
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | miao miao hao | + 1109 word(s) | 1109 | 2021-06-18 09:37:08 | | | |
| 2 | Enzi Gong | Meta information modification | 1109 | 2021-07-02 06:21:33 | | |
Video Upload Options
Plant cytoplasmic male sterility (CMS) is a maternally inherited trait maintaining female fertility but producing abortive pollen. CMS is widespread among plants and widely used to produce hybrids with significant heterosis. CMS is usually associated with chimeric open reading frames (ORF) caused by mitochondrial genome reorganization.
1. Overview
Heterosis utilization is very important in hybrid seed production. An AL-type cytoplasmic male sterile (CMS) line has been used in wheat-hybrid seed production, but its sterility mechanism has not been explored. In the present study, we sequenced and verified the candidate CMS gene in the AL-type sterile line (AL18A) and its maintainer line (AL18B). In the late uni-nucleate stage, the tapetum cells of AL18A showed delayed programmed cell death (PCD) and termination of microspore at the bi-nucleate stage. As compared to AL18B, the AL18A line produced 100% aborted pollens. The mitochondrial genomes of AL18A and AL18B were sequenced using the next generation sequencing such as Hiseq and PacBio. It was found that the mitochondrial genome of AL18A had 99% similarity with that of Triticum timopheevii, AL18B was identical to that of Triticum aestivum cv. Chinese Yumai. Based on transmembrane structure prediction, 12 orfs were selected as candidate CMS genes, including a previously suggested orf256. Only the lines harboring orf279 showed sterility in the transgenic Arabidopsis system, indicating that orf279 is the CMS gene in the AL-type wheat CMS lines. These results provide a theoretical basis and data support to further analyze the mechanism of AL-type cytoplasmic male sterility in wheat.
2. Plant Cytoplasmic Male Sterility
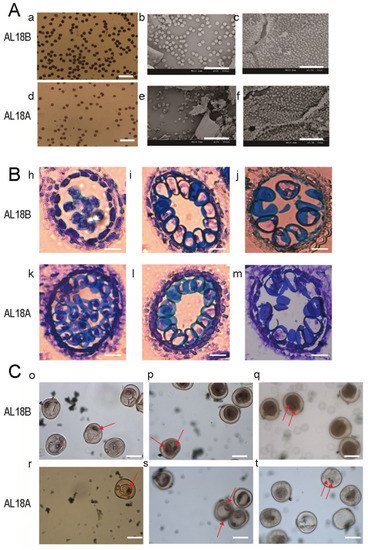
3. The Wheat AL-CMS Line AL18A Is the Typical Sterile Type
References
- Chen, L.; Liu, Y.-G. Male Sterility and Fertility Restoration in Crops. Annu. Rev. Plant Biol. 2014, 65, 579–606.
- Ivanov, M.K.; Dymshits, G.M. Cytoplasmic male sterility and restoration of pollen fertility in higher plants. Russ. J. Genet. 2007, 43, 354–368.
- Luo, D.; Xu, H.; Liu, Z.; Guo, J.; Li, H.; Chen, L.; Fang, C.; Zhang, Q.; Bai, M.; Yao, N.; et al. A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat. Genet. 2013, 45, 573–577.
- Schnable, P.S.; Wise, R.P. The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Plant Sci. 1998, 3, 175–180.
- Hanson, M.R.; Bentolila, S. Interactions of mitochondrial and nuclear genes that affect male gametophyte development. Plant Cell 2004, 16, S154–S169.
- L’Homme, Y.; Stahl, R.J.; Li, X.-Q.; Hameed, A.; Brown, G.G. Brassica nap cytoplasmic male sterility is associated with expression of a mtDNA region containing a chimeric gene similar to the pol CMS-associated orf224 gene. Curr. Genet. 1997, 31, 325–335.
- Nivison, H.T.; Sutton, C.A.; Wilson, R.K.; Hanson, M.R. Sequencing, processing, and localization of the petunia CMS-associated mitochondrial protein. Plant J. 1994, 5, 613–623.
- Wang, Z.; Zou, Y.; Li, X.; Zhang, Q.; Chen, L.; Wu, H.; Su, D.; Chen, Y.; Guo, J.; Luo, D.; et al. Cytoplasmic Male Sterility of Rice with Boro II Cytoplasm Is Caused by a Cytotoxic Peptide and Is Restored by Two Related PPR Motif Genes via Distinct Modes of mRNA Silencing. Plant Cell 2006, 18, 676–687.
