Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kyungsu Kang | + 1437 word(s) | 1437 | 2021-05-27 08:25:50 | | | |
| 2 | Bruce Ren | -21 word(s) | 1416 | 2021-06-01 06:11:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Kang, K. Natural Photosensitizers in APDT. Encyclopedia. Available online: https://encyclopedia.pub/entry/10182 (accessed on 08 February 2026).
Kang K. Natural Photosensitizers in APDT. Encyclopedia. Available at: https://encyclopedia.pub/entry/10182. Accessed February 08, 2026.
Kang, Kyungsu. "Natural Photosensitizers in APDT" Encyclopedia, https://encyclopedia.pub/entry/10182 (accessed February 08, 2026).
Kang, K. (2021, May 27). Natural Photosensitizers in APDT. In Encyclopedia. https://encyclopedia.pub/entry/10182
Kang, Kyungsu. "Natural Photosensitizers in APDT." Encyclopedia. Web. 27 May, 2021.
Copy Citation
Antimicrobial photodynamic therapy (APDT) is constantly evolving and can minimize this antimicrobial resistance problem. Reactive oxygen species produced when nontoxic photosensitizers are exposed to light are the main functional components of APDT responsible for microbial destruction; therefore, APDT has a broad spectrum of target pathogens, such as bacteria, fungi, and viruses. Various photosensitizers, including natural extracts, compounds, and their synthetic derivatives, are being investigated. Light, oxygen, and PSs in precise cooperation are the key factors determining APDT efficiency and are responsible for ROS production and the inactivation of the targeted cells.
antimicrobial photodynamic therapy
natural photosensitizers
natural extracts
antibiotic resistance
model organisms
biophotonics
light
1. Introduction
After penicillin was identified as a product of Penicillium notatum by Alexander Fleming in 1928, its widespread consumption was noted in the early 1940s. In 1944, 50% of the clinical isolates of Staphylococci sp. were unexpectedly shown to exhibit resistance to penicillin [1]. Extensive antibiotic (mis)use has led to the spread of more resistant bacteria to the environment, and antimicrobial resistance (AMR) is an increasing threat to humans. By 2050, 10 million deaths per year are expected to be related to AMR [2]. Antibiotic-resistant pathogens are emerging threats to human life and are classified as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecalis, multidrug-resistant mycobacteria, Gram-negative pathogens, and fungi [1].
Antibiotic resistance and antibiotic pollution are common problems worldwide. Antibiotic resistance in infections potentially results in sepsis and even ineluctable systemic inflammation and organ failure [3]. Medicine is not the only source of antibiotic resistance. Aquaculture production, livestock, and pets consume and waste a high rate of antibiotics. Some probiotics and plant extracts containing essential oils have been introduced as alternative treatments to overcome excess antibiotic usage in aquaculture [4]. Notably, every use of antibiotics can create selective pressure for mutation and the development of drug resistance [5]. Overuse or misuse of antibiotics can increase the rate of AMR. Some country-based strategies have been implemented to overcome AMR. Additionally, in 2014, the World Health Organization (WHO) published a global report on AMR [6].
Antimicrobial photodynamic therapy (APDT) is a challenging method to overcome excess antibiotic consumption and limit antibiotic resistance gene transfer. Quorum sensing, vaccines, lectin inhibition, and iron chelation have been used as treatments for drug-resistant microorganisms, and APDT might be considered a favorable technique among them. The photodynamic effect was first described by Oscar Raab in 1904 and first successfully used to treat cancer cells in 1905 [7]. By the time PDT focused on cancer cell treatment, APDT was focused on overcoming antibiotic resistance by targeting bacteria, algae, yeasts, and viruses [8].
