
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chao-Yi Wu | + 3239 word(s) | 3239 | 2021-02-08 04:55:48 | | | |
| 2 | Peter Tang | Meta information modification | 3239 | 2021-02-24 13:42:57 | | |
Video Upload Options
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease mainly involving synovial inflammation and articular bone destruction. RA is a heterogeneous disease with diverse clinical presentations, prognoses and therapeutic responses. Following the first discovery of rheumatoid factors (RFs) 80 years ago, the identification of both anti-citrullinated protein antibodies (ACPAs) and anti-carbamylated protein antibodies (anti-CarP Abs) has greatly facilitated approaches toward RA, especially in the fields of early diagnosis and prognosis prediction of the disease.
1. Introduction
Rheumatoid arthritis (RA) is a systemic inflammatory disorder that mainly involves articular inflammation. Approximately 0.5% to 1% of the population worldwide is affected by the disease, which may result in joint destruction and disability [1]. In addition, approximately 40% of patients present with extra-articular manifestations, complicating disease progression and mortality [2]. Autoantibodies isolated from patient serum and synovial fluid play critical roles in the pathogenesis of RA [3]. According to the literature, 70–80% of patients with RA are positive for autoantibodies (autoAbs), such as rheumatoid factors (RFs) and anti-citrullinated protein antibodies (ACPAs) [4]. Although they are not necessarily present in all patients, antibodies reacting with self-antigens, including immunoglobulins and posttranslational modified (PTM) protein epitopes, have been known for over 80 years to exist in RA [3]. With the advancement of our knowledge on the correlation of autoAbs and RA, these antibodies have become critical biomarkers widely utilized in clinical practice for disease prediction and diagnosis and may assist in choosing therapeutic regimens, as depicted in Figure 1.
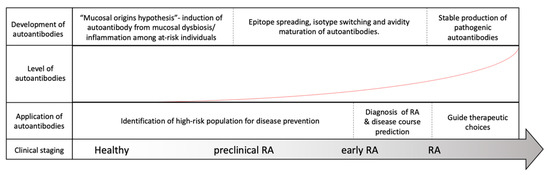
Figure 1. Evolution of rheumatoid arthritis (RA) and its correlation with RA-associated autoantibodies. Among at-risk populations, antigen exposure from the mucosal area helps form T-cells and assists in the induction of RA-associated autoantibodies many years before the onset of RA. The development of RA, however, requires further autoantibody maturation, including epitope spreading, isotype switching, avidity maturation and antibody glycosylation, before they are pathogenically transformed. As our understanding of autoantibodies advances, the application of the autoantibodies may be utilized to assist in RA diagnosis, to predict disease course, to guide treatment and possibly to prevent or delay RA development before its onset.
RFs were the first autoAb reported in RA. They were described by Waaler in 1940 as factors with hemagglutinating activity in the serum of a patient with RA [5] and named by Pike in 1949 for their associations with RA [6]. Subsequent studies have revealed that the presence of RF is associated with a more severe and erosive RA phenotype. Compellingly, utilizing an analytical ultracentrifuge technique, Kunkel and his colleagues later discovered that RF is an antibody to antigen–antibody complexes [7][8]. Moreover, scientists discovered that RF targets antigenic epitopes within the crystallizable fragment (Fc) region of immunoglobulin G (IgG) and is present in various isotypes [9]. Although the specificity of RF for RA is only approximately 60–70% and the conditions required to break tolerance to IgG are not yet fully understood [10], RF was included in the 1987 ACR classification criteria for RA [11] and considered the paradigm of autoAb clinical significance in RA.
The identification of ACPAs can be traced back to 1964, when Nienhuis and Mandema reported anti-perinuclear factors within the sera of RA patients [12]. Approximately 30 years later, Hoet and colleagues characterized them with regard to their reactivity toward citrullinated peptides [13]. Following the discovery of multiple protein candidates eligible for peptidyl arginine deiminase (PAD) citrullination, “RA citrullinome”, referring to the collection of hundreds of citrullinated proteins identified in the serum and synovial fluid of RA patients, was introduced to the field of RA research in recent decades [14][15][16][17][18]. With its superb diagnostic specificity, immunopathogenic relevance and excellent clinical correlations [19][20][21][22], ACPAs were included in the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) RA classification criteria [23]. Alongside the classical RA autoAb RF, ACPA was also considered an important hallmark of the disease in the past decade.
In recent years, growing evidence has supported that proteins resulting from PTMs, other than citrullination, are also capable of triggering autoimmune responses important for the development of RA [24]. Indeed, autoantibodies against carbamylated protein (anti-CarP Ab), acetylated proteins, malondialdehyde, malondialdehyde-acetaldehyde and other posttranslated modified epitopes, such as anti-hinge antibodies, have attracted attention due to their clinical significance and their potential use in refining RA diagnosis and care [3][11][25][26][27]. Among them, anti-CarP Ab, described in 2011 [28], is perhaps one of the best studied anti-PTM autoantibodies.
The pathogenic roles of RA-associated autoAbs have been critically reviewed by us and other researchers [22][23][24][25][26][27][28][29].
2. Characteristics of RF, ACPA and anti-CarP Ab
The function of an antibody is governed by the specific antigen it recognizes and the specific isotype determine by its crystallizable fragment (Fc) region. We first examine differences and similarities among RA-associated autoAbs for antibody characterization.
2.1. Rheumatoid Factors
Rheumatoid factors, despite their name, are not specific to RA. In fact, RFs are commonly produced during immunizations and secondary immune responses to infections to aid in pathogen removal [30][31][32]. Other rheumatic conditions, such as systemic lupus erythematosus, Sjogren’s disease and sarcoidosis, are also associated with the presence of RF [33]. Among healthy individuals, moreover, the presence of RF increases with age and exceeds 25% among elderly individuals over the age of 85 [34]. In contrast to the physiological RFs in healthy individuals, RFs isolated from RA patients have been reported to be relatively monoreactive, possessing higher affinity, harboring more somatic mutations, and utilizing more heterogeneous V-genes and more frequently become isotype switched [35]. Although a spectrum of immunoglobulins, including IgG, IgA, IgM and IgE, are all found among RFs, IgM, comprising the majority of RF isotypes in RA, is detected in 60–80% of RA patients, followed by IgA and IgG [36][37].
Interestingly, while it is generally believed that RF targets the CH2-CH3 Fc portion of IgGs [38], a recent study reported by Maibom-Thomsen et al. showed that RF does not interact with native IgGs in their soluble form when they are not bound to their corresponding antigens [39]. Through further mass spectrometry analysis, it was found that the binding of antibodies to pathogen surfaces likely induces a conformational change and exposes the RF epitopes within the Fc region (Figure 2) [39]. This novel finding provided an opportunity for a better understanding of the antibody structures and the functional physiology of antibodies in the human body.
