmex, Kobe, Japan). The measured values of WFA+ -M2BP conjugated to WFA were indexed with the obtained values using the following equation: Cutoff index (C.O.I) = ([WFA+-M2BP]sample-[WFA+-M2BP]NC)/([WFA+-M2BP]PC − [WFA+ -M2BP]NC), where the [WFA+-M2BP] sample is the WFA+-M2BP count of the serum samples, PC is positive control, and NC is the negative control. The positive control was supplied as a calibration solution preliminarily standardized to yield a cutoff value of 1.0 [20]. The clinical application of the Wisteria floribunda agglutinin-positive Mac-2-binding protein (WFA+-M2BP) has been widely promoted after Japanese public health insurance began to cover its expenses in 2015. M2BPGi has been used as a liver fibrosis marker for various liver diseases [21], including NAFLD [20][22]. Accumulating evidence suggests that higher levels of serum M2BPGi can predict HCC incidence in patients with chronic hepatitis B [23][24][25][26][27][28]. According to a report by Kawanaka et al. [29], the carcinogenic rate was as high as 6.8% at 5 years and 21.1% at 10 years in NAFLD cases where M2BPGi was 1.26 or higher, while the rate was as low as 1.7% at 5 years and 1.7% at 10 years in patients with M2BPGi below 1.26 [29]. It has been suggested that M2BPGi may be a predictor of hepatocarcinogenesis, as well as fibrosis, but its mechanism has not been clarified.
2.2. GALAD Score
In Japan, AFP, the AFP-L3 fraction, and PIVKA-II (des-γ-carboxy pro-thrombin [DCP] in foreign countries) have been used for many years as tumor markers. According to Toyoda et al. the sensitivity was 60% and the specificity was 85% in HCC stage 1 (n = 235) when these three types of tumor markers were combined [30]. In Japan, the combination of these tumor markers is followed by a combination of imaging tests as a surveillance method for HCC. The GALAD score calculated from the age, sex, AFP, AFP-L3 fraction, and DCP has been reported to be useful in the early diagnosis of HCC globally [31]. Moreover, PIVKA-II 1 mAU/mL = DCP 0.012 ng/mL can be calculated. In a study comparing NASH with HCC and without HCC at eight facilities in Germany, the GALAD score provided a better diagnosis of HCC (AUROC 0.93) than AFP (AUROC 0.88), AFP-L3 fractionation (AUROC 0.86), or PIVKA-II alone (AUROC 0.87) [32]. The GALAD score remained useful independent of the existence of liver cirrhosis; a cutoff value of 0.63 was appropriate, even though only 25 patients were examined using the Milan criteria. The sensitivity was good at 68%, the specificity at 95%, and an AUROC of 0.91. In a prospective study of 392 NAFLD patients (of which 17 experienced HCC incidence during the course) at Ogaki Municipal Hospital, the GALAD score was characterized by an upward trend from one and a half years before the diagnosis of HCC. The GALAD score was effective for the surveillance of NASH patients [32]. These are data from only a single facility; a multi-center validation study is needed in the future.
2.3. Apoptosis Inhibitor of Macrophages
The apoptosis inhibitor of macrophages (AIM) is a protein with a molecular weight of about 40 kD that was discovered by Professor Miyazaki of the University of Tokyo in 1999 [33]. AIM is produced by Kupffer cells in the liver and macrophages in the abdominal cavity [34]. IgM behaves as a carrier of the AIM protein, storing a large amount of the inactivated form of AIM in the blood. Under certain disease conditions, AIM can dissociate from IgM locally or systemically to exert its functions, inducing the removal of various biological debris, such as excess fat, bacteria, cancer cells, or dead cell debris [35]. In patients with NASH-HCC, AIM is more strongly dissociated from the IgM pentamer compared to non-tumor-bearing patients, while IgM-unbound AIM (free AIM) in blood increases in NASH-HCC [36]. Since free AIM (cutoff value: 1.6 μg/mL) can detect HCC with a higher sensitivity (88.5%) than PIVKA-II (53.8%) or AFP (26.9%), AIM is expected to be used as a diagnostic marker for detecting NASH-HCC. Since AIM may be used as a predictor of carcinogenesis in the future, researches should focus on future data collection. Since AIM has an inhibitory effect on HCC carcinogenesis in animal models [37][38], the clinical application of AIM for HCC treatment is also anticipated. The increased blood free AIM in NASH-HCC, moreover, may be a biodefense response.