In addition to combating AMR and excess antibiotic consumption, APDT can provide strong antibiotic potency. APDT inhibits a broad spectrum of pathogens because its antimicrobial activity originates from reactive oxygen species (ROS) production induced by a unique photochemical reaction. Viera et al. [9] studied APDT efficiency against a broad range of organisms, including bacteria, fungi, and viruses, and reported that APDT is effective against a wide range of organisms. Tissue specificity is another advantage of APDT. Generally, no toxicity is observed in nonphotosensitizer-treated cells or in cells that are not exposed to light. Photosensitizers (PSs) are known to be taken up predominantly by target cells rather than nontarget cells. Only the infected tissue is irradiated, and the PS in unirradiated locations is pharmacodynamically passive. In addition, Park et al. [10] reported that APDT can be used effectively without damaging resident flora or human tissue.
2. Key Factors in Antimicrobial Photodynamic Therapy (APDT)
Light, oxygen, and PSs in precise cooperation are the key factors determining APDT efficiency and are responsible for ROS production and the inactivation of the targeted cells [11].
2.1. Light Sources
Photobiomodulation has been applied to relieve pain, decrease inflammation, and stimulate the healing of living tissues [12]. Light sources significantly affect PDT. Sunlight with diverse wavelengths activates the PS and causes shallow tissue penetration, thermal effects, and difficulties in controlling the dose [13]. Xenon lamps, light emitting diodes (LEDs), laser beams, and fiber optic devices are alternatives to sunlight and can overcome many problems associated with sunlight exposure. A xenon lamp illuminates a wide range, while a laser beam illuminates a narrow area. Near-infrared (NIR) (700–810 nm), red (600–700 nm), yellow (550–600 nm), green (490–550 nm), blue (400–490 nm), and ultraviolet A (UVA) (330–400 nm) light have been applied for APDT [14]. A broad range of light has been used for APDT; however, longer wavelengths are preferred because of the deeper tissue penetration [15].
LEDs have become attractive light sources because they are easy to operate, safe, and inexpensive. Moreover, some innovative methods have been developed as alternatives to classic light sources. For example, wearable light-emitting fabrics facilitate the delivery of total light over a much longer time with a much lower power density, overcoming the problem of depletion of the available oxygen supply by decreasing the oxygen consumption rate [14]. The sufficient intensity of light for antibacterial photosensitization ranges from 5–1000 W/m2, as higher light intensities potentially result in thermal problems. The exposure time varies from seconds to minutes, depending on the light intensity [16].
2.2. Oxygen
PSs and light irradiation are the main mechanisms of photodynamic therapy (PDT), and the ROS generated and the singlet oxygen (1O2) converted from molecular oxygen by PSs are responsible for bacterial damage. Singlet oxygen damages organelles and causes programmed cell death in human cells [17].
Molecular oxygen (O2), a nonpolar small molecule that diffuses across biological membranes, is used by aerobic organisms for oxidation and respiration. After O2 passes through the membranes, oxidative phosphorylation and adenosine triphosphate generation occur, and oxygen is reduced to produce energy. Respiratory flavoenzymes are the main catalytic redox cofactors that participate directly in ROS formation by transferring ē to O2 to produce superoxide (O2●−) and H2O2. Afterwards, a Fenton reaction, in which H2O2 is oxidized with the available ferrous iron (Fe2+) to generate OH●, occurs [18]. Sodium azide (NaN3) and histidine are 1O2 quenchers, and thiourea and dimethyl sulfoxide are free radical scavengers that have been used in photodynamic inactivation therapies [19].
Singlet oxygen (1O2) is an extremely short-lived and reactive form of oxygen that is involved in photochemical reduction processes. In type I reactions, electrons are stripped from biological macromolecules and •OH is transformed into hydroxide ions [1]. Superoxide dismutase (SOD) converts O•− into HO and O, and H2O2 participates in the Fenton reaction, resulting in the homolytic fission of the oxygen–oxygen bond in H2O2 to yield a hydroxide ion [1]. In type II reactions, 1O2 interacts with double bonds, sulfur moieties, and aromatic components of macromolecules in Diels–Alder cycloadditions [1]. Type I and type II reactions involving natural PSs are illustrated in Figure 1, and each step is explained below.