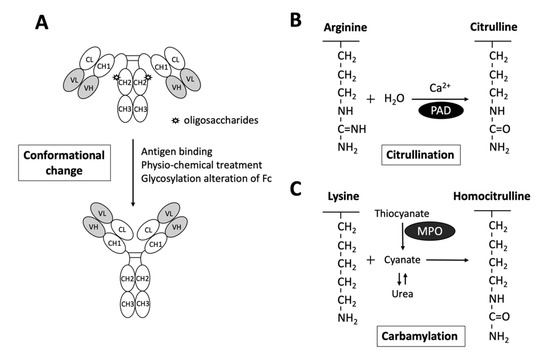
Figure 2. Schematic diagram illustrating the exposure of RF epitopes and posttranslational modification of peptide targets for autoantibody interactions in rheumatoid arthritis. (A) The epitopes of RFs reside in the Fc portion of IgG mostly in the CH2/CH3 or the CH3/CH3 groove. Recent evidence has suggested that conformational changes via altered Fc glycosylation, physicochemical treatment, antigen binding, or physical adsorption onto a hydrophobic surface promote the exposure of RF epitopes on circulating IgGs. (B) Citrullinated peptides are generated by the enzymatic activity of peptidylarginine deiminase (PAD), converting arginine to citrulline in the presence of calcium. (C) Carbamylation is the conversion of a lysine to homocitrulline upon the binding of cyanate in the form of isocyanic acid to lysine, without enzymatic catabolism. Urea and thiocyanate catabolized by myeloperoxidase (MPO) are important sources of cyanate.
2.2. Anti-Citrullinated Protein Antibodies
As depicted in Figure 2, citrullination is an enzymatic reaction mediated by PAD that converts arginine to citrulline. Keratin, fibrinogen, vimentin, fibronectin, α-enolase and 78-kDa glucose-regulated protein (GRP78) are well-known substrates for citrullination [14][15][16][17][18][40]. With the expanding collection of citrullinated proteins identified from the synovial fluid and serum of patients with RA, the term “RA citrullinome” has been introduced in recent decades [14][15][17][16][18]. However, despite its name, the spectrum of epitopes recognized by ACPAs is not limited to the “RA citrullinome” but also to peptides that undergo other PTMs, including carbamylation and acetylation [41][42]. Overlapping reactivity is commonly believed to explain the broad reactivity of ACPAs. Recently, Li and colleagues discovered that one-third of ACPAs recognize only monotargets, with limited overlapping reactivity [43]. Further evidence suggests that the majority of ACPAs in the sera of RA patients reacting to citrulline side chains have no functional role [44]. In fact, only ACPAs interacting or cross-reacting with citrulline and proximal amino acid side chains of articular proteins display arthritogenic capacity [44]. Additionally, the diversity and avidity of ACPAs toward citrullinated peptides have been noted to change and evolve over time. Kongpachith and colleagues found that somatic hypermutations accumulating during affinity maturation by clonally related B cells alter the antibody paratope to mediate “epitope spreading” and the polyreactivity of the ACPA response in patients with RA [45].
Similarly to the broad spectrum of RF isotypes, enrichment of IgA and IgG ACPA, particularly IgG1, occurs in the serum of RA patients and precedes the development of the disease [37][46][47][48]. Nevertheless, while glycosylation of the antigen-binding fragment (Fab) was estimated to be approximately 14% among global IgGs [49], more than 80% of citrulline-reactive B cells were found to contain glycosylation sites in their variable domains [50], and the variable domains of more than 90% of ACPAs indeed carried glycans [51]. This N-linked glycosylation in the Fab portion has been shown to critically predict the development of RA [52]. Glycosylation of the Fc fragment is also a unique feature of ACPAs [46][53]. Specifically, increasing core fucosylation and decreasing galactosylation and sialylation have been observed in the Fc fragment of ACPAs, directing molecular interactions and functions of ACPAs [46][53].
ACPAs, as compared to the classic RA antibody RF, have a superior diagnostic specificity and can be detected in approximately two-thirds of RA patients [19][20]. While 1–3% of healthy subjects may also test positive for ACPAs, their levels are usually in the lower range. Moreover, in comparison to the ACPAs isolated from the RA patients, those found in healthy individuals usually recognize a narrow spectrum of citrullinated antigens with altered avidities [43][54][55][56].
2.3. Anti-Carbamylated protein Antibodies
Anti-CarP Ab is another well-studied RA-related autoantibody. In contrast to citrullination, carbamylation is a nonenzymatic PTM of proteins that requires the presence of cyanate (Figure 2). Upon the binding of cyanate in the form of isocyanic acid to the ε-NH2 group side chain of lysine, homocitrulline is produced via the carbamylation reaction [57]. Nevertheless, under physiological conditions, the level of cyanate is generally too low to efficiently induce carbamylation. As urea is a source of cyanate, the level of cyanate in patients with elevated blood urea nitrogen and renal dysfunctions may be increased, promoting carbamylation [58]. Additionally, cyanate can be derived from the transformation of thiocyanate mediated by myeloperoxidase (MPO). Under chronic inflammatory conditions, MPOs may be released from neutrophils to enhance carbamylation [57].
Carbamylated alpha-1 anti-trypsin, fibrinogen, vimentin, alpha-enolase and GRP78 are some of the targets of anti-CarP Ab identified in the sera of patients with RA [57][59][60][61][62]. The avidity of ACPAs is considered low, and the even lower avidity of anti-CarP Abs suggests that despite proper class switching, no or little avidity maturation occurs at the time of the latter’s production [63][64]. Notably, anti-CarP Ab is found in nearly 45% of early RA patients positive for ACPAs, possibly because of close similarity between citrulline and homocitrulline [28][65][66]. Regardless, a large proportion of ACPA and anti-CarP Abs that interact only with citrullinated or carbamylated proteins can also be found alongside those that cross-react with one another in the serum of double-positive RA patients [42]. Through the investigation of a small RA cohort, Shi et al. reported a median percentage of noncross-reactive anti-CarP Ab as high as 70% [42]. Nonetheless, MC Lu discovered that while there was a positive correlation of ACPA with anti-GRP78 antibody in patients with RA. Similar correlation was not seen with anti-CarP GRP78 antibody [62]. Taken together, these findings suggest that anti-CarP Abs and ACPAs are not the same.
Specifically, the sensitivity of anti-CarP Ab is 18–26% and 27–46% prior to and after RA diagnosis, respectively, and its specificity is approximately 90% and above in RA [67]. Approximately 8–16% of ACPA-negative patients test positive for anti-CarP Ab [28][65]. Although anti-CarP Abs can also be found in healthy individuals and in other rheumatic conditions [68][69][70][71][72], the prevalence of anti-CarP Ab in RA patients is relatively high [71][73][74]. Similar to that of ACPA, isotypes of anti-CarP Ab display a broad spectrum. IgA, IgM and IgGs, including IgG1, IgG2, IgG3 and IgG4 anti-CarP Ab have all been discovered in patients with RA [73]. Specifically, anti-CarP IgG comprises all anti-CarP IgG subclasses, and IgA can be found in nearly 45% of RA patients, whereas anti-CarP IgM was detected in only 16% [73].