2.4. SNP Combo
Various SNPs can relate to hepatocarcinogenesis in NAFLD. PNPLA3 SNP, which has the most abundant evidence, contributes not only to the development of hepatic fibrosis but also to hepatocarcinogenesis [39][40][41]. In a study from the United Kingdom, 100 Caucasian NAFLD associated HCC cases were reported. A study on 275 NAFLD non-carcinoma cases diagnosed by liver biopsy revealed that CG hetero carriers have a 2.52-fold higher risk, and GG homo carriers have a 12.19-fold higher risk of liver carcinogenesis than PNPLA3 CC homo carriers [40]. Since PNPLA3 GG homozygotes are a risk factor, even when examined in patients only with liver cirrhosis, PNPLA3 SNP GG homozygotes present a high risk of hepatocarcinogenesis independent of liver fibrosis. The cumulative hepatocarcinogenesis rate of 238 Japanese NAFLD patients diagnosed by biopsy was examined in PNPLA3 SNP [39]. GG homozygotes were shown to have significantly higher hepatocarcinogenesis rates than C allele carriers. A study on the risk of hepatocarcinogenesis among obese individuals using an obesity cohort in Sweden revealed that the G allele carriers had 5.9 (95% CI: 1.5–23.8) times higher hepatocarcinogenesis rates [42]. An analysis of the risk of hepatocarcinogenesis among confirmed Japanese diabetic patients revealed that the JAZF1 G allele and PNPLA3 SNP GG homozygotes are risk factors [43]. It has been reported that the T allele of membrane-bound O-acyl-transferase domain circulating 7 (MBOAT7) is involved in hepatocarcinogenesis in patients without cirrhosis [44]. We previously reported that a combination of PNPLA3 and dysferlin in patients with NAFLD in Japan presented a high risk of developing HCC for both risk alleles [45], but further cases need to be studied for validation. PNPLA3 G alleles are prevalent in Japan, South Korea, Taiwan, and Mexico [7]. Thus, there is concern that NASH-HCC incidence will increase in these countries. A report from Europe indicated that the risk alleles of PNPLA3, transmembrane 6 superfamily member 2 (TM6SF2) and hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13), indicate 29 times the risk of hepatocarcinogenesis compared to the general population [23]. NAFLD patients with the TM6SF2 risk allele accumulate hepatic steatosis, but atherosclerosis is low in those with NAFLD risk alleles due to their excretion as very low density lipoprotein (VLDL). HSD17B13 modulates the action of the PNPLA3 gene. Genotype of HSD17B13 are classified to allele homozygotes (T/T), heterozygotes (T/TA), and alternate allele homozygotes (TA/TA). When patients have the PNPLA3G allele with a TA variant of HSD17B13, inflammation and fibrosis are suppressed [46]. In this way, it is important to incorporate this SNP combo into risk assessment. However, this SNP poses problems for daily clinical practice, such as cost and the protection of personal information. It is important to use the family history of HCC and cirrhosis as a simple alternative method [47].
2.5. Noninvasive Liquid Biopsy
The concept of liquid biopsy was developed to address the need for reliable, minimally invasive methods for diagnosis, prognosis, and overall disease monitoring. Liquid biopsy is a modality where bodily fluid samples, instead of solid tissue, are used for pathophysiological or molecular analyses. This method has been introduced in many clinically relevant fields, including cancer research, and, in general, any bodily fluids can be used as potential samples for a liquid biopsy. The term “liquid biopsy” can also apply to cancer by-products, including circulating tumor cells (CTCs), cell-free DNA (cfDNA), cell-free RNA (cfRNA), microRNA (miRNA), extracellular vesicles (EVs), and tumor-derived metabolites [48]. The most widely used markers are CTCs and ctDNA. ctDNA that carries cancer-specific genetic and epigenetic aberrations may facilitate a noninvasive liquid biopsy for the diagnosis and monitoring of cancer [49][50].
3. Algorithm for HCC Surveillance in Nonalcoholic Fatty Liver Disease
We constructed an algorithm for HCC surveillance in NAFLD referring to the AGA expert review (Figure 3). NAFLD patients with cirrhosis should underdo HCC surveillance. NAFLD patients who are likely to have advanced fibrosis revealed by NITs (FIB-4 index, ELF test, VCTE, and MRE) should consider HCC surveillance. US is the first method for the surveillance of HCC, but the adequacy of US should be documented because of its difficulty to use among obese patients. In NAFLD patients with a high likelihood of an inadequate US (or if an US is attempted but inadequate), a CT or MRI may be utilized [51]. Tumor markers such as PIVKA-II, AFP, and AFP-L3 may help identify those with a high risk of HCC in NAFLD. NAFLD patients who are unlikely to have advanced fibrosis evaluated by NITs should not undergo routine surveillance.