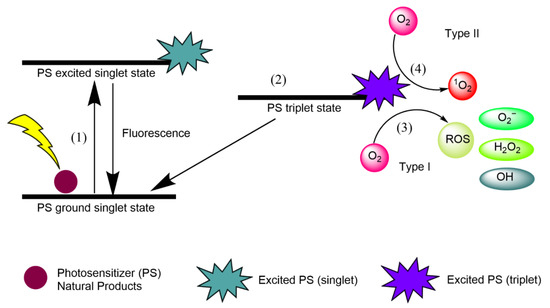
Figure 1. Type I and type II reactions in APDT. (1) Excitation from ground singlet state; (2) intersystem crossing into the triplet excited state; (3) Type I reaction; (4) Type II reaction.
- (1)
-
Natural product PSs are converted from the ground singlet state into the excited singlet state when exposed to a specific wavelength of light.
- (2)
-
If the PS in the excited singlet state does not return to the initial ground state, it can be subjected to intersystem crossing into the triplet excited state.
- (3)
-
A type I reaction comprises transferring a hydrogen atom from PS to an organic molecule to form radicals, and the reduced PS interacts with oxygen through a redox reaction, forming ROS and a superoxide anion radical (O2•−) [20].
- (4)
-
The type II reaction comprises direct energy transfer from the activated PS to molecular oxygen to form singlet oxygen (1O2) and is simpler than the type I reaction.
2.3. Photosensitizers (PSs)
PSs play an important role in photodynamic reactions as absorbers of light energy [13]. PSs are divided into three subgroups, namely, first-, second-, and third-generation PSs. Water-soluble porphyrins called “hematoporphyrins” are characterized as first-generation PSs, and methylene blue, toluidine blue, photosense®, Foscan®, and 5′-aminolevulinic acid (ALA) are examples of second-generation PSs. They possess a higher singlet oxygen quantum yield, chemical purity, and selectivity than first-generation PSs [7]. Third-generation PSs have been investigated recently with the main aims of reducing damage to healthy cells and increasing bioavailability. These systems generally consist of drug delivery systems, gene engineering-based technologies, or monoclonal antibody receptor combinations.
An ideal PS should:
-
Have a strong absorption peak in the red to near-infrared spectral region (between 650 and 800 nm) [15];
-
Possess a substantial triplet quantum yield leading to good ROS production upon irradiation [21];
-
Have high tissue selectivity [22];
-
Exhibit no dark toxicity [23];
-
Have ideal solubility to maintain lipophilic ability to cross the phospholipid membrane and prevent self-aggregation [24];
-
Exhibit high stability under storage conditions [25];
-
Kill microorganisms sufficiently without damaging eukaryotic host cells [26];
-
Display optimal absorption, distribution, metabolism, and excretion (ADME) [24];
-
Have a small size to enable microbial membrane permeation [2]; and
-
Have low manufacturing costs [23].
References
- Tyler G St. Denis; Tianhong Dai; Leonid Izikson; Christos Astrakas; Richard Rox Anderson; Michael R Hamblin; George P Tegos; All you need is light. Virulence 2011, 2, 509-520, 10.4161/viru.2.6.17889.
- Nidia Maldonado-Carmona; Tan-Sothea Ouk; Mário J. F. Calvete; Mariette M. Pereira; Nicolas Villandier; Stephanie Leroy-Lhez; Conjugating biomaterials with photosensitizers: advances and perspectives for photodynamic antimicrobial chemotherapy. Photochemical & Photobiological Sciences 2020, 19, 445-461, 10.1039/c9pp00398c.
- Darshna Yagnik; Vlad Serafin; Ajit J. Shah; Antimicrobial activity of apple cider vinegar against Escherichia coli, Staphylococcus aureus and Candida albicans; downregulating cytokine and microbial protein expression. Scientific Reports 2018, 8, 1-12, 10.1038/s41598-017-18618-x.