3. Risk Factors for the Production of RF, ACPA and Anti-CarP Ab
3.1. Genetic Risk Factors
Genetic background is an important factor influencing the development of RA. There is an estimated 40–50% familial risk among seropositive RA patients with strong risk noted in first-degree relatives [75]. In addition to the high correlation for RF reported among identical twins with RA [76], a number of studies have identified differences in genetic components between RA patients positive or negative for RF [77][78]. However, controversies were noted, as some studies demonstrated that RA patients, regardless of the presence of RF, may harbor similar human leukocyte antigen (HLA) susceptibility alleles [79][80].
A conserved amino acid sequence at positions 70 and 74 within the HLA-DRB1 molecule, namely, the “shared epitope (SE)”, is a leading genetic risk factor for ACPA-positive RA [81]. Furthermore, a dose effect of SE alleles on risk of RA among ACPA-positive RA patients was documented by Huizinga and others [82]. Utilizing paired samples of presymptomatic individuals, Kissel and others recently discovered that SE alleles are associated with ACPA Fab glycosylation in the predisease phase [83]. In addition to SE, the protein tyrosine phosphatase nonreceptor type 22 risk allele displays a synergistic action along with the SE alleles [84]. Furthermore, single-nucleotide polymorphisms and long noncoding RNAs are associated with the presence of ACPAs in RA patients [85][86]. Tumor necrosis factor (TNF) receptor-associated factor 1 C5 region, TNF-α-induced protein, CD40, C-C motif chemokine ligand 2, antisense noncoding RNA in the INK4 locus, and peptidyl arginine-deiminase 4 are some of the well-documented genetic factors linked to ACPA-seropositive RA [81].
In contrast, the genetic risk for anti-CarP Ab is less known. While HLA-DR3 is found more commonly in individuals with ACPA-negative RA than in the healthy population [87], HLA-DR3 is also associated with RA cases positive for anti-CarP Ab and negative for ACPA [88].
3.2. Environmental Risk Factors
3.2.1. Tobacco Smoking
The significance of tobacco smoking as a risk factor for RA has been well accepted [89][90]. Interestingly, although smoking has been associated with all seropositive RA [54], it is known to be associated with the presence of RF, even in the absence of RA [91]. Large population studies and twin studies have described the association between smoking and ACPA positivity [92], with a dominant risk being in individuals positive for SE [92][93]. Aside from the inflammatory changes in the lungs subsequently causing activation of PADs [94], in the setting of SE, smoking confers a risk of high ACPA levels and suggests a distinct mechanism of ACPA production different from that of RF [91][95].
Moreover, smoking is suspected to promote carbamylation in patients with RA. While smoking has been demonstrated to promote MPO-mediated conversion of thiocyanate to cyanate [96], the level of anti-CarP Ab, however, was not significantly higher [68]. Perhaps critical factors (genetic or environmental exposures) other than carbamylation alone are required for the induction of anti-CarP Ab.
3.2.2. Microbial Triggers
As RF is physiologically important to enhance immune complex clearance, to assist B cell uptake for antigen presentation and to facilitate complement fixation, an increased level of RF has been detected in individuals with chronic or indolent infection, including hepatitis B or C virus infection or infective endocarditis [97][98]. Nonetheless, the production of RF under such conditions typically ceases following resolution of the infection. Due to the action of bacterial pore-forming virulence and calcium ionophores in triggering calcium influx and generating nontolerized neocitrullinated epitopes [99][100][101], the role of periodontitis-causing bacteria and intestinal microbiota in ACPA-associated RA was recently summarized [102][103][104][105][106]. Although infection is likely to trigger the release of MPO from activated neutrophils during infection episodes, no direct evidence has linked acute infection to anti-CarP Ab-positive RA, and the risk of infection in autoantibody-mediated RA is still under investigation.
4. Predictive Values of RF, ACPA and Anti-CarP Ab in the Diagnosis of Presymptomatic RA Patients
Although not necessarily required for the development of RA, autoAbs can readily be found many years before the onset of symptomatic disease in the evolution model of RA [107][108][109]. During the presymptomatic phase of the disease, B cells undergo a series of selection, expansion, epitope spreading and antibody maturation processes [22][29], providing physicians an opportunity for early detection and prevention of the disease.
4.1. Rheumatoid Factor
The detection of RFs in the form of IgM, IgA, and IgG was found to predate the onset of RA by years across patients of various ethnicities [110][111][112]. Interestingly, their appearance in serum is sequential before the diagnosis of RA: IgM RF first, followed by IgA RF, and finally IgG RF [112][113]. In a Danish cohort study, healthy individuals with elevated RF levels had up to 26-fold higher long-term risk of RA and up to 32% 10-year absolute risk of developing RA [110]. Moreover, the positive predictive value (PPV) for RF ranges from 36–97%, with most values falling between 70% and 80%; the negative predictive value (NPV) is 69–95% [114].
4.2. Anti-Citrullinated Protein Antibodies
Similar to RF, ACPAs can be detected in serum samples up to 14 years before the onset of articular symptoms of RA and precede the presence of IgM RF by up to a decade [115]. Careful investigation of pre-RA blood donors and blood samples collected from patients before their diagnosis suggested that an increase in the level of ACAP can generally be found 1–3 years prior to the onset of symptoms [115][116]. In addition to the occurrence of ACPAs, the relevance of antibody levels and characteristics have been discussed in the prediction of RA development [117][118][119][120]. For example, while the PPV for the development of RA within a period of 2–6 years ranges from 30% to 70%, the best PPVs were those with higher antibody levels or those positive for both ACPA and RF [120][121]. In a study analyzing 260 IgM-RF-negative patients with early arthritis, the PPV of a positive ACPA test was 91.7% after a one-year follow-up [122].
In 2010, Van der Woude et al. discovered an excess amount of citrullinated epitopes recognizable by ACPA in the sera of pre-RA patients [123], reporting that the ACPAs isolated from those who were later confirmed to have RA recognized considerably more citrullinated targets [123]. Utilizing citrullinated peptide tetramers, a similar phenomenon was also reported recently by Kongpachith and colleagues [45]. Leaving a trail during the process of antibody maturation, the amount of ACPA epitope spreading was increased in the presymptomatic phase, predicting progression to RA [124][125]. Moreover, N-linked glycosylation in the Fab segment of ACPA is known to be critical in predicting RA development [52]. By studying a subset of first-degree relatives of indigenous North American RA patients, Hafkenscheid et al. discovered that extensive glycosylation of the IgG ACPA V domain predisposed individuals to the development of RA [52]. Altered glycosylation in the Fc portion as well as decreasing galactosylation and increasing fucosylation of serum ACPA IgG1 are also reported to precede the onset of RA [46].