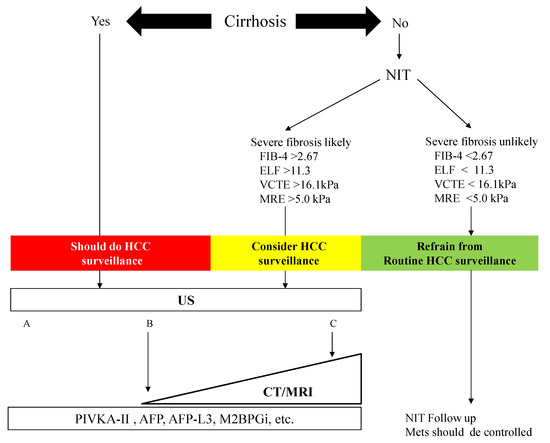
Figure 3. Algorithm for HCC surveillance in Nonalcoholic Fatty Liver Disease. NIT: noninvasive test, FIB-4: fibrosis-4, ELF: enhanced liver fibrosis, VCTE: vibration-controlled transient elastography, MRE: magnetic resonance elastography, HCC: hepatocellular carcinoma, US: ultrasonography, CT: computed tomography, MRI: magnetic resonance imaging, PIVKA-II: protein induced by vitamin K, AFP: α-fetoprotein, M2BPGi: Mac-2 binding protein glycosylated isomer. Mets: metabolic syndrome. The visualization score of the ultrasound for HCC screening is graded into the following categories: A—no or minimal limitations; B—moderate limitations, as the examination may obscure small masses; and C—severe limitation, as the examination may miss focal liver lesions [51].
4. Conclusions
The surveillance of HCC is unreasonable for all patients with NAFLD, who are estimated to total more than 2 billion worldwide. For cases of cirrhosis, suspected advanced fibrosis according to NITs, and cases of diabetes mellitus, HCC should be surveyed by semi-annual US and measurements of tumor markers such as PIVKA-II, AFP, and AFP-L3 (Figure 3). Because it is difficult to visualize the HCC in NAFLD patients by abdominal US because of obesity, alternative imaging such as CT or MRI should be considered. Early identification through surveillance provides more curative treatment options. If SNP measurements can be performed in general clinical settings, more efficient surveillance can be expected, but a cost-benefit analysis of this surveillance will be necessary in the future [52][53]. We also hope to establish innovative parameters for HCC surveillance, such as M2bpGi, the GALAD score, and free AIM. Precision tools that can better predict the development of HCC in individual patients with NAFLD are needed.
References
- Tateishi, R.; Uchino, K.; Fujiwara, N.; Takehara, T.; Okanoue, T.; Seike, M.; Yoshiji, H.; Yatsuhashi, H.; Shimizu, M.; Torimura, T.; et al. A nationwide survey on non-B, non-C hepatocellular carcinoma in Japan: 2011–2015 update. J. Gastroenterol. 2019, 54, 367.
- Shim, J.H. Should you advocate for hepatocellular carcinoma surveillance in patients with alcohol-related liver disease or non-alcoholic fatty liver disease? Clin. Mol. Hepatol. 2020, 26, 183–184.
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202.
- Finelli, C.; Tarantino, G. What is the role of adiponectin in obesity related non-alcoholic fatty liver disease? World J. Gastroenterol. 2013, 19, 802–812.
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; et al. Global Nonalcoholic Steatohepatitis Council. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin. Gastroenterol. Hepatol. 2019, 17, 748–755.
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84.
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20.
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011, 140, 124–131.
- Yatsuji, S.; Hashimoto, E.; Tobari, M.; Taniai, M.; Tokushige, K.; Shiratori, K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J. Gastroenterol. Hepatol. 2009, 24, 248–254.
- Sumida, Y.; Yoneda, M.; Tokushige, K.; Kawanaka, M.; Fujii, H.; Yoneda, M.; Imajo, K.; Takahashi, H.; Eguchi, Y.; Ono, M.; et al. Estimated Prevalence of Advanced Hepatic Fibrosis by Elastography in Patients with Type 2 Diabetes. Interv. Obes. Diabetes 2020, 3, 287.