- Wise, R.P.; Fliss, A.E.; Pring, D.R.; Gengenbach, B.G. urf13-T of T cytoplasm maize mitochondria encodes a 13 kD polypeptide. Plant Mol. Biol. 1987, 9, 121–126.
- Wise, R.P.; Dill, C.L.; Schnable, P. Mutator-Induced Mutations of the Rf1 Nuclear Fertility Restorer of T-Cytoplasm Maize Alter the Accumulation of T-Urf13 Mitochondrial Transcripts. Genetics 1996, 143, 1383–1394.
- Wolf-Litman, O.; Soferman, O.; Tabib, Y.; Izhar, S. Interaction of the mitochondrial S-Pcf locus for cytoplasmic male sterility in Petunia with multiple fertility-restoration genes in somatic hybrid plants. Theor. Appl. Genet. 1992, 84, 829–834.
- Wang, K.; Gao, F.; Ji, Y.; Liu, Y.; Dan, Z.; Yang, P.; Zhu, Y.; Li, S. ORFH79 impairs mitochondrial function via interaction with a subunit of electron transport chain complex III in Honglian cytoplasmic male sterile rice. New Phytol. 2013, 198, 408–418.
- Xiao, S.; Zang, J.; Pei, Y.; Liu, J.; Liu, J.; Song, W.; Shi, Z.; Su, A.; Zhao, J.; Chen, H. Activation of Mitochondrial orf355 Gene Expression by a Nuclear-Encoded DREB Transcription Factor Causes Cytoplasmic Male Sterility in Maize. Mol. Plant 2020, 13, 1270–1283.
- Bentolila, S.; Alfonso, A.A.; HansonM, R. A pentatricopep tide repeat containing gene restores fertility to cytoplasmic male sterile plants. Proc. Natl. Acad. Sci. USA 2002, 99, 10887–10892.
- Sun, L.; Yao, F.; Li, C.; Li, L.; Liu, B.; Gao, Q. RAPD analysing of CMS-T, K, V mtDNAs and cloning of mtDNA fragments associated with CMS-K in wheat. Acta Agron. Sin. 2001, 27, 144–148.
- Song, J.; Hedgcoth, C. A chimeric gene (orf256) is expressed as protein only in cytoplasmic male-sterile lines of wheat. Plant Mol. Biol. 1994, 26, 535–539.
- Hedgcoth, C.; El-Shehawi, A.M.; Wei, P.; Clarkson, M.; Tamalis, D. A chimeric open reading frame associated with cytoplasmic male sterility in alloplasmic wheat with Triticum timopheevi mitochondria is present in several Triticum and Aegilops species, barley, and rye. Curr. Genet. 2002, 41, 357–366.
- Gupta, P.K.; Balyan, H.S.; Gahlaut, V.; Saripalli, G.; Pal, B.; Basnet, B.R.; Joshi, A.K. Hybrid wheat: Past, present and future. Theor. Appl. Genet. 2019, 132, 2463–2483.
- Melonek, J.; Duarte, J.; Martin, J.; Beuf, L.; Murigneux, A.; Varenne, P.; Comadran, J.; Specel, S.; Levadoux, S.; Bernath-Levin, K.; et al. The genetic basis of cytoplasmic male sterility and fertility restoration in wheat. Nat. Commun. 2021, 12, 1–14.
- Liu, H.; Cui, P.; Zhan, K.; Lin, Q.; Zhuo, G.; Guoyin, Z.; Ding, F.; Yang, W.; Liu, D.; Hu, S.; et al. Comparative analysis of mitochondrial genomes between a wheat K-type cytoplasmic male sterility (CMS) line and its maintainer line. BMC Genom. 2011, 12, 163.
- Gui, A.; Nie, Y.; Ma, L.; Xu, H.; Li, R.; Liu, X.; Kong, D.; Zhang, X.; Mu, P. Mapping QTLs of fertility restoring genes for AL-type male sterility line AL18A in wheat. J. Triticeae Crop. 2017, 37, 1–6.
- Nie, Y.; Kong, D.; Cui, F.; Sang, W.; Mu, P.; Xu, H.; Tian, X. Study on the outcross rate of wheat AL type cytoplasmic male sterile lines. J. N. Am. Agric. 2018, 46, 1–6.
- Nie, Y.; Tian, X.; Han, X.; Xu, H.; Mu, P.; Sang, W.; Cui, F.; Xiang, J.; Zhou, B. New hybrid wheat variety with high yield and good quality, Xindong 43. J. Triticeae Crop. 2014, 34, 1450.