4.3. Anti-Carbamylated Protein Antibodies
Similarly, anti-CarP Ab, which can be detected before the onset of RA, is also applicable in the setting of preclinical screening [25][126][127][128][129]. In fact, anti-CarP Ab can be detected more than a decade prior to the onset of RA, at approximately the same time as ACPA and before IgM RF can be detected [126][130]. Specifically, in comparison with those targeting carbamylated fibrinogen, the level of anti-CarP Ab screening against carbamylated fetal calf serum is more associated with future RA diagnosis according to Brink’s observation [129], who reported sensitivities of 13.9% and 42.2% for anti-CarP Ab in presymptomatic individuals and after the development of RA, respectively. Moreover, Pecani et al. recently reported a PPV of 88% and an NPV of 60% for anti-CarP Ab in RA patients [131].
4.4. Additive Values of the RF, ACPA and Anti-CarP Ab
Additive values of RF and ACPA in predicting the development of RA have been reported by Dahlqvist et al. and have been reproducibly confirmed [114][132]. In comparison to controls, the specificity for future development of classifiable RA with the combination of both ACPA and RF of any isotype can reach as high as 99% [112].
References
- Lacis, A.A.; Schmidt, G.A.; Rind, D.; Ruedy, R.A. Atmospheric CO2: Principal Control Knob Governing Earth’s Temperature. Science 2010, 330, 356–359.
- Huang, J.; Yu, H.; Dai, A.; Wei, Y.; Kang, L. Drylands face potential threat under 2 °C global warming target. Nat. Clim. Chang. 2017, 7, 417–422.
- Rogelj, J.; Den Elzen, M.; Höhne, N.; Fransen, T.; Fekete, H.; Winkler, H.; Schaeffer, R.; Sha, F.; Riahi, K.; Meinshausen, M. Paris Agreement climate proposals need a boost to keep warming well below 2 °C. Nature 2016, 534, 631–639.
- Heede, R. Tracing anthropogenic carbon dioxide and methane emissions to fossil fuel and cement producers, 1854-2010. Clim. Chang. 2014, 122, 229–241.
- Liu, Z.; Feng, K.; Davis, S.J.; Guan, D.; Chen, B.; Hubacek, K.; Yan, J. Understanding the energy consumption and greenhouse gas emissions and the implication for achieving climate change mitigation targets. Appl. Energy 2016, 184, 741–747.
- Anderson, K.; Peters, G. The trouble with negative emissions. Science 2016, 354, 182–183.
- Souza, G.M.; Ballester, M.V.R.; de Brito Cruz, C.H.; Chum, H.; Dale, B.; Dale, V.H.; Fernandes, E.C.M.; Foust, T.; Karp, A.; Lynd, L.; et al. The role of bioenergy in a climate-changing world. Environ. Dev. 2017, 23, 57–64.
- Fajardy, M.; Mac Dowell, N. Can BECCS deliver sustainable and resource efficient negative emissions? Energy Environ. Sci. 2017, 10, 1389–1426.
- Laibach, N.; Börner, J.; Bröring, S. Exploring the future of the bioeconomy: An expert-based scoping study examining key enabling technology fields with potential to foster the transition toward a bio-based economy. Technol. Soc. 2019, 58, 101118.
- Vandermeulen, V.; der Steen, M.; Stevens, C.V.; Van Huylenbroeck, G. Industry expectations regarding the transition toward a biobased economy. Biofuels Bioprod. Biorefining 2012, 6, 453–464.
- Zanchi, G.; Pena, N.; Bird, N. Is woody bioenergy carbon neutral? A comparative assessment of emissions from consumption of woody bioenergy and fossil fuel. GCB Bioenergy 2012, 4, 761–772.
- Dhillon, R.S.; von Wuehlisch, G. Mitigation of global warming through renewable biomass. Biomass Bioenergy 2013, 48, 75–89.
- Saba, N.; Jawaid, M.; Sultan, M.T.H.; Alothman, O.Y. Green Biocomposites for Structural Applications. In Green Biocomposites: Design and Applications; Springer: Berlin, Germany, 2017; ISBN 9783319493817.
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46.
- Lal, R. Crop residues as soil amendments and feedstock for bioethanol production. Waste Manag. 2008, 28, 747–758.
- Scarlat, N.; Martinov, M.; Dallemand, J.F. Assessment of the availability of agricultural crop residues in the European Union: Potential and limitations for bioenergy use. Waste Manag. 2010, 30, 1889–1897.
- Mitchell, R.B.; Schmer, M.R.; Anderson, W.F.; Jin, V.; Balkcom, K.S.; Kiniry, J.; Coffin, A.; White, P. Dedicated Energy Crops and Crop Residues for Bioenergy Feedstocks in the Central and Eastern USA. Bioenergy Res. 2016, 9, 384–398.
- Van der Weijde, T.; Alvim Kamei, C.L.; Torres, A.F.; Vermerris, W.; Dolstra, O.; Visser, R.G.F.; Trindade, L.M. The potential of C4 grasses for cellulosic biofuel production. Front. Plant Sci. 2013, 4, 1–18.
- Masters, M.D.; Black, C.K.; Kantola, I.B.; Woli, K.P.; Voigt, T.; David, M.B.; DeLucia, E.H. Soil nutrient removal by four potential bioenergy crops: Zea mays, Panicum virgatum, Miscanthus × giganteus, and prairie. Agric. Ecosyst. Environ. 2016, 216, 51–60.
- Tilman, D.; Socolow, R.; Foley, J.A.; Hill, J.; Larson, E.; Lynd, L.; Pacala, S.; Reilly, J.; Searchinger, T.; Somerville, C.; et al. Beneficial biofuels-The food, energy, and environment trilemma. Science 2009, 325, 270–271.
- Sims, R.E.H.; Mabee, W.; Saddler, J.N.; Taylor, M. An overview of second generation biofuel technologies. Bioresour. Technol. 2010, 101, 1570–1580.
- Pancaldi, F.; Trindade, L.M. Marginal Lands to Grow Novel Bio-Based Crops: A Plant Breeding Perspective. Front. Plant Sci. 2020, 11, 227.
- Field, C.B.; Campbell, J.E.; Lobell, D.B. Biomass energy: The scale of the potential resource. Trends Ecol. Evol. 2008, 23, 65–72.
- Searchinger, T.; Heimlich, R.; Houghton, R.A.; Dong, F.; Elobeid, A.; Fabiosa, J.; Tokgoz, S.; Hayes, D.; Yu, T.-H. Use of U.S. Croplands for Biofuels Increases Greenhouse Gases Through Emissions from Land-Use Change. Science 2008, 319, 1238–1240.
- Zeri, M.; Anderson-Teixeira, K.; Hickman, G.; Masters, M.; DeLucia, E.; Bernacchi, C.J. Carbon exchange by establishing biofuel crops in Central Illinois. Agric. Ecosyst. Environ. 2011, 144, 319–329.
