
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ghasan Huseien | + 4107 word(s) | 4107 | 2021-07-12 05:48:25 | | | |
| 2 | Vivi Li | Meta information modification | 4107 | 2021-07-13 06:22:53 | | |
Video Upload Options
Uses of novel technologies for improving the durability and lifespan of the construction materials have emerged as viable solutions toward the sustainable future wherein the coating industry plays a significant role in economy growth and better livelihoods. Thus, the continual innovation of various technologies to introduce diverse market products has become indispensable. Properties of materials like color stability under UV, elevated temperatures and aggressive environments, and skid and abrasion resistance are the main challenges faced by commercial coating materials, leading to more demand of natural materials as sustainable agents. Lately, nanostructured core–shell pigments with unique compositions have widely been utilized in composite materials to enhance their properties. Core–shell particles exhibit smart properties and have immense benefits when combined with building materials.
1. Introduction
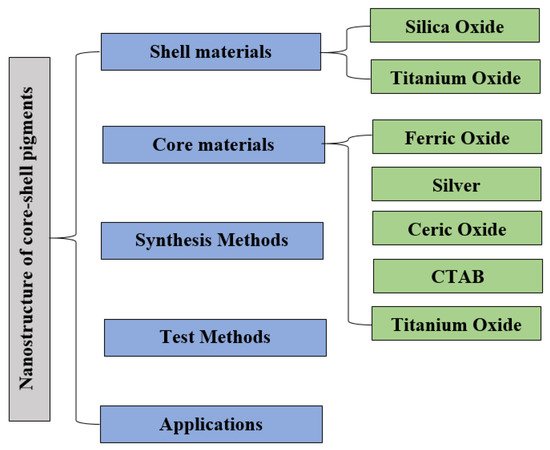
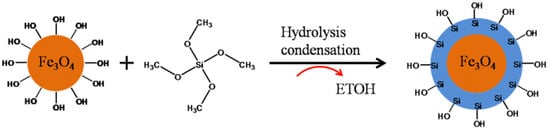
2. Core–Shell Nanoparticle: Synthesis Approach and Importance
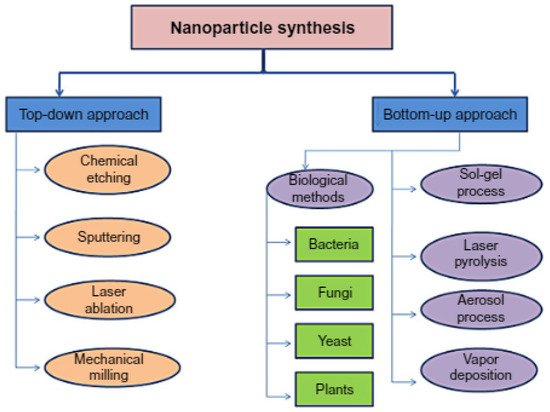
3. Core–Shell Synthesis Methods
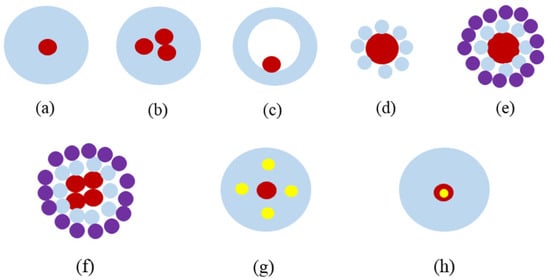
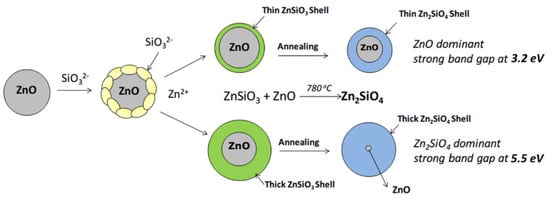
References
- Lin, C.; Li, Y.; Yu, M.; Yang, P.; Lin, J. A facile synthesis and characterization of monodisperse spherical pigment particles with a core/shell structure. Adv. Funct. Mater. 2007, 17, 1459–1465.
- Torres-Cavanillas, R.; Lima-Moya, L.; Tichelaar, F.D.; Zandbergen, H.W.; Giménez-Marqués, M.; Coronado, E. Downsizing of robust 2 spin-crossover nanoparticles with ultrathin shells. Dalton Trans. 2019, 48, 15465–15469.