- Hakan Turker; Arzu Birinci Yıldırım; Screening for antibacterial activity of some Turkish plants against fish pathogens: a possible alternative in the treatment of bacterial infections. Biotechnology & Biotechnological Equipment 2015, 29, 281-288, 10.1080/13102818.2015.1006445.
- Zheng Pang; Renee Raudonis; Bernard R. Glick; Tong-Jun Lin; Zhenyu Cheng; Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnology Advances 2019, 37, 177-192, 10.1016/j.biotechadv.2018.11.013.
- Stephan Harbarth; For The World Healthcare-Associated Infections Resistance Forum Participants; Hanan H. Balkhy; Herman Goossens; Vincent Jarlier; Jan A J W Kluytmans; Ramanan Laxminarayan; Mirko Saam; Alex Van Belkum; Didier Pittet; et al. Antimicrobial resistance: one world, one fight!. Antimicrobial Resistance & Infection Control 2015, 4, 49, 10.1186/s13756-015-0091-2.
- Andrea Sowa; Jens Voskuhl; Host-guest complexes – Boosting the performance of photosensitizers. International Journal of Pharmaceutics 2020, 586, 119595, 10.1016/j.ijpharm.2020.119595.
- Thaila Quatrini Corrêa; Kate Cristina Blanco; Jennifer Machado Soares; Natalia Mayumi Inada; Cristina Kurachi; Marjorie De Assis Golim; Elenice Deffune; Vanderlei Salvador Bagnato; Photodynamic inactivation for in vitro decontamination of Staphylococcus aureus in whole blood. Photodiagnosis and Photodynamic Therapy 2019, 28, 58-64, 10.1016/j.pdpdt.2019.08.013.
- Cátia Vieira; Adriele Santos; Mariana Q. Mesquita; Ana Teresa Peixoto De Campos Gomes; M. Graça P. M. S. Neves; M. Amparo F. Faustino; Adelaide Almeida; Advances in aPDT based on the combination of a porphyrinic formulation with potassium iodide: Effectiveness on bacteria and fungi planktonic/biofilm forms and viruses. Journal of Porphyrins and Phthalocyanines 2019, 23, 534-545, 10.1142/s1088424619500408.
- Danbi Park; Min Kim; Jin Woo Choi; Jeong-Hwa Baek; Seoung Hoon Lee; Kyunghwa Baek; Antimicrobial photodynamic therapy efficacy against specific pathogenic periodontitis bacterial species. Photodiagnosis and Photodynamic Therapy 2020, 30, 101688, 10.1016/j.pdpdt.2020.101688.
- João C. S. Simões; Sophia Sarpaki; Panagiotis Papadimitroulas; Bruno Therrien; George Loudos; Conjugated Photosensitizers for Imaging and PDT in Cancer Research. Journal of Medicinal Chemistry 2020, 63, 14119-14150, 10.1021/acs.jmedchem.0c00047.
- Laura Marinela Ailioaie; Gerhard Litscher; Curcumin and Photobiomodulation in Chronic Viral Hepatitis and Hepatocellular Carcinoma. International Journal of Molecular Sciences 2020, 21, 7150, 10.3390/ijms21197150.
- Xiangsheng Zhang; Tang Liu; Zhihong Li; Progress of photodynamic therapy applications in the treatment of musculoskeletal sarcoma (Review). Oncology Letters 2014, 8, 1403-1408, 10.3892/ol.2014.2332.
- Nasim Kashef; Ying-Ying Huang; Michael R. Hamblin; Advances in antimicrobial photodynamic inactivation at the nanoscale. Nanophotonics 2017, 6, 853-879, 10.1515/nanoph-2016-0189.
- Balaji Babu; John Mack; Tebello Nyokong; Sn(iv) N-confused porphyrins as photosensitizer dyes for photodynamic therapy in the near IR region. Dalton Transactions 2020, 49, 15180-15183, 10.1039/d0dt03296d.