- Qin, Z.; Zhuang, Q.; Zhu, X. Carbon and nitrogen dynamics in bioenergy ecosystems: 2. Potential greenhouse gas emissions and global warming intensity in the conterminous United States. GCB Bioenergy 2015, 7, 25–39.
- Fanous, J.; Moomaw, W.R. A Critical Look at Forest Bioenergy: Exposing a high carbon “climate solution”. GDAE Clim. Policy Br. 2018, 1–8.
- Norton, M.; Baldi, A.; Buda, V.; Carli, B.; Cudlin, P.; Jones, M.B.; Korhola, A.; Michalski, R.; Novo, F.; Oszlányi, J.; et al. Serious mismatches continue between science and policy in forest bioenergy. GCB Bioenergy 2019, 1256–1263.
- Zhuang, Q.; Qin, Z.; Chen, M. Biofuel, land and water: Maize, switchgrass or Miscanthus? Environ. Res. Lett. 2013, 8.
- Clifton-Brown, J.; Chiang, Y.-C.; Hodkinson, T. Miscanthus: Genetic Resources and Breeding Potential to Enhance Bioenergy Production. In Genetic Improvement of Bioenergy Crops; Springer: Berlin, Germany, 2008; pp. 273–294. ISBN 978-0-387-70804-1.
- Hodkinson, T.R.; Klaas, M.; Jones, M.B.; Prickett, R.; Barth, S. Miscanthus: A case study for the utilization of natural genetic variation. Plant Genet. Resour. Characterisation Util. 2015, 13, 219–237.
- Hodkinson, T.R.; Chase, M.W.; Lledó, M.D.; Salamin, N.; Renvoize, S.A. Phylogenetics of Miscanthus, Saccharum and related genera (Saccharinae, Andropogoneae, Poaceae) based on DNA sequences from ITS nuclear ribosomal DNA and plastid trnL intron and trnL-F intergenic spacers. J. Plant Res. 2002, 115, 381–392.
- Clark, L.V.; Brummer, J.E.; Głowacka, K.; Hall, M.C.; Heo, K.; Peng, J.; Yamada, T.; Yoo, J.H.; Yu, C.Y.; Zhao, H.; et al. A footprint of past climate change on the diversity and population structure of Miscanthus sinensis. Ann. Bot. 2014, 114, 97–107.
- Lewandowski, I.; Clifton-Brown, J.C.; Scurlock, J.M.O.; Huisman, W. Miscanthus: European experience with a novel energy crop. Biomass Bioenergy 2000, 19, 209–227.
- Beale, C.V.; Long, S.P. Can perennial C4 grasses attain high efficiencies of radiant energy conversion in cool climates? Plant. Cell Environ. 1995, 18, 641–650.
- Naidu, S.L.; Moose, S.P.; AL-Shoaibi, A.K.; Raines, C.A.; Long, S.P. Cold Tolerance of C 4 photosynthesis in Miscanthus × giganteus: Adaptation in Amounts and Sequence of C 4 Photosynthetic Enzymes. Plant Physiol. 2003, 132, 1688–1697.
- Hamilton, S.K.; Hussain, M.Z.; Bhardwaj, A.K.; Basso, B.; Robertson, G.P. Comparative water use by maize, perennial crops, restored prairie, and poplar trees in the US Midwest. Environ. Res. Lett. 2015, 10, 64015.
- Kørup, K.; Lærke, P.E.; Baadsgaard, H.; Andersen, M.N.; Kristensen, K.; Münnich, C.; Didion, T.; Jensen, E.S.; Mårtensson, L.M.; Jørgensen, U. Biomass production and water use efficiency in perennial grasses during and after drought stress. GCB Bioenergy 2018, 10, 12–27.
- Oliveira, J.A.; West, C.P.; Afif, E.; Palencia, P. Comparison of miscanthus and switchgrass cultivars for biomass yield, soil nutrients, and nutrient removal in northwest Spain. Agron. J. 2017, 109, 122–130.
- Chung, J.-H.; Kim, D.-S. Miscanthus as a potential bioenergy crop in East Asia. J. Crop Sci. Biotechnol. 2012, 15, 65–77.
- Clifton-Brown, J.C.; Lewandowski, I.; Andersson, B.; Basch, G.; Christian, D.G.; Kjeldsen, J.B.; Jørgensen, U.; Mortensen, J.V.; Riche, A.B.; Schwarz, K.U.; et al. Performance of 15 Miscanthus genotypes at five sites in Europe. Agron. J. 2001, 93, 1013–1019.
- Jeżowski, S.; Mos, M.; Buckby, S.; Cerazy-Waliszewska, J.; Owczarzak, W.; Mocek, A.; Kaczmarek, Z.; McCalmont, J.P. Establishment, growth, and yield potential of the perennial grass Miscanthus × Giganteus on degraded coal mine soils. Front. Plant Sci. 2017, 8, 1–8.
- Yost, M.A.; Randall, B.K.; Kitchen, N.R.; Heaton, E.A.; Myers, R.L. Yield potential and nitrogen requirements of miscanthus × giganteus on eroded soil. Agron. J. 2017, 109, 684–695.
- Amaducci, S.; Facciotto, G.; Bergante, S.; Perego, A.; Serra, P.; Ferrarini, A.; Chimento, C. Biomass production and energy balance of herbaceous and woody crops on marginal soils in the Po Valley. GCB Bioenergy 2017, 9, 31–45.
- Barbosa, B.; Boléo, S.; Sidella, S.; Costa, J.; Duarte, M.P.; Mendes, B.; Cosentino, S.L.; Fernando, A.L. Phytoremediation of Heavy Metal-Contaminated Soils Using the Perennial Energy Crops Miscanthus spp. and Arundo donax L. Bioenergy Res. 2015, 8, 1500–1511.
- Pidlisnyuk, V.; Stefanovska, T.; Lewis, E.E.; Erickson, L.E.; Davis, L.C. Miscanthus as a Productive Biofuel Crop for Phytoremediation. CRC. Crit. Rev. Plant Sci. 2014, 33, 1–19.
- Nakajima, T.; Yamada, T.; Anzoua, K.G.; Kokubo, R.; Noborio, K. Carbon sequestration and yield performances of Miscanthus × giganteus and Miscanthus sinensis. Carbon Manag. 2018, 9, 415–423.
- Robertson, A.D.; Whitaker, J.; Morrison, R.; Davies, C.A.; Smith, P.; McNamara, N.P. A Miscanthus plantation can be carbon neutral without increasing soil carbon stocks. GCB Bioenergy 2017, 9, 645–661.
- Xue, S.; Kalinina, O.; Lewandowski, I. Present and future options for Miscanthus propagation and establishment. Renew. Sustain. Energy Rev. 2015, 49, 1233–1246.
- Beccari, G.; Covarelli, L.; Balmas, V.; Tosi, L. First report of Miscanthus giganteus rhizome rot caused by Fusarium avenaceum, Fusarium oxysporum and Mucor hiemalis. Australas. Plant Dis. Notes 2010, 5, 28–29.