- Alexander, M.; Mackechnie, J.; Yam, W. Carbonation of concrete bridge structures in three South African localities. Cem. Concr. Compos. 2007, 29, 750–759.
- Sadeghi-Niaraki, S.; Ghasemi, B.; Ghasemi, E.; Ghahari, M. Preparation of (Fe, Cr)2O3@TiO2 cool pigments for energy saving applications. J. Alloys Compd. 2019, 779, 367–379.
- Soranakom, P.; Vittayakorn, N.; Rakkwamsuk, P.; Supothina, S.; Seeharaj, P. Effect of surfactant concentration on the formation of Fe2O3@SiO2 NIR-reflective red pigments. Ceram. Int. 2021, 47, 13147–13155.
- Dong, X.; Zhang, X.; Yu, X.; Jiang, Z.; Liu, X.; Li, C.; Sun, Z.; Zheng, S.; Dionysiou, D.D. A novel rutile TiO2/AlPO4 core–shell pigment with substantially suppressed photoactivity and enhanced dispersion stability. Powder Technol. 2020, 366, 537–545.
- Yao, B.; Geng, S.; Wang, J.; Wang, L. Synthesis, Characterization, and Optical Properties of Near-Infrared Reflecting Composite Inorganic Pigments Composed of TiO2/CuO Core–Shell Particles. Aust. J. Chem. 2018, 71, 373–379.
- Huseien, G.F.; Shah, K.W.; Sam, A.R.M. Sustainability of nanomaterials based self-healing concrete: An all-inclusive insight. J. Build. Eng. 2019, 23, 155–171.
- Izu, N.; Uchida, T.; Itoh, T.; Shin, W. Decreasing the shell ratio of core–shell type nanoparticles with a ceria core and polymer shell by acid treatment. Solid State Sci. 2018, 85, 32–37.
- Ahmed, N.; Fathi, A.; Mohamed, M.; Abd El-Gawad, W. Evaluation of new core–shell pigments on the anticorrosive performance of coated reinforced concrete steel. Prog. Org. Coat. 2020, 140, 105530.
- Sanchis-Gual, R.; Torres-Cavanillas, R.; Puchau, M.C.; Giménez-Marqués, M.; Coronado, E. Plasmon-assisted spin transition in gold crossover heterostructures. J. Mater. Chem. C 2021.
- Torres-Cavanillas, R.; Sanchis-Gual, R.; Dugay, J.; Coronado-Puchau, M.; Giménez-Marqués, M.; Coronado, E. Design of Bistable Core–Shell Nanoparticles Showing Large Electrical Responses for the Spin Switching. Adv. Mater. 2019, 31, 1900039.
- Ahmed, N.M.; Mohamed, M.G.; Abd El-Gawad, W.M. Corrosion protection performance of silica fume waste-phosphates core–shell pigments. Pigment Resin Technol. 2018, 47, 261–271.
- Sertchook, H.; Avnir, D. Submicron silica/polystyrene composite particles prepared by a one-step sol-gel process. Chem. Mater. 2003, 15, 1690–1694.
- Zhuang, Z.; Sheng, W.; Yan, Y. Synthesis of monodispere 3O4 core–shell nanocrystals and their enhanced catalytic activity for oxygen evolution reaction. Adv. Mater. 2014, 26, 3950–3955.
- Zhong, Z.; Yin, Y.; Gates, B.; Xia, Y. Preparation of mesoscale hollow spheres of TiO2 and SnO2 by templating against crystalline arrays of polystyrene beads. Adv. Mater. 2000, 12, 206–209.
- Samal, A.K.; Polavarapu, L.; Rodal-Cedeira, S.; Liz-Marzán, L.M.; Pérez-Juste, J.; Pastoriza-Santos, I. Size Tunable core–shell nanoparticles: Synthesis and surface-enhanced raman scattering properties. Langmuir 2013, 29, 15076–15082.
- Parashar, M.; Shukla, V.K.; Singh, R. Metal oxides nanoparticles via sol-gel method: A review on synthesis, characterization and applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749.
- Dugay, J.; Evers, W.; Torres-Cavanillas, R.; Giménez-Marqués, M.; Coronado, E.; Van der Zant, H.S. Charge Mobility and Dynamics in Spin-Crossover Nanoparticles Studied by Time-Resolved Microwave Conductivity. J. Phys. Chem. Lett. 2018, 9, 5672–5678.
