
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Megan O'Connor | + 2155 word(s) | 2155 | 2021-08-16 11:23:42 | | | |
| 2 | Amina Yu | Meta information modification | 2155 | 2021-08-26 05:23:28 | | |
Video Upload Options
SIV and SHIV-infected NHPs exhibit a range of viral burdens, pathologies, and responses to combinatorial antiretroviral therapy (cART) regimens and the choice of the NHP model for AIDS could influence outcomes in studies investigating interventions. Previously, in rhesus macaques (RMs) we showed that maintenance of mucosal Th17/Treg homeostasis during SIV infection correlated with a better virological response to cART. Here, in RMs we compared viral kinetics and dysregulation of gut homeostasis, defined by T cell subset disruption, during highly pathogenic SIVΔB670 compared to SHIV-1157ipd3N4 infection.SHIV infection resulted in lower acute viremia and less disruption to gut CD4 T-cell homeostasis. Additionally, 24/24 SHIV-infected versus 10/19 SIV-infected animals had sustained viral suppression <100 copies/mL of plasma after 5 months of cART. Significantly, the more profound viral suppression during cART in a subset of SIV and all SHIV-infected RMs corresponded with less gut immune dysregulation during acute SIV/SHIV infection, defined by maintenance of the Th17/Treg ratio. These results highlight significant differences in viral control during cART and gut dysregulation in NHP AIDS models and suggest that selection of a model may impact the evaluation of candidate therapeutic interventions for HIV treatment and cure strategies.
1. Introduction
To determine whether impaired mucosal responses also occurred in a SHIV model, we sought to compare viral replication, cART viral suppression, and gut T cell immune dysregulation during SHIV-1157ipd3N4 infection with SIVΔB670 infection in RM, as previously reported [11]. SIVΔB670 is highly pathogenic and was passaged in vitro to be less pathogenic, but animals still progress to AIDS in less than a year [14][15]. SHIV-1157ipd3N4 is a CCR5-tropic SHIV containing an HIV clade C envelope and was adapted for use in NHPs by passage in vivo and causes progression to AIDS in 2–5 years [16][17]. We demonstrate that both strains support high levels of viral replication in rhesus macaques, with SHIV-1157ipd3N4-infected animals showing lower acute viral burdens and greater virologic control, defined as sustained suppression of viral load below 100 viral RNA copies/mL, during cART. Analysis of mucosal CD4 T-cells during acute infection and following >5 months of cART revealed lower immune dysregulation, defined by an imbalance of Th17 and Treg cells, and lower levels of T-cell activation during cART in SHIV-1157ipd3N4-infected animals indicating overall slower SHIV-related gut disease progression. These results indicate that selection of a pre-clinical NHP model can influence outcomes in the evaluation of HIV treatment and prevention strategies.
2. Lower Acute Gut Immune Dysfunction in SIV/SHIV-Infected Macaques Is Associated with Greater Viral Control by cART
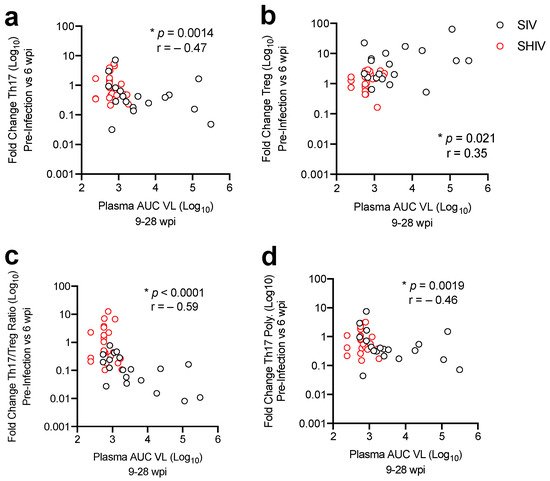
3. Discussion
References
- Evans, D.T.; Silvestri, G. Non-Human Primate Models in AIDS Research. Curr. Opin. HIV AIDS 2013, 8, 255–261.
- Hatziioannou, T.; Evans, D.T. Animal Models for HIV/AIDS Research. Nat. Rev. Microbiol. 2012, 10, 852–867.
- Bar, K.J.; Coronado, E.; Hensley-McBain, T.; O’Connor, M.A.; Osborn, J.M.; Miller, C.; Gott, T.M.; Wangari, S.; Iwayama, N.; Ahrens, C.Y.; et al. Simian-Human Immunodeficiency Virus SHIV.CH505 Infection of Rhesus Macaques Results in Persistent Viral Replication and Induces Intestinal Immunopathology. J. Virol. 2019, 93.
- Favre, D.; Lederer, S.; Kanwar, B.; Ma, Z.-M.; Proll, S.; Kasakow, Z.; Mold, J.; Swainson, L.; Barbour, J.D.; Baskin, C.R.; et al. Critical Loss of the Balance between Th17 and T Regulatory Cell Populations in Pathogenic SIV Infection. PLoS Pathog. 2009, 5, e1000295.