- Falter, C.; Voigt, C.A. Comparative Cellular Analysis of Pathogenic Fungi with a Disease Incidence in Brachypodium distachyon and Miscanthus x giganteus. Bioenergy Res. 2014, 7, 958–973.
- Scauflaire, J.; Gourgue, M.; Foucart, G.; Renard, F.; Vandeputte, F.; Munaut, F. Fusarium miscanthi and other Fusarium species as causal agents of Miscanthus × giganteus rhizome rot. Eur. J. Plant Pathol. 2013, 137, 1–3.
- Mekete, T.; Reynolds, K.; Lopez-Nicora, H.D.; Gray, M.E.; Niblack, T.L. Plant-parasitic nematodes are potential pathogens of Miscanthus × giganteus and Panicum virgatum used for biofuels. Plant Dis. 2011, 95, 413–418.
- Clifton-Brown, J.C.; Lewandowski, I. Overwintering problems of newly established Miscanthus plantations can be overcome by identifying genotypes with improved rhizome cold tolerance. New Phytol. 2000, 148, 287–294.
- Kucharik, C.J.; VanLoocke, A.; Lenters, J.D.; Motew, M.M. Miscanthus Establishment and Overwintering in the Midwest USA: A Regional Modeling Study of Crop Residue Management on Critical Minimum Soil Temperatures. PLoS ONE 2013, 8.
- De Melo Peixoto, M.; Friesen, P.C.; Sage, R.F. Winter cold-tolerance thresholds in field-grown Miscanthus hybrid rhizomes. J. Exp. Bot. 2015, 66, 4415–4425.
- Van der Weijde, T.; Dolstra, O.; Visser, R.G.F.; Trindade, L.M. Stability of Cell Wall Composition and Saccharification Efficiency in Miscanthus across Diverse Environments. Front. Plant Sci. 2017, 7, 1–14.
- Hodgson, E.M.; Fahmi, R.; Yates, N.; Barraclough, T.; Shield, I.; Allison, G.; Bridgwater, A.V.; Donnison, I.S. Miscanthus as a feedstock for fast-pyrolysis: Does agronomic treatment affect quality? Bioresour. Technol. 2010, 101, 6185–6191.
- Lewandowski, I.; Kicherer, A. Combustion quality of biomass: Practical relevance and experiments to modify the biomass quality of Miscanthus x giganteus. Eur. J. Agron. 1997, 6, 163–177.
- Moll, L.; Wever, C.; Völkering, G.; Pude, R. Increase of Miscanthus cultivation with new roles in materials production—A review. Agronomy 2020, 10, 308.
- Van der Weijde, T.; Kiesel, A.; Iqbal, Y.; Muylle, H.; Dolstra, O.; Visser, R.G.F.; Lewandowski, I.; Trindade, L.M. Evaluation of Miscanthus sinensis biomass quality as feedstock for conversion into different bioenergy products. GCB Bioenergy 2017, 9, 176–190.
- Kiesel, A.; Wagner, M.; Lewandowski, I. Environmental performance of miscanthus, switchgrass and maize: Can C4 perennials increase the sustainability of biogas production? Sustainability 2017, 9, 5.
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid hydrolysis of lignocellulosic biomass: Sugars and furfurals formation. Catalysts 2020, 10, 437.
- Cudjoe, E.; Hunsen, M.; Xue, Z.; Way, A.E.; Barrios, E.; Olson, R.A.; Hore, M.J.A.; Rowan, S.J. Miscanthus Giganteus: A commercially viable sustainable source of cellulose nanocrystals. Carbohydr. Polym. 2017, 155, 230–241.
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344.
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215.
- Wang, K.T.; Jing, C.; Wood, C.; Nagardeolekar, A.; Kohan, N.; Dongre, P.; Amidon, T.E.; Bujanovic, B.M. Toward complete utilization of miscanthus in a hot-water extraction-based biorefinery. Energies 2018, 11, 39.
- Messaoudi, Y.; Smichi, N.; Bouachir, F.; Gargouri, M. Fractionation and Biotransformation of Lignocelluloses-Based Wastes for Bioethanol, Xylose and Vanillin Production. Waste Biomass Valorization 2019, 10, 357–367.
- Tu, W.C.; Hallett, J.P. Recent advances in the pretreatment of lignocellulosic biomass. Curr. Opin. Green Sustain. Chem. 2019, 20, 11–17.
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467.
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Lignocellulosic Biorefineries in Europe: Current State and Prospects. Trends Biotechnol. 2019, 37, 231–234.
- Adney, W.S.; Rivard, C.J.; Shiang, M.; Himmel, M.E. Anaerobic digestion of lignocellulosic biomass and wastes - Cellulases and related enzymes. Appl. Biochem. Biotechnol. 1991, 30, 165–183.
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476.
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53.
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353.
- Lewandowski, I.; Clifton-Brown, J.; Trindade, L.M.; Van Der Linden, G.C.; Schwarz, K.U.; Müller-Sämann, K.; Anisimov, A.; Chen, C.L.; Dolstra, O.; Donnison, I.S.; et al. Progress on optimizing miscanthus biomass production for the european bioeconomy: Results of the EU FP7 project OPTIMISC. Front. Plant Sci. 2016, 7, 1–23.
- Clifton-Brown, J.; Harfouche, A.; Casler, M.D.; Dylan Jones, H.; Macalpine, W.J.; Murphy-Bokern, D.; Smart, L.B.; Adler, A.; Ashman, C.; Awty-Carroll, D.; et al. Breeding progress and preparedness for mass-scale deployment of perennial lignocellulosic biomass crops switchgrass, miscanthus, willow and poplar. GCB Bioenergy 2019, 11, 118–151.
- Clifton-Brown, J.; Schwarz, K.U.; Awty-Carroll, D.; Iurato, A.; Meyer, H.; Greef, J.; Gwyn, J.; Mos, M.; Ashman, C.; Hayes, C.; et al. Breeding strategies to improve Miscanthus as a sustainable source of biomass for bioenergy and biorenewable products. Agronomy 2019, 9, 673.
- Jiang, J.; Guan, Y.; McCormick, S.; Juvik, J.; Lubberstedt, T.; Fei, S.Z. Gametophytic self-incompatibility is operative in Miscanthus sinensis (poaceae) and is affected by pistil age. Crop Sci. 2017, 57, 1948–1956.
- Sacks, E.J.; Juvik, J.A.; Lin, Q.; Stewart, J.R.; Yamada, T. The Gene Pool of Miscanthus Species and Its Improvement. In Genomics of the Saccharinae; Paterson, A.H., Ed.; Springer: New York, NY, USA, 2013; pp. 73–101. ISBN 978-1-4419-5947-8.
- Trindade, L.M.; Dolstra, O.; Van Loo, E.N.; Visser, R.G.F. Plant breeding and its role in a biobased economy. In The Biobased Economy: Biofuels, Materials and Chemicals in the Post-oil Era; Earthscan Ltd.: London, UK, 2010; pp. 67–82.