- Diehl, A.M.; Day, C. Cause, Pathogenesis, and Treatment of Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2017, 377, 2063–2072.
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–775.
- Piscaglia, F.; Svegliati-Baroni, G.; Barchetti, A. HCC-NAFLD Italian Study Group Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology 2016, 63, 827–838.
- Mittal, S.; Sada, Y.; El-Serag, H.B.; Kanwal, F.; Duan, Z.; Temple, S.; May, S.B.; Kramer, J.R.; Richardson, P.A.; Davila, J.A.; et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the Veteran Affairs Population. Clin. Gastroenterol. Hepatol. 2015, 13, 594–601.
- Kim, G.A.; Lee, H.C.; Choe, J.; Kim, M.J.; Lee, M.J.; Chang, H.S.; Bae, I.Y.; Kim, H.K.; An, J.; Shim, J.H.; et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 2018, 68, 140–146.
- Kawaguchi, T.; Tokushige, K.; Hyogo, H.; Aikata, H.; Nakajima, T.; Ono, M.; Kawanaka, M.; Sawada, K.; Imajo, K.; Honda, K.; et al. A Data Mining-based Prognostic Algorithm for NAFLD-related Hepatoma Patients: A Nationwide Study by the Japan Study Group of NAFLD. Sci. Rep. 2018, 8, 10434.
- Tavakoli, H.; Robinson, A.; Liu, B.; Bhuket, T.; Younossi, Z.; Saab, S.; Ahmed, A.; Wong, R.J. Cirrhosis Patients with Nonalcoholic Steatohepatitis Are Significantly Less Likely to Receive Surveillance for Hepatocellular Carcinoma. Dig. Dis. Sci. 2017, 62, 2174–2181.
- Younossi, Z.M.; Otgonsuren, M.; Henry, L.; Venkatesan, C.; Mishra, A.; Erario, M.; Hunt, S. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 2015, 62, 1723–1730.
- Narimatsu, H. Development of M2BPGi: A novel fibrosis serum glycobiomarker for chronic hepatitis/cirrhosis diagnostics. Expert Rev. Proteom. 2015, 12, 683–693.
- Alkhouri, N.; Johnson, C.; Adams, L.; Kitajima, S.; Tsuruno, C.; Colpitts, T.L.; Hatcho, K.; Lawitz, E.; Lopez, R.; Feldstein, A.E. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein levels predict the presence of fibrotic nonalcoholic steatohepatitis (NASH) and NASH cirrhosis. PLoS ONE 2018, 13, e0202226.
- Ito, K.; Murotani, K.; Nakade, Y.; Inoue, T.; Nakao, H.; Sumida, Y.; Kamada, Y.; Yoneda, M. Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein levels and liver fibrosis: A meta-analysis. J. Gastroenterol. Hepatol. 2017, 32, 1922–1930.
- Abe, M.; Miyake, T.; Kuno, A.; Imai, Y.; Sawai, Y.; Hino, K.; Hara, Y.; Hige, S.; Sakamoto, M.; Yamada, G.; et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J. Gastroenterol. 2015, 50, 776–784.
- Tseng, T.C.; Peng, C.Y.; Hsu, Y.C.; Su, T.H.; Wang, C.C.; Liu, C.J.; Yang, H.C.; Yang, W.T.; Lin, C.H.; Yu, M.L.; et al. Baseline Mac-2 Binding Protein Glycosylation Isomer Level Stratifies Risks of Hepatocellular Carcinoma in Chronic Hepatitis B Patients with Oral Antiviral Therapy. Liver Cancer 2020, 9, 207–220.
- Kawaguchi, K.; Honda, M.; Ohta, H.; Terashima, T.; Shimakami, T.; Arai, K.; Yamashita, T.; Sakai, Y.; Yamashita, T.; Mizukoshi, E.; et al. Serum Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts hepatocellular carcinoma incidence and recurrence in nucleos(t)ide analogue therapy for chronic hepatitis B. J. Gastroenterol. 2018, 53, 740–751.
- Kim, S.U.; Heo, J.Y.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Han, K.H.; Park, J.Y.; Kim, D.Y.; Han, K.H.; Ahn, S.H.; et al. Wisteria floribunda agglutinin-positive human Mac-2 binding protein predicts the risk of HBV-related liver cancer development. Liver Int. 2017, 37, 879–887.