- Khowawisetsut, L.; Pattanapanyasat, K.; Onlamoon, N.; Mayne, A.E.; Little, D.M.; Villinger, F.; Ansari, A.A. Relationships between IL-17(+) Subsets, Tregs and PDCs That Distinguish among SIV Infected Elite Controllers, Low, Medium and High Viral Load Rhesus Macaques. PLoS ONE 2013, 8, e61264.
- Amedee, A.M.; Lacour, N.; Gierman, J.L.; Martin, L.N.; Clements, J.E.; Bohm, R.; Harrison, R.M.; Murphey-Corb, M. Genotypic Selection of Simian Immunodeficiency Virus in Macaque Infants Infected Transplacentally. J. Virol. 1995, 69, 7982–7990.
- Hong, F.F.; Mellors, J.W. Changes in HIV Reservoirs during Long-Term Antiretroviral Therapy. Curr. Opin. HIV AIDS 2015, 10, 43–48.
- Hunt, P.W. Th17, Gut, and HIV: Therapeutic Implications. Curr. Opin. HIV AIDS 2010, 5, 189–193.
- Fuller, D.H.; Rajakumar, P.; Che, J.W.; Narendran, A.; Nyaundi, J.; Michael, H.; Yager, E.J.; Stagnar, C.; Wahlberg, B.; Taber, R.; et al. Therapeutic DNA Vaccine Induces Broad T Cell Responses in the Gut and Sustained Protection from Viral Rebound and AIDS in SIV-Infected Rhesus Macaques. PLoS ONE 2012, 7, e33715.
- Tunggal, H.C.; Munson, P.V.; O’Connor, M.A.; Hajari, N.; Dross, S.E.; Bratt, D.; Fuller, J.T.; Bagley, K.; Fuller, D.H. Effects of Therapeutic Vaccination on the Control of SIV in Rhesus Macaques with Variable Responsiveness to Antiretroviral Drugs. PLoS ONE 2021, 16, e0253265.
- O’Connor, M.A.; Munson, P.V.; Tunggal, H.; Hajari, N.; Lewis, T.B.; Bratt, D.; Moats, C.; Smedley, J.; Bagley, K.; Mullins, J.I.; et al. Mucosal T Helper 17 and T Regulatory Cell Homeostasis Correlates with Acute Simian Immunodeficiency Virus Viremia and Responsiveness to Antiretroviral Therapy in Macaques. AIDS Res. Hum. Retrovir. 2019, 35, 295–305.
- Falivene, J.; Ghiglione, Y.; Laufer, N.; Eugenia Socías, M.; Pía Holgado, M.; Julia Ruiz, M.; Maeto, C.; Inés Figueroa, M.; Giavedoni, L.D.; Cahn, P.; et al. Th17 and Th17/Treg Ratio at Early HIV Infection Associate with Protective HIV-Specific CD8(+) T-Cell Responses and Disease Progression. Sci. Rep. 2015, 5, 11511.
- Ponte, R.; Mehraj, V.; Ghali, P.; Couëdel-Courteille, A.; Cheynier, R.; Routy, J.-P. Reversing Gut Damage in HIV Infection: Using Non-Human Primate Models to Instruct Clinical Research. EBioMedicine 2016, 4, 40–49.
- Baskin, G.B.; Murphey-Corb, M.; Watson, E.A.; Martin, L.N. Necropsy Findings in Rhesus Monkeys Experimentally Infected with Cultured Simian Immunodeficiency Virus (SIV)/Delta. Vet. Pathol. 1988, 25, 456–467.
- Murphey-Corb, M.; Martin, L.N.; Rangan, S.R.; Baskin, G.B.; Gormus, B.J.; Wolf, R.H.; Andes, W.A.; West, M.; Montelaro, R.C. Isolation of an HTLV-III-Related Retrovirus from Macaques with Simian AIDS and Its Possible Origin in Asymptomatic Mangabeys. Nat. Cell Biol. 1986, 321, 435–437.
- Song, R.J.; Chenine, A.-L.; Rasmussen, R.A.; Ruprecht, C.R.; Mirshahidi, S.; Grisson, R.D.; Xu, W.; Whitney, J.B.; Goins, L.M.; Ong, H.; et al. Molecularly Cloned SHIV-1157ipd3N4: A Highly Replication- Competent, Mucosally Transmissible R5 Simian-Human Immunodeficiency Virus Encoding HIV Clade C Env. J. Virol. 2006, 80, 8729–8738.
- Humbert, M.; Rasmussen, R.A.; Song, R.; Ong, H.; Sharma, P.; Chenine, A.L.; Kramer, V.G.; Siddappa, N.B.; Xu, W.; Else, J.G.; et al. SHIV-1157i and Passaged Progeny Viruses Encoding R5 HIV-1 Clade C Env Cause AIDS in Rhesus Monkeys. Retrovirology 2008, 5, 94.
- Grimm, D.U.S. Labs Using a Record Number of Monkeys. Science 2018, 362, 630.