- Dong, H.; Green, S.V.; Nishiwaki, A.; Yamada, T.; Stewart, J.R.; Deuter, M.; Sacks, E.J. Winter hardiness of Miscanthus (I): Overwintering ability and yield of new Miscanthus ×giganteus genotypes in Illinois and Arkansas. GCB Bioenergy 2019, 11, 691–705.
- Clark, L.V.; Dwiyanti, M.S.; Anzoua, K.G.; Brummer, J.E.; Ghimire, B.K.; Głowacka, K.; Hall, M.; Heo, K.; Jin, X.; Lipka, A.E.; et al. Biomass yield in a genetically diverse Miscanthus sinensis germplasm panel evaluated at five locations revealed individuals with exceptional potential. GCB Bioenergy 2019, 1125–1145.
- Atienza, S.G.; Satovic, Z.; Petersen, K.K.; Dolstra, O.; Martin, A. Influencing combustion quality in Miscanthus sinensis Anderss.: Identification of QTLs for calcium, phosphorus and sulphur content. Plant Breed. 2003, 122, 141–145.
- Gifford, J.M.; Chae, W.B.; Swaminathan, K.; Moose, S.P.; Juvik, J.A. Mapping the genome of Miscanthus sinensis for QTL associated with biomass productivity. GCB Bioenergy 2015, 7, 797–810.
- Van der Weijde, T.; Kamei, C.L.A.; Severing, E.I.; Torres, A.F.; Gomez, L.D.; Dolstra, O.; Maliepaard, C.A.; McQueen-Mason, S.J.; Visser, R.G.F.; Trindade, L.M. Genetic complexity of miscanthus cell wall composition and biomass quality for biofuels. BMC Genom. 2017, 18, 1–15.
- Dong, H.; Liu, S.; Clark, L.V.; Sharma, S.; Gifford, J.M.; Juvik, J.A.; Lipka, A.E.; Sacks, E.J. Genetic mapping of biomass yield in three interconnected Miscanthus populations. GCB Bioenergy 2018, 10, 165–185.
- Slavov, G.T.; Nipper, R.; Robson, P.; Farrar, K.; Allison, G.G.; Bosch, M.; Clifton-Brown, J.C.; Donnison, I.S.; Jensen, E. Genome-wide association studies and prediction of 17 traits related to phenology, biomass and cell wall composition in the energy grass Miscanthus sinensis. New Phytol. 2014, 201, 1227–1239.
- Clark, L.V.; Dwiyanti, M.S.; Anzoua, K.G.; Brummer, J.E.; Ghimire, B.K.; Głowacka, K.; Hall, M.; Heo, K.; Jin, X.; Lipka, A.E.; et al. Genome-wide association and genomic prediction for biomass yield in a genetically diverse Miscanthus sinensis germplasm panel phenotyped at five locations in Asia and North America. GCB Bioenergy 2019, 988–1007.
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809.
- Chadwick, D.T.; McDonnell, K.P.; Brennan, L.P.; Fagan, C.C.; Everard, C.D. Evaluation of infrared techniques for the assessment of biomass and biofuel quality parameters and conversion technology processes: A review. Renew. Sustain. Energy Rev. 2014, 30, 672–681.
- Vogel, J. Unique aspects of the grass cell wall. Curr. Opin. Plant Biol. 2008, 11, 301–307.
- Zhong, R.; Cui, D.; Ye, Z.H. Secondary cell wall biosynthesis. New Phytol. 2019, 221, 1703–1723.
- Zhong, R.; Ye, Z.H. Secondary cell walls: Biosynthesis, patterned deposition and transcriptional regulation. Plant Cell Physiol. 2015, 56, 195–214.
- Li, Z.; Fernie, A.R.; Persson, S. Transition of primary to secondary cell wall synthesis. Sci. Bull. 2016, 61, 838–846.
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376.
- Van der Weijde, T.; Torres, A.F.; Dolstra, O.; Dechesne, A.; Visser, R.G.F.; Trindade, L.M. Impact of Different Lignin Fractions on Saccharification Efficiency in Diverse Species of the Bioenergy Crop Miscanthus. Bioenergy Res. 2016, 9, 146–156.
- Allison, G.G.; Morris, C.; Clifton-Brown, J.; Lister, S.J.; Donnison, I.S. Genotypic variation in cell wall composition in a diverse set of 244 accessions of Miscanthus. Biomass Bioenergy 2011, 35, 4740–4747.
- Battaglia, M.; Fike, J.; Fike, W.; Sadeghpour, A.; Diatta, A. Miscanthus ×giganteus biomass yield and quality in the Virginia Piedmont. Grassl. Sci. 2019, 1–8.
- Da Costa, R.M.F.; Lee, S.J.; Allison, G.G.; Hazen, S.P.; Winters, A.; Bosch, M. Genotype, development and tissue-derived variation of cell-wall properties in the lignocellulosic energy crop Miscanthus. Ann. Bot. 2014, 114, 1265–1277.
- Qin, J.; Yang, Y.; Jiang, J.; Yi, Z.; Xiao, L.; Ai, X.; Chen, Z. Comparison of lignocellulose composition in four major species of Miscanthus. Afr. J. Biotechnol. 2012, 11, 12529–12537.
- Adams, J.M.M.; Winters, A.L.; Hodgson, E.M.; Gallagher, J.A. What cell wall components are the best indicators for Miscanthus digestibility and conversion to ethanol following variable pretreatments? Biotechnol. Biofuels 2018, 11, 1–14.
- De Souza, A.P.; Kamei, C.L.A.; Torres, A.F.; Pattathil, S.; Hahn, M.G.; Trindade, L.M.; Buckeridge, M.S. How cell wall complexity influences saccharification efficiency in Miscanthus sinensis. J. Exp. Bot. 2015, 66, 4351–4365.
- Da Costa, R.M.F.; Pattathil, S.; Avci, U.; Lee, S.J.; Hazen, S.P.; Winters, A.; Hahn, M.G.; Bosch, M. A cell wall reference profile for Miscanthus bioenergy crops highlights compositional and structural variations associated with development and organ origin. New Phytol. 2017, 213, 1710–1725.
- Somerville, C. Cellulose Synthesis in Higher Plants. Annu. Rev. Cell Dev. Biol. 2006, 22, 53–78.
- Ochoa-Villarreal, M.; Aispuro-Hernández, E.; Vargas-Arispuro, I.; Martínez-Téllez, M.Á. Plant cell wall polymers: Function, structure and biological activity of their derivatives. Polymerization 2012, 4, 63–86.
- Lygin, A.V.; Upton, J.; Dohleman, F.G.; Juvik, J.; Zabotina, O.A.; Widholm, J.M.; Lozovaya, V.V. Composition of cell wall phenolics and polysaccharides of the potential bioenergy crop -Miscanthus. GCB Bioenergy 2011, 3, 333–345.