- Zivile Luksiene; Lubov Brovko; Antibacterial Photosensitization-Based Treatment for Food Safety. Food Engineering Reviews 2013, 5, 185-199, 10.1007/s12393-013-9070-7.
- Jiabao Zhuang; Hanxiao Yang; Yue Li; Bing Wang; Nan Li; Na Zhao; Efficient photosensitizers with aggregation-induced emission characteristics for lysosome- and Gram-positive bacteria-targeted photodynamic therapy. Chemical Communications 2020, 56, 2630-2633, 10.1039/d0cc00394h.
- Mohammad Yousef Memar; Reza Ghotaslou; Mohammad Samiei; Khosro Adibkia; Antimicrobial use of reactive oxygen therapy: current insights. Infection and Drug Resistance 2018, ume 11, 567-576, 10.2147/idr.s142397.
- Liyi Huang; Yi Xuan; Yuichiro Koide; Timur Zhiyentayev Ms; Masamitsu Tanaka; Michael R. Hamblin; Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers in Surgery and Medicine 2012, 44, 490-499, 10.1002/lsm.22045.
- Rigo Baluyot Villacorta; Kristine Faith Javier Roque; Giovanni Alarkon Tapang; Sonia Donaldo Jacinto; Plant extracts as natural photosensitizers in photodynamic therapy: in vitro activity against human mammary adenocarcinoma MCF-7 cells. Asian Pacific Journal of Tropical Biomedicine 2017, 7, 358-366, 10.1016/j.apjtb.2017.01.025.
- Zhijia Wang; Jianzhang Zhao; Bodipy–Anthracene Dyads as Triplet Photosensitizers: Effect of Chromophore Orientation on Triplet-State Formation Efficiency and Application in Triplet–Triplet Annihilation Upconversion. Organic Letters 2017, 19, 4492-4495, 10.1021/acs.orglett.7b02047.
- Stanisław Kwiatkowski; Bartosz Knap; Dawid Przystupski; Jolanta Saczko; Ewa Kędzierska; Karolina Knap-Czop; Jolanta Kotlińska; Olga Michel; Krzysztof Kotowski; Julita Kulbacka; et al. Photodynamic therapy – mechanisms, photosensitizers and combinations. Biomedicine & Pharmacotherapy 2018, 106, 1098-1107, 10.1016/j.biopha.2018.07.049.
- Heidi Abrahamse; Michael R. Hamblin; New photosensitizers for photodynamic therapy. Biochemical Journal 2016, 473, 347-364, 10.1042/bj20150942.
- Qicai Xiao; Juan Wu; Xin Pang; Yue Jiang; Pan Wang; Albert W. Leung; Liqian Gao; Sheng Jiang; Chuanshan Xu; Juan Wu Qicai Xiao; et al. Discovery and Development of Natural Products and their Derivatives as Photosensitizers for Photodynamic Therapy. Current Medicinal Chemistry 2018, 25, 839-860, 10.2174/0929867324666170823143137.
- F. Vázquez-Hernández; D.A. Granada-Ramírez; J.S. Arias-Cerón; P. Rodriguez-Fragoso; J.G. Mendoza-Álvarez; E. Ramón-Gallegos; A. Cruz-Orea; J.P. Luna-Arias; Use of nanostructured materials in drug delivery. Nanobiomaterials 2018, 2018, 503-549, 10.1016/b978-0-08-100716-7.00020-9.
- Fabian Cieplik; Dongmei Deng; Wim Crielaard; Wolfgang Buchalla; Elmar Hellwig; Ali Al-Ahmad; Tim Maisch; Antimicrobial photodynamic therapy – what we know and what we don’t. Critical Reviews in Microbiology 2018, 44, 571-589, 10.1080/1040841x.2018.1467876.
More
Information
Subjects:
Medicine, General & Internal
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.2K
Revisions:
2 times
(View History)
Update Date:
01 Jun 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




