
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lucía Fernández | + 2163 word(s) | 2163 | 2020-12-29 07:19:53 | | | |
| 2 | Vicky Zhou | Meta information modification | 2163 | 2021-01-05 05:19:08 | | |
Video Upload Options
The quick spread of infectious diseases and their unpredictable consequences, in terms of human lives and economic losses, will require a change in our strategy, both at the clinical and the research level. Ultimately, we should be ready to fight against infectious diseases affecting a huge number of people in different parts of the world. This new scenario will require rapid, inexpensive diagnostic systems, applicable anywhere in the world and, preferably, without the need for specialized personnel. Also, treatments for these diseases must be versatile, easily scalable, cheap, and easy to apply. All this will only be possible with the joint support of governments, which will have to make the requirements for the approval of new therapies more flexible. Meanwhile, the pharmaceutical sector must commit to prioritizing products of global interest over the most profitable ones. Extreme circumstances demand a vehement response, and any profit losses may well pay dividends going forward.
1. Introduction
Recent advances in basic scientific research, together with the development of molecular biology techniques, have not only improved infectious disease diagnosis, but have also provided relevant data about pathogenesis and epidemiology. As such, science offers the necessary tools for appropriate disease prevention and control. However, the progress made in this field over the past century is now facing a new challenge. The available techniques for disease detection and treatment must be adapted to deal with global health problems. In response to this challenge, several molecular assays have been developed to address pathogen detection and quantitation with high sensitivity and specificity. These include, for instance, some nucleic acid-based detection methods that exhibit a high sensitivity, specificity, accuracy, efficiency, and versatility [1].
Another aspect to consider is that the rising antimicrobial resistance and the heightened risk of viral pandemics might contribute to the increased incidence of bacterial infections. For example, the scarce data available to date show that some coronavirus patients (1% to 10%) contract secondary bacterial infections [2]. On the other hand, the increase in hygiene practices may, at least initially, limit the spread of different microbes, including antibiotic resistant pathogens. However, a more frequent use of biocides may favor antibiotic resistance selection in the long-term due to cross-resistance. The antimicrobial resistance crisis has been further complicated by the dearth in the development and commercialization of novel antibiotics. For instance, only 11 new antimicrobials have been approved by the U.S. Food and Drug Administration (U.S. FDA) since 2017 [3]. In this scenario, it will be essential to promote global initiatives aimed at delivering new compounds that can effectively substitute or complement the current therapeutics.
Overall, to break the vicious circle of resistance, it will be necessary to implement enhanced stewardship policies and design novel, effective antimicrobials. Also, disease risk assessment will help to identify which areas require a greater effort from the public health system to minimize the weakness of the population in the near future.
2. State-of-the-Art of New Therapies for Fighting Infectious Bacterial Diseases
The use of conventional antibiotics to treat bacterial infections should be just one strategy among many. Instead, it currently remains the only available option in most cases. Fortunately, several new approaches to fighting pathogenic bacteria are already in different stages of development. We can only hope that some of these strategies prove to be successful before bacterial infections become untreatable.