- Ahmed, J.; Sharma, S.; Ramanujachary, K.V.; Lofland, S.E.; Ganguli, A.K. Microemulsion-mediated synthesis of cobalt (pure fcc and hexagonal phases) and cobalt–nickel alloy nanoparticles. J. Colloid Interface Sci. 2009, 336, 814–819.
- El-Safty, S.A. Synthesis, characterization and catalytic activity of highly ordered hexagonal and cubic composite monoliths. J. Colloid Interface Sci. 2008, 319, 477–488.
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433.
- Han, W.; Yi, L.; Zhao, N.; Tang, A.; Gao, M.; Tang, Z. Synthesis and shape-tailoring of copper sulfide/indium sulfide-based nanocrystals. J. Am. Chem. Soc. 2008, 130, 13152–13161.
- Lee, H.; Kim, C.; Yang, S.; Han, J.W.; Kim, J. Shape-controlled nanocrystals for catalytic applications. Catal. Surv. Asia 2012, 16, 14–27.
- Libor, Z.; Zhang, Q. The synthesis of nickel nanoparticles with controlled morphology and SiO2/Ni core–shell structures. Mater. Chem. Phys. 2009, 114, 902–907.
- Kim, Y.; Pee, J.-H.; Chang, J.H.; Choi, K.; Kim, K.J.; Jung, D.-Y. Silica effect on coloration of hematite nanoparticles for red pigments. Chem. Lett. 2009, 38, 842–843.
- Liu, R.; Priestley, R.D. Rational design and fabrication of core–shell nanoparticles through a one-step/pot strategy. J. Mater. Chem. A 2016, 4, 6680–6692.
- Chen, S.; Cheng, M.; Lang, Y.; Tian, C.; Wei, H.; Wang, C.-A. Preparation and characterization of monodispersed spherical Fe2O3@SiO2 reddish pigments with core–shell structure. J. Adv. Ceram. 2019, 8, 39–46.
- Dodd, A.C. A comparison of mechanochemical methods for the synthesis of nanoparticulate nickel oxide. Powder Technol. 2009, 196, 30–35.
- Deng, W.; Xia, W.; Li, C.; Tang, Y. Formation of ultra-fine grained materials by machining and the characteristics of the deformation fields. J. Mater. Process. Technol. 2009, 209, 4521–4526.
- Salari, M.; Marashi, P.; Rezaee, M. Synthesis of TiO2 nanoparticles via a novel mechanochemical method. J. Alloys Compd. 2009, 469, 386–390.
- Sasikumar, R.; Arunachalam, R. Synthesis of nanostructured aluminium matrix composite (AMC) through machining. Mater. Lett. 2009, 63, 2426–2428.
- Wang, Y.; Cai, K.; Yao, X. Facile synthesis of PbTe nanoparticles and thin films in alkaline aqueous solution at room temperature. J. Solid State Chem. 2009, 182, 3383–3386.
- Yoo, S.-H.; Liu, L.; Park, S. Nanoparticle films as a conducting layer for anodic aluminum oxide template-assisted nanorod synthesis. J. Colloid Interface Sci. 2009, 339, 183–186.
- Oldenburg, S.; Averitt, R.; Westcott, S.; Halas, N. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247.
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346.
- Pal, G.; Rai, P.; Pandey, A. Green synthesis of nanoparticles: A greener approach for a cleaner future. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1, pp. 1–26.
- Zhang, Y.; Fan, L.; Chen, H.; Zhang, J.; Zhang, Y.; Wang, A. Learning from ancient Maya: Preparation of stable palygorskite/methylene 2 Maya Blue-like pigment. Microporous Mesoporous Mater. 2015, 211, 124–133.
- Hayes, R.; Ahmed, A.; Edge, T.; Zhang, H. Core–shell particles: Preparation, fundamentals and applications in high performance liquid chromatography. J. Chromatogr. A 2014, 1357, 36–52.
- Mao, W.-X.; Zhang, W.; Chi, Z.-X.; Lu, R.-W.; Cao, A.-M.; Wan, L.-J. Core–shell structured Ce2S3@ZnO and its potential as a pigment. J. Mater. Chem. A 2015, 3, 2176–2180.
- Liu, J.; Qiao, S.Z.; Chen, J.S.; Lou, X.W.D.; Xing, X.; Lu, G.Q.M. Yolk/shell nanoparticles: New platforms for nanoreactors, drug delivery and lithium-ion batteries. Chem. Commun. 2011, 47, 12578–12591.