- Le Ngoc Huyen, T.; Rémond, C.; Dheilly, R.M.; Chabbert, B. Effect of harvesting date on the composition and saccharification of Miscanthus × giganteus. Bioresour. Technol. 2010, 101, 8224–8231.
- Jung, S.J.; Kim, S.H.; Chung, I.M. Comparison of lignin, cellulose, and hemicellulose contents for biofuels utilization among 4 types of lignocellulosic crops. Biomass Bioenergy 2015, 83, 322–327.
- Van der Weijde, T.; Huxley, L.M.; Hawkins, S.; Sembiring, E.H.; Farrar, K.; Dolstra, O.; Visser, R.G.F.; Trindade, L.M. Impact of drought stress on growth and quality of miscanthus for biofuel production. GCB Bioenergy 2017, 9, 770–782.
- Da Costa, R.M.F.; Pattathil, S.; Avci, U.; Winters, A.; Hahn, M.G.; Bosch, M. Desirable plant cell wall traits for higher-quality miscanthus lignocellulosic biomass. Biotechnol. Biofuels 2019, 12, 1–18.
- Hallac, B.B.; Ragauskas, A.J. Analyzing cellulose degree of polymerization and its relevancy to cellulosic ethanol. Biofuels Bioprod. Biorefining 2011, 5, 215–225.
- Gismatulina, Y.A.; Budaeva, V.V.; Veprev, S.G.; Sakovich, G.V.; Shumny, V.K. Cellulose from various parts of Soranovskii Miscanthus. Russ. J. Genet. Appl. Res. 2015, 5, 60–68.
- Gismatulina, Y.A.; Budaeva, V.V. Chemical composition of five Miscanthus sinensis harvests and nitric-acid cellulose therefrom. Ind. Crops Prod. 2017, 109, 227–232.
- Waliszewska, H.; Zborowska, M.; Waliszewska, B.; Borysiak, S.; Antczak, A.; Czekała, W. Transformation of Miscanthus and Sorghum cellulose during methane fermentation. Cellulose 2018, 25, 1207–1216.
- Sun, D.; Alam, A.; Tu, Y.; Zhou, S.; Wang, Y.; Xia, T.; Huang, J.; Li, Y.; Zahoor; Wei, X.; et al. Steam-exploded biomass saccharification is predominately affected by lignocellulose porosity and largely enhanced by Tween-80 in Miscanthus. Bioresour. Technol. 2017, 239, 74–81.
- Zhang, W.; Yi, Z.; Huang, J.; Li, F.; Hao, B.; Li, M.; Hong, S.; Lv, Y.; Sun, W.; Ragauskas, A.; et al. Three lignocellulose features that distinctively affect biomass enzymatic digestibility under NaOH and H2SO4 pretreatments in Miscanthus. Bioresour. Technol. 2013, 130, 30–37.
- Teeri, T.T. Crystalline cellulose degradation: New insight into the function of cellobiohydrolases. Trends Biotechnol. 1997, 15, 160–167.
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous cellulose - Structure and characterization. Cellul. Chem. Technol. 2011, 45, 13–21.
- Bansal, P.; Hall, M.; Realff, M.J.; Lee, J.H.; Bommarius, A.S. Multivariate statistical analysis of X-ray data from cellulose: A new method to determine degree of crystallinity and predict hydrolysis rates. Bioresour. Technol. 2010, 101, 4461–4471.
- Yoshida, M.; Liu, Y.; Uchida, S.; Kawarada, K.; Ukagami, Y.; Ichinose, H.; Kaneko, S.; Fukuda, K. Effects of cellulose crystallinity, hemicellulose, and lignin on the enzymatic hydrolysis of Miscanthus sinensis to monosaccharides. Biosci. Biotechnol. Biochem. 2008, 72, 805–810.
- Xu, N.; Zhang, W.; Ren, S.; Liu, F.; Zhao, C.; Liao, H.; Xu, Z.; Huang, J.; Li, Q.; Tu, Y.; et al. Hemicelluloses negatively affect lignocellulose crystallinity for high biomass digestibility under NaOH and H2SO4 pretreatments in Miscanthus. Biotechnol. Biofuels 2012, 5, 1.
- Shiga, T.M.; Xiao, W.; Yang, H.; Zhang, X.; Olek, A.T.; Donohoe, B.S.; Liu, J.; Makowski, L.; Hou, T.; McCann, M.C.; et al. Enhanced rates of enzymatic saccharification and catalytic synthesis of biofuel substrates in gelatinized cellulose generated by trifluoroacetic acid. Biotechnol. Biofuels 2017, 10, 1–15.
- Qin, L.; Li, W.C.; Zhu, J.Q.; Liang, J.N.; Li, B.Z.; Yuan, Y.J. Ethylenediamine pretreatment changes cellulose allomorph and lignin structure of lignocellulose at ambient pressure. Biotechnol. Biofuels 2015, 8, 1–15.
- Jeoh, T.; Ishizawa, C.I.; Davis, M.F.; Himmel, M.E.; Adney, W.S.; Johnson, D.K. Cellulase digestibility of pretreated biomass is limited by cellulose accessibility. Biotechnol. Bioeng. 2007, 98, 112–122.
- Pauly, M.; Gille, S.; Liu, L.; Mansoori, N.; de Souza, A.; Schultink, A.; Xiong, G. Hemicellulose biosynthesis. Planta 2013, 238, 627–642.
- Li, X.; Liao, H.; Fan, C.; Hu, H.; Li, Y.; Li, J.; Yi, Z.; Cai, X.; Peng, L.; Tu, Y. Distinct geographical distribution of the Miscanthus accessions with varied biomass enzymatic saccharification. PLoS ONE 2016, 11, 1–15.
- Schäfer, J.; Sattler, M.; Iqbal, Y.; Lewandowski, I.; Bunzel, M. Characterization of Miscanthus cell wall polymers. GCB Bioenergy 2019, 11, 191–205.
- Wang, Y.; Huang, J.; Li, Y.; Xiong, K.; Wang, Y.; Li, F.; Liu, M.; Wu, Z.; Tu, Y.; Peng, L. Ammonium oxalate-extractable uronic acids positively affect biomass enzymatic digestibility by reducing lignocellulose crystallinity in Miscanthus. Bioresour. Technol. 2015, 196, 391–398.
- Cheng, K.; Sorek, H.; Zimmermann, H.; Wemmer, D.E.; Pauly, M. Solution-state 2D NMR spectroscopy of plant cell Walls enabled by a dimethylsulfoxide- d 6/1-Ethyl-3-methylimidazolium acetate solvent. Anal. Chem. 2013, 85, 3213–3221.
- Gírio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Bogel-Łukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800.
- Hatfield, R.D.; Rancour, D.M.; Marita, J.M. Grass cell walls: A story of cross-linking. Front. Plant Sci. 2017, 7.