Recent developments in this field allow the design of a complete tactic for prevention and treatment of these infections. Bacterial disease prevention remains a key challenge for various reasons. For instance, vaccines are not always easy to obtain, and reducing the contact with dangerous bacteria would be akin to creating a sterile environment, which would only be possible under specific conditions. Therefore, pathogenic microorganisms and their potential ability to develop drug resistance will always be a problem. With this in mind, there is ongoing research on various strategies to prevent and/or treat infectious diseases, the most important being the following (Figure 1):
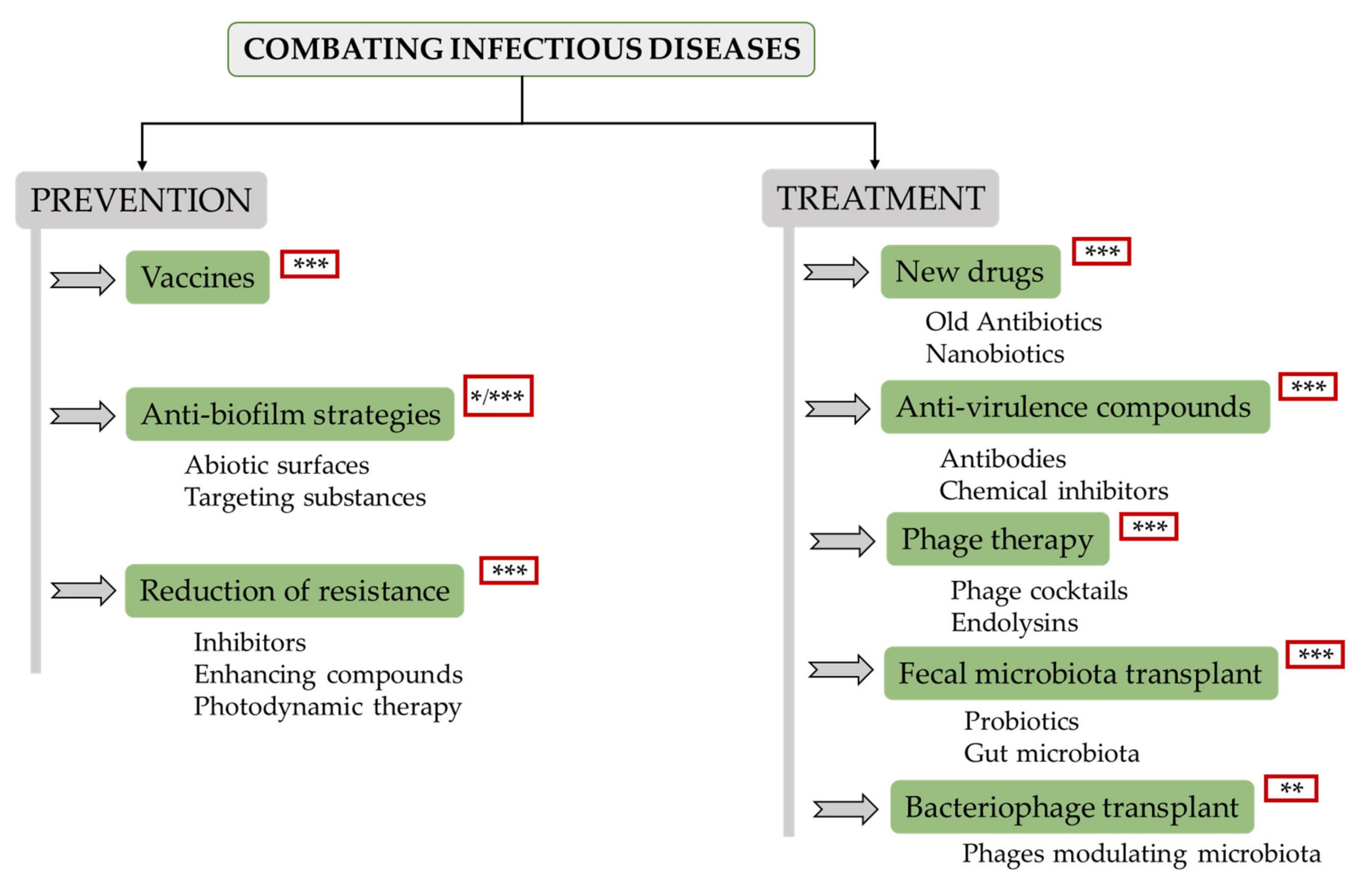
2.1. Vaccines
Vaccination remains one of the most effective systems for infectious disease prevention. Additionally, the use of vaccines also contributes to mitigating the problems derived from infections caused by antibiotic-resistant bacteria. This is even the case for vaccinations against viral infections, as they result in a reduced frequency of superinfections with bacteria. A lesser prevalence of bacterial infections ultimately leads to a decrease in antibiotic prescription, thereby diminishing the selective pressure for antibiotic resistance. At the moment, vaccine development focuses on pathogens that represent a global threat to human health, antimicrobial-resistant ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) and pathogenic bacteria from the WHO priority list [4]. As a result of this effort, several vaccines against a number of pathogens are now in the pipeline
2.2. Antibiofilm Strategies
Biofilms are involved in multiple infections on both biotic (heart valves, tissues) and abiotic (catheters or prosthetic joints) surfaces. These structures are very difficult to eradicate because they exhibit multiple properties that hinder antibiotic activity. These include the physiological state of bacteria, the presence of persister cells, and the extracellular matrix. There are two routes of action to prevent biofilm formation: the development of abiotic surfaces and/or coatings that inhibit bacterial adherence to medical devices [5], and by targeting substances required for surface colonization, including adhesins, extracellular matrix components and regulatory signals [6][7]. Interference with regulatory signals may be achieved, for instance, by applying quorum sensing (QS) inhibitors. These compounds can act by preventing synthesis of the signaling molecules or by blocking the interactions between QS molecules and their receptors.