- Hoenigl, M.; Chaillon, A.; Moore, D.J.; Morris, S.R.; Mehta, S.R.; Gianella, S.; Amico, K.R.; Little, S.J. Rapid HIV Viral Load Suppression in Those Initiating Antiretroviral Therapy at First Visit after HIV Diagnosis. Sci. Rep. 2016, 6, 32947.
- Pahar, B.; Kuebler, D.; Rasmussen, T.; Wang, X.; Srivastav, S.K.; Das, A.; Veazey, R.S. Quantification of Viral RNA and DNA Positive Cells in Tissues From Simian Immunodeficiency Virus/Simian Human Immunodeficiency Virus Infected Controller and Progressor Rhesus Macaques. Front. Microbiol. 2019, 10, 2933.
- Mannioui, A.; Bourry, O.; Sellier, P.; Delache, B.; Brochard, P.; Andrieu, T.; Vaslin, B.; Karlsson, I.; Roques, P.; Le Grand, R. Dynamics of Viral Replication in Blood and Lymphoid Tissues during SIVmac251 Infection of Macaques. Retrovirology 2009, 6, 106.
- Wong, J.K.; Yukl, S.A. Tissue Reservoirs of HIV. Curr. Opin. HIV AIDS 2016, 11, 362–370.
- Del Prete, G.Q.; Lifson, J.D. Considerations in the Development of Nonhuman Primate Models of Combination Antiretroviral Therapy for Studies of AIDS Virus Suppression, Residual Virus, and Curative Strategies. Curr. Opin. HIV AIDS 2013, 8, 262–272.
- Del Prete, G.Q.; Shoemaker, R.; Oswald, K.; Lara, A.; Trubey, C.M.; Fast, R.; Schneider, D.K.; Kiser, R.; Coalter, V.; Wiles, A.; et al. Effect of Suberoylanilide Hydroxamic Acid (SAHA) Administration on the Residual Virus Pool in a Model of Combination Antiretroviral Therapy-Mediated Suppression in SIVmac239-Infected Indian Rhesus Macaques. Antimicrob. Agents Chemother 2014, 58, 6790–6806.
- Podany, A.T.; Scarsi, K.K.; Fletcher, C.V. Comparative Clinical Pharmacokinetics and Pharmacodynamics of HIV-1 Integrase Strand Transfer Inhibitors. Clin. Pharmacokinet. 2017, 56, 25–40.
- Del Prete, G.Q.; Smedley, J.; Macallister, R.; Jones, G.S.; Li, B.; Hattersley, J.; Zheng, J.; Piatak, M.; Keele, B.F.; Hesselgesser, J.; et al. Short Communication: Comparative Evaluation of Coformulated Injectable Combination Antiretroviral Therapy Regimens in Simian Immunodeficiency Virus-Infected Rhesus Macaques. AIDS Res. Hum. Retrovir. 2016, 32, 163–168.
- Crakes, K.R.; Jiang, G. Gut Microbiome Alterations During HIV/SIV Infection: Implications for HIV Cure. Front. Microbiol. 2019, 10, 1104.
- Younas, M.; Psomas, C.; Reynes, J.; Corbeau, P. Immune Activation in the Course of HIV-1 Infection: Causes, Phenotypes and Persistence under Therapy. HIV Med. 2016, 17, 89–105.
- Schuetz, A.; Deleage, C.; Sereti, I.; Rerknimitr, R.; Phanuphak, N.; Phuang-Ngern, Y.; Estes, J.D.; Sandler, N.G.; Sukhumvittaya, S.; Marovich, M.; et al. Initiation of ART during Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation. PLoS Pathog. 2014, 10, e1004543.
- November 25, C.S.H. govDate Last Updated; 2020 Global Statistics. Available online: https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics (accessed on 12 August 2021).
- Park, L.S.; Tate, J.P.; Sigel, K.; Brown, S.T.; Crothers, K.; Gibert, C.; Goetz, M.B.; Rimland, D.; Rodriguez-Barradas, M.C.; Bedimo, R.J.; et al. Association of Viral Suppression With Lower AIDS-Defining and Non-AIDS-Defining Cancer Incidence in HIV-Infected Veterans: A Prospective Cohort Study. Ann. Intern. Med. 2018, 169, 87–96.
- Martin, G.E.; Gossez, M.; Williams, J.P.; Stöhr, W.; Meyerowitz, J.; Leitman, E.M.; Goulder, P.; Porter, K.; Fidler, S.; Frater, J.; et al. Post-Treatment Control or Treated Controllers? Viral Remission in Treated and Untreated Primary HIV Infection. AIDS 2017, 31, 477–484.
- Namazi, G.; Fajnzylber, J.M.; Aga, E.; Bosch, R.J.; Acosta, E.P.; Sharaf, R.; Hartogensis, W.; Jacobson, J.M.; Connick, E.; Volberding, P.; et al. The Control of HIV After Antiretroviral Medication Pause (CHAMP) Study: Posttreatment Controllers Identified From 14 Clinical Studies. J. Infect. Dis. 2018, 218, 1954–1963.