- Shinkai, N.; Nojima, M.; Iio, E.; Matsunami, K.; Toyoda, H.; Murakami, S.; Inoue, T.; Ogawa, S.; Kumada, T.; Tanaka, Y. High levels of serum Mac-2-binding protein glycosylation isomer (M2BPGi) predict the development of hepatocellular carcinoma in hepatitis B patients treated with nucleot(s)ide analogues. J. Gastroenterol. 2018, 53, 883–889.
- Cheung, K.S.; Seto, W.K.; Wong, D.K.; Mak, L.Y.; Lai, C.L.; Yuen, M.F. Wisteria floribunda agglutinin-positive human Mac-2 binding protein predicts liver cancer development in chronic hepatitis B patients under antiviral treatment. Oncotarget 2017, 8, 47507–47517.
- Mak, L.Y.; Ko, M.; To, E.; Wong, D.K.; Ma, J.H.; Hui, T.L.; Seto, W.K.; Fung, J.; Lai, C.L.; Yuen, M.F. Serum Mac-2-binding protein glycosylation isomer and risk of hepatocellular carcinoma in entecavir-treated chronic hepatitis B patients. J. Gastroenterol. Hepatol. 2019, 34, 1817–1823.
- Kawanaka, M.; Tomiyama, Y.; Hyogo, H.; Koda, M.; Shima, T.; Tobita, H.; Hiramatsu, A.; Nishino, K.; Okamoto, T.; Sato, S.; et al. Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts the development of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. Hepatol. Res. 2018, 48, 521.
- Toyoda, H.; Kumada, T.; Tada, T.; Kaneoka, Y.; Maeda, A.; Kanke, F.; Satomura, S. Clinical utility of highly sensitive Lens culinaris agglutinin-reactive alpha-fetoprotein in hepatocellular carcinoma patients with alpha-fetoprotein <20 ng/mL. Cancer Sci. 2011, 102, 1025–1031.
- Johnson, P.J.; Pirrie, S.J.; Cox, T.F.; Berhane, S.; Teng, M.; Palmer, D.; Morse, J.; Hull, D.; Patman, G.; Kagebayashi, C.; et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol. Biomark. Prev. 2014, 23, 144–153.
- Best, J.; Bechmann, L.P.; Sowa, J.P.; Sydor, S.; Dechêne, A.; Pflanz, K.; Bedreli, S.; Schotten, C.; Geier, A.; Berg, T.; et al. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients With Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2020, 18, 728–735.
- Miyazaki, T.; Hirokami, Y.; Matsuhashi, N.; Takatsuka, H.; Naito, M. Increased susceptibility of thymocytes to apoptosis in mice lacking AIM, a novel murine macrophage-derived soluble factor belonging to the scavenger receptor cysteine-rich domain superfamily. J. Exp. Med. 1999, 189, 413–422.
- Arai, S.; Miyazaki, T. Impacts of the apoptosis inhibitor of macrophage (AIM) on obesity-associated inflammatory diseases. Semin. Immunopathol. 2014, 36, 3–12.
- Miyazaki, T.; Yamazaki, T.; Sugisawa, R.; Gershwin, M.E.; Arai, S. AIM associated with the IgM pentamer: Attackers on stand-by at aircraft carrier. Cell. Mol. Immunol. 2018, 15, 563–574.
- Koyama, N.; Yamazaki, T.; Kanetsuki, Y.; Hirota, J.; Asai, T.; Mitsumoto, Y.; Mizuno, M.; Shima, T.; Kanbara, Y.; Arai, S.; et al. Activation of apoptosis inhibitor of macrophage is a sensitive diagnostic marker for NASH-associated hepatocellular carcinoma. J. Gastroenterol. 2018, 53, 770.
- Maehara, N.; Arai, S.; Mori, M.; Iwamura, Y.; Kurokawa, J.; Kai, T.; Kusunoki, S.; Taniguchi, K.; Ikeda, K.; Ohara, O.; et al. Circulating AIM prevents hepatocellular carcinoma through complement activation. Cell Rep. 2014, 9, 61–74.
- Ozawa, T.; Maehara, N.; Kai, T.; Arai, S.; Miyazaki, T. Dietary fructose-induced hepatocellular carcinoma development manifested in mice lacking apoptosis inhibitor of macrophage (AIM). Genes Cells. 2016, 21, 1320–1332.