2.3. Reduction of Antibiotic Resistance
A straight approach to treating infections caused by antibiotic resistant bacteria is the reduction of their ability to survive in the presence of antibiotics or under attack by the host’s immune system. One of these strategies is the use of compounds to specifically inhibit antibiotic resistance mechanisms, the classical example being the use of β-lactamase inhibitors in combination with a β-lactam antibiotic (e.g., amoxycillin and clavulanic acid) [8]. Similarly, it is possible to inhibit antibiotic resistance mediated by efflux pumps [9]. A second method to enhance antibiotic activity is by combining antibiotics with other substances. For instance, cyslabdan, a compound produced by Streptomyces sp. K04-0144, increases the activity of imipenem against methicillin-resistant S. aureus (MRSA) strains [10]. Finally, the use of photodynamic therapy (PDT) is an alternative strategy for destroying antibiotic resistant bacteria as effectively as susceptible cells by employing a non-toxic dye (photosensitizer) and low-intensity visible light. Illumination of the infected area in the presence of oxygen results in the production of cytotoxic molecules. In some cases, the photosensitizer increases the action of antibiotics.
2.4. New Drugs
Due to the difficulty of finding new molecules with antibiotic activity, an alternative is the use of old antibiotics, antibiotic combinations or even the application of compounds commonly used to treat other diseases [11]. The progress in high-throughput screening technologies and software development allows the identification of therapeutically interesting compounds by using in silico approaches, such as structure- (SBDD) and ligand-based drug design (LBDD), which has led to the development of several already approved therapeutics [12]. Additionally, a promising future is envisaged for nanobiotics, nanoparticles generated from a polymer-antibiotic conjugate, which releases the active drug upon hydrolysis in specific environments. For instance, some nanobiotics release their contents under acidic conditions such as those found within macrophages and granulomas [13]. Other types of nanoparticles (colloidal forms of silver, zinc, copper, titanium and vanadium) can also exert antimicrobial activity through the generation of reactive oxygen species, cell membrane permeation, triggering DNA damage or interrupting transmembrane electron transport [14].
2.5. Antivirulence Treatments
This strategy is based on the use of compounds targeting virulence factors produced by pathogenic bacteria. As a result, antivirulence compounds reduce pathogenicity and, consequently, contribute to bacterial clearance by the host’s immune system or antibiotic treatment. Common targets include toxins, adhesins, QS molecules, virulence-dedicated secretion and regulatory proteins, siderophores, and immune evasion factors. There are two main types of treatments, namely antibodies and chemical inhibitors. A nice example of toxin-directed antibodies is raxibacumab (GlaxoSmithKline), an already approved monoclonal antibody used to treat inhalational anthrax, a form of this infectious disease caused by breathing in the spores of the bacterium Bacillus anthracis [15]. Another example is the FDA- and EMA-approved human monoclonal antibody Bezlotoxumab (Merck), which blocks Clostridium difficile toxin B and prevents recurrent infections by this pathogen [16]. Similar antibodies are being assayed to target toxins secreted by other pathogens such as S. aureus. Chemical inhibitors are usually directed against toxin secretion systems to inhibit their activity [17]. ZnO nanoparticles have an inhibitory effect on QS-regulated genes controlling virulence factor secretion [18]. Monoclonal antibodies can also be directed against these targets. For instance, a monoclonal antibody (MEDI3902, Medimmune) targets the P. aeruginosa T3SS needle tip protein to prevent injection of toxins into the host cells and, at the same time, the Psl exopolysaccharide, which is involved in bacterial colonization, thus inhibiting attachment to epithelial cells [19]. The use of MEDI3902 to prevent VAP caused by P. aeruginosa is currently undergoing phase II clinical trials. A further example is the monoclonal antibody mAb 514G3, able to neutralize S. aureus protein A (SpA), whose effectiveness for the treatment of staphylococcal bloodstream infections is the focus of a phase I/II clinical study (XBiotech NCT02357966) [20]. A different strategy is to favor pathogen removal by the host immune system or improve the action of antibiotics. An example is the high-density lipoprotein (HDL) that binds to streptococcal collagen-like protein enhancing phagocytosis [21]. Other non-traditional approaches include the use of immunomodulating agents such as Reltecimod, a peptide that reduces acute inflammation and protects from superantigen toxins. This therapy is in phase III clinical trials (AtoxBio) for the treatment of patients with necrotizing soft-tissue infections in addition to the standard treatment (https://www.globenewswire.com/news-release/2020/07/10/2060386/0/en/Atox-Bio-Announces-a-Positive-Effect-of-Reltecimod-on-Resolution-of-Organ-Dysfunction-in-Phase-3-ACCUTE-Trial-for-Patients-with-Necrotizing-Soft-Tissue-Infection-Flesh-Eating-Disea.html).
2.6. Phage Therapy
Bacteriophages can theoretically be used as therapeutics against any bacterial infection. Recently, phage therapy has attracted a lot of attention due to the successful treatment of a modest number of patients under compassionate use programs by administration of personalized phage preparations [22]. So far, several publications have reported the use of phage therapy against a wide variety of infectious diseases, including gastrointestinal infections, burns, chronic venous ulcers, as well as systemic, urogenital, respiratory, otorhinolaryngeal, and osteoarticular infections [23][24]. In view of this increasing number of successful cases, several companies are putting effort and investment into the development of phage cocktails; e.g., Pherecydes Pharma is working on a therapy for osteomyelitis and prosthesis infections. Moreover, phage-derived proteins (endolysins) are also gaining attention because of their efficient bacteriolytic activity. Endolysins are being developed as a pharmaceutical product for application against human and animal pathogens (companies ContraFect, Intron Biotechnology, GangaGen and Micreos are conducting phase II clinical trials), as well as in the cosmetic industry (the GladSkin brand with Staphefekt ™ against S. aureus commercialized by Micreos). Recent clinical trials in humans (phase I) have been conducted with two endolysins: iNtRON-N-Rephasin® SAL200 (Tonabacase) (iNtRON Biotecnology, 2020) and Exebacase (CF-301) (ContraFect Corporation, 2019) against S. aureus bacteremia without observing any adverse effect. These two proteins are currently undergoing phase II and phase III trials, respectively. Although phages have been used historically in infection treatment, important challenges and scientific questions (phage specificity, pharmacokinetics, resistance development, or potential virulence transfer) should be solved before translating this therapy to routine clinical practice. Nonetheless, the future of phage therapy is promising because there are new possibilities of adapting these antimicrobials by using genetic tools such as CRISPR–Cas systems [25]. Similarly, extensive protein engineering will allow the use of endolysins against Gram-negative bacteria [26].
2.7. Fecal Microbiota Transplant
Nowadays, the importance of the microbiota in human health has been well established. In fact, a significant number of examples show a correlation between some diseases (diabetes, cardiovascular disease, mental health) and the microbiota state. Moreover, the use of probiotics and fecal microbiota transplantation have been shown to reduce the incidence and severity of some bacterial infections, especially those more difficult to eradicate or caused by antibiotic resistant bacteria, such as C. difficile [27]. Furthermore, synthetic biology allows the specific genetic engineering of probiotics [28]. Despite promising results from controlled trials, the efficacy of probiotics for disease prevention has not been fully demonstrated yet. In contrast, fecal microbiota transplants have been shown to restore the gut microbiota in patients and significantly reverse the predisposition for colonization with multiresistant pathogens [29].
2.8. Bacteriophage Transplant
Bacteriophages are in dynamic interaction with microorganisms colonizing the human gut; therefore, it is theoretically possible to use them for modulating the microbiota. This effect can be achieved by performing bacteriophage transplants. Research on this topic is still in the first stages, but experiments carried out in animal models have found that phage predation impacts on the number of susceptible bacteria and, indirectly, on other bacterial species, which has noticeable consequences for the gut metabolome [30]. Moreover, there is evidence about the persistence of bacteriophages and their impact on long-term microbial dynamics in human beings after fecal microbiota transplantation [31].