- Seko, Y.; Sumida, Y.; Tanaka, S.; Mori, K.; Taketani, H.; Ishiba, H.; Hara, T.; Okajima, A.; Umemura, A.; Nishikawa, T.; et al. Development of hepatocellular carcinoma in Japanese patients with biopsy-proven non-alcoholic fatty liver disease: Association between PNPLA3 genotype and hepatocarcinogenesis/fibrosis progression. Hepatol. Res. 2019, 47, 1083.
- Liu, Y.L.; Patman, G.L.; Leathart, J.B.; Piguet, A.C.; Burt, A.D.; Dufour, J.F.; Day, C.P.; Daly, A.K.; Reeves, H.L.; Anstee, Q.M. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J. Hepatol. 2014, 61, 75.
- Seko, Y.; Yamaguchi, K.; Itoh, Y. The genetic backgrounds in nonalcoholic fatty liver disease. Clin. J. Gastroenterol. 2018, 11, 97–102.
- Burza, M.A.; Pirazzi, C.; Maglio, C.; Sjöholm, K.; Mancina, R.M.; Svensson, P.A.; Jacobson, P.; Adiels, M.; Baroni, M.G.; Borén, J.; et al. PNPLA3 I148M (rs738409) genetic variant is associated with hepatocellular carcinoma in obese individuals. Dig. Liver Dis. 2012, 44, 1037.
- Ueyama, M.; Nishida, N.; Korenaga, M.; Korenaga, K.; Kumagai, E.; Yanai, H.; Adachi, H.; Katsuyama, H.; Moriyama, S.; Hamasaki, H.; et al. The impact of PNPLA3 and JAZF1 on hepatocellular carcinoma in non-viral hepatitis patients with type 2 diabetes mellitus. J. Gastroenterol. 2016, 51, 370.
- Donati, B.; Dongiovanni, P.; Romeo, S.; Meroni, M.; McCain, M.; Miele, L.; Petta, S.; Maier, S.; Rosso, C.; De Luca, L.; et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci. Rep. 2017, 7, 4492.
- Kawaguchi, T.; Shima, T.; Mizuno, M.; Mitsumoto, Y.; Umemura, A.; Kanbara, Y.; Tanaka, S.; Sumida, Y.; Yasui, K.; Takahashi, M.; et al. Risk estimation model for nonalcoholic fatty liver disease in the Japanese using multiple genetic markers. PLoS ONE 2018, 13, e0185490.
- Gellert-Kristensen, H.; Richardson, T.G.; Davey Smith, G.; Nordestgaard, B.G.; Tybjaerg-Hansen, A.; Stender, S. Combined Effect of PNPLA3, TM6SF2, and HSD17B13 Variants on Risk of Cirrhosis and Hepatocellular Carcinoma in the General Population. Hepatology 2020, in press.
- Caussy, C.; Soni, M.; Cui, J.; Bettencourt, R.; Schork, N.; Chen, C.H.; Ikhwan, M.A.; Bassirian, S.; Cepin, S.; Gonzalez, M.P.; et al. Familial NAFLD Cirrhosis Research Consortium. Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. J. Clin. Investig. 2017, 127, 2697–2704.
- Mocan, T.; Simão, A.L.; Castro, R.E.; Rodrigues, C.; Słomka, A.; Wang, B.; Strassburg, C.; Wöhler, A.; Willms, A.G.; Kornek, M. Liquid Biopsies in Hepatocellular Carcinoma: Are We Winning? J. Clin. Med. 2020, 9, 1541.
- Xu, R.H.; Wei, W.; Krawczyk, M.; Wang, W.; Luo, H.; Flagg, K.; Yi, S.; Shi, W.; Quan, Q.; Li, K.; et al. Circulating tumor DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat. Mater. 2017, 16, 1155–1161.
- Li, X.; Wang, H.; Li, T.; Wang, L.; Wu, X.; Liu, J.; Xu, Y.; Wei, W. Circulating tumor DNA/circulating tumor cells and the applicability in different causes induced hepatocellular carcinoma. Curr. Probl. Cancer 2020, 44, 100516.
- Loomba, R.; Lim, J.K.; Patton, H.; El-Serag, H.B. AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2020, 158, 1822.
- Srivastava, A.; Jong, S.; Gola, A.; Gailer, R.; Morgan, S.; Sennett, K.; Tanwar, S.; Pizzo, E.; O’Beirne, J.; Tsochatzis, E.; et al. Cost-comparison analysis of FIB-4, ELF and fibroscan in community pathways for non-alcoholic fatty liver disease. BMC Gastroenterol. 2019, 19, 122.
- European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402.