References
- Zhu, C.S.; Liu, C.Y.; Qiu, X.Y.; Xie, S.S.; Li, W.Y.; Zhu, L.; Zhu, L.Y. Novel nucleic acid detection strategies based on CRISPR-Cas systems: From construction to application. Biotechn. Bioeng. 2020, 117, 2279–2294.
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924.
- CenterWatch. FDA Approved Drugs. 2020. Available online: https://www.centerwatch.com/directories/1067-fda-approved-drugs/topic/546-bacterial-infections (accessed on 10 January 2020).
- WHO. 2017. Available online: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 15 January 2020).
- Diaz Blanco, C.; Ortner, A.; Dimitrov, R.; Navarro, A.; Mendoza, E.; Tzanov, T. Building an antifouling zwitterionic coating on urinary catheters using an enzymatically triggered bottom-up approach. ACS Appl. Mater Interfaces 2014, 6, 11385–11393.
- Murugayah, S.A.; Gerth, M.L. Engineering quorum quenching enzymes: Progress and perspectives. Biochem. Soc. Trans. 2019, 47, 793–800.
- Defraine, V.; Fauvart, M.; Michiels, J. Fighting bacterial persistence: Current and emerging anti-persister strategies and therapeutics. Drug Resist Updat. 2018, 38, 12–26.
- Bush, K.; Bradford, P.A. Interplay between β-lactamases and new β-lactamase inhibitors. Nat. Rev. Microbiol. 2019, 17, 295–306.
- Grimsey, E.M.; Fais, C.; Marshall, R.L.; Ricci, V.; Ciusa, M.L.; Stone, J.W.; Ivens, A.; Malloci, G.; Ruggerone, P.; Vargiu, A.V.; et al. Chlorpromazine and amitriptyline are substrates and inhibitors of the acrb multidrug efflux pump. MBio 2020, 11, e00465-20.
- Fukumoto, A.; Kim, Y.P.; Hanaki, H.; Shiomi, K.; Tomoda, H.; Omura, S. Cyslabdan, a new potentiator of imipenem activity against methicillin-resistant Staphylococcus aureus, produced by Streptomyces sp. K04-0144: II. Biological activities. J. Antibiot (Tokyo) 2008, 61, 7–10.
- Cheng, Y.S.; Sun, W.; Xu, M.; Shen, M.; Khraiwesh, M.; Sciotti, R.J.; Zheng, W. Repurposing screen identifies unconventional drugs with activity against multidrug resistant Acinetobacter baumannii. Front. Cell Infect. Microbiol. 2019, 8, 438.
- Talele, T.T.; Khedkar, S.A.; Rigby, A.C. Successful applications of computer aided drug discovery: Moving drugs from concept to the clinic. Curr. Top. Med. Chem. 2010, 10, 127–141.
- Batalha, I.L.; Bernut, A.; Schiebler, M.; Ouberai, M.M.; Passemar, C.; Klapholz, C.; Kinna, S.; Michel, S.; Sader, K.; Castro-Hartmann, P.; et al. Polymeric nanobiotics as a novel treatment for mycobacterial infections. J. Control. Release 2019, 314, 116–124.
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292.
- Skoura, N.; Wang-Jairaj, J.; Della Pasqua, O.; Chandrasekaran, V.; Billiard, J.; Yeakey, A.; Smith, W.; Steel, H.; Tan, L.K. Effect of raxibacumab on immunogenicity of Anthrax Vaccine Adsorbed: A phase 4, open-label, parallel-group, randomised non-inferiority study. Lancet Infect. Dis. 2020, 20, 983–991.
- Wilcox, M.H.; Gerding, D.N.; Poxton, I.R.; Kelly, C.; Nathan, R.; Birch, T.; Cornely, O.A.; Rahav, G.; Bouza, E.; Lee, C.; et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N. Engl. J. Med. 2017, 376, 305–317.
- Marshall, N.C.; Brett Finlay, B. Targeting the type III secretion system to treat bacterial infections. Expert Opin. Targets 2014, 18, 137–152.
- Saleh, M.M.; Sadeq, R.A.; Abdel Latif, H.K.; Abbas, H.A.; Askoura, M. Zinc oxide nanoparticles inhibits quorum sensing and virulence in Pseudomonas aeruginosa. Afr. Health Sci. 2019, 19, 2043–2055.
- DiGiandomenico, A.; Keller, A.E.; Gao, C.; Rainey, G.J.; Warrener, P.; Camara, M.M.; Bonnell, J.; Fleming, R.; Bezabeh, B.; Dimasi, N.; et al. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci. Transl. Med. 2014, 6, 262ra155.
- Varshney, A.K.; Kuzmicheva, G.A.; Lin, J.; Sunley, K.M.; Bowling, R.A., Jr.; Kwan, T.Y.; Mays, H.R.; Rambhadran, A.; Zhang, Y.; Martin, R.L.; et al. A natural human monoclonal antibody targeting Staphylococcus protein A protects against Staphylococcus aureus bacteremia. PLoS ONE 2018, 13, e0190537.
- Liu, L.; Zhou, L.; Li, Y.; Bai, W.; Liu, N.; Li, W.; Gao, Y.; Liu, Z.; Han, R. High-density lipoprotein acts as an opsonin to enhance phagocytosis of group A Streptococcus by U937 cells. Microbiol. Immunol. 2015, 59, 419–425.
- Pirnay, J.P.; Verbeken, G.; Ceyssens, P.J.; Huys, I.; De Vos, D.; Ameloot, C.; Fauconnier, A. The Magistral Phage. Viruses 2018, 10, 64.
- Ferry, T.; Boucher, F.; Fevre, C.; Perpoint, T.; Chateau, J.; Petitjean, C.; Josse, J.; Chidiac, C.; L’hostis, G.; Leboucher, G.; et al. Innovations for the treatment of a complex bone and joint infection due to XDR Pseudomonas aeruginosa including local application of a selected cocktail of bacteriophages. J. Antimicrob. Chemother. 2018, 73, 2901–2903.
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733.
- Hatoum-Aslan, A. Phage genetic engineering using CRISPR–Cas systems. Viruses 2018, 10, 335.
- Briers, Y.; Walmagh, M.; Van Puyenbroeck, V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliveira, H.; Azeredo, J.; Verween, G.; Pirnay, J.P.; et al. Engineered endolysin-based ‘Artilysins’ to combat multidrug-resistant gram-negative pathogens. MBio 2014, 5, e01379–14.
- Hvas, C.L.; Dahl Jørgensen, S.M.; Jørgensen, S.P.; Storgaard, M.; Lemming, L.; Hansen, M.M.; Erikstrup, C.; Dahlerup, J.F. Fecal microbiota transplantation is superior to fidaxomicin for treatment of recurrent Clostridium difficile infection. Gastroenterology 2019, 156, 1324–1332.e3.
- Ozdemir, T.; Fedorec, A.J.H.; Danino, T.; Barnes, C.P. Synthetic biology and engineered live biotherapeutics: Toward increasing system complexity. Cell Syst. 2018, 7, 5–16.
- Bar-Yoseph, H.; Carasso, S.; Shklar, S.; Korytny, A.; Even Dar, R.; Daoud, H.; Nassar, R.; Maharshak, N.; Hussein, K.; Geffen, Y.; et al. Oral capsulized fecal microbiota transplantation for eradication of carbapenemase-producing Enterobacteriaceae colonization with a metagenomic perspective. Clin. Infect. Dis. 2020, cia737.
- Hsu, B.B.; Gibson, T.E.; Yeliseyev, V.; Liu, Q.; Lyon, L.; Bry, L.; Silver, P.A.; Gerber, G.K. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host. Microbe. 2019, 25, 803–814.e5.
- Draper, L.A.; Ryan, F.J.; Smith, M.K.; Jalanka, J.; Mattila, E.; Arkkila, P.A.; Ross, R.P.; Satokari, R.; Hill, C. Long-term colonisation with donor bacteriophages following successful faecal microbial transplantation. Microbiome 2018, 6, 220.