- Niu, H.-Y.; Li, W.-H.; Shi, Y.-L.; Cai, Y.-Q. A core–shell magnetic mesoporous silica sorbent for organic targets with high extraction performance and anti-interference ability. Chem. Commun. 2011, 47, 4454–4456.
- Insin, N.; Tracy, J.B.; Lee, H.; Zimmer, J.P.; Westervelt, R.M.; Bawendi, M.G. Incorporation of iron oxide nanoparticles and quantum dots into silica microspheres. ACS Nano 2008, 2, 197–202.
- Wang, J.; Yang, N.; Tang, H.; Dong, Z.; Jin, Q.; Yang, M.; Kisailus, D.; Zhao, H.; Tang, Z.; Wang, D. Accurate control of multishelled Co3O4 hollow microspheres as high-performance anode materials in lithiumion batteries. Angew. Chem. 2013, 125, 6545–6548.
- Lai, X.; Li, J.; Korgel, B.A.; Dong, Z.; Li, Z.; Su, F.; Du, J.; Wang, D. General synthesis and gas-sensing properties of multiple-shell metal oxide hollow microspheres. Angew. Chem. Int. Ed. 2011, 50, 2738–2741.
- Wang, D.; Xin, H.L.; Hovden, R.; Wang, H.; Yu, Y.; Muller, D.A.; DiSalvo, F.J.; Abruña, H.D. Structurally ordered intermetallic platinum-cobalt core–shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013, 12, 81–87.
- Chae, H.S.; Kim, S.D.; Piao, S.H.; Choi, H.J. Core-shell structured Fe3O4@SiO2 nanoparticles fabricated by sol-gel method and their magnetorheology. Colloid Polym. Sci. 2016, 294, 647–655.
- Guo, X.; Canet, J.-L.; Boyer, D.; Gautier, A.; Mahiou, R. Sol-gel emulsion synthesis of biphotonic core–shell nanoparticles based on lanthanide doped organic-inorganic hybrid materials. J. Mater. Chem. 2012, 22, 6117–6122.
- Li, Y.-M.; Liu, S.-G.; Song, F.-S.; Wang, Z.-M.; Shen, Z.-Y.; Xie, Z.-X. Preparation and thermal stability of silica layer multicoated γ-Ce2S3 red pigment microparticles. Surf. Coat. Technol. 2018, 345, 70–75.
- Nikolaev, P.; Bronikowski, M.J.; Bradley, R.K.; Rohmund, F.; Colbert, D.T.; Smith, K.; Smalley, R.E. Gas-phase catalytic growth of single-walled carbon nanotubes from carbon monoxide. Chem. Phys. Lett. 1999, 313, 91–97.
- El-Gendy, A.; Ibrahim, E.; Khavrus, V.; Krupskaya, Y.; Hampel, S.; Leonhardt, A.; Büchner, B.; Klingeler, R. The synthesis of carbon coated Fe, Co and Ni nanoparticles and an examination of their magnetic properties. Carbon 2009, 47, 2821–2828.
- Mendes, R.G.; Koch, B.; Bachmatiuk, A.; El-Gendy, A.A.; Krupskaya, Y.; Springer, A.; Klingeler, R.; Schmidt, O.; Büchner, B.; Sanchez, S. Synthesis and toxicity characterization of carbon coated iron oxide nanoparticles with highly defined size distributions. Biochim. Biophys. Acta BBA Gen. Subj. 2014, 1840, 160–169.
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69.
- Bogush, G.; Tracy, M.; Zukoski Iv, C. Preparation of monodisperse silica particles: Control of size and mass fraction. J. Non-Cryst. Solids 1988, 104, 95–106.
- Drašar, P. The Sol-Gel Handbook: Synthesis, Characterization and Applications; David, L., Marcos, Z., Eds.; Wiley: Hoboken, NJ, USA, 2016.
- Du, G.-H.; Liu, Z.; Xia, X.; Chu, Q.; Zhang, S. Characterization and application of Fe3O4/SiO2 nanocomposites. J. Sol. Gel. Sci. Technol. 2006, 39, 285–291.
- Li, Z.; Wanjala, B.; Cernigliaro, G.; Nawrocki, D.; Gu, Z. Synthesis of Zn2SiO4@ZnO core–shell nanoparticles and the effect of shell thickness on band-gap transition. Mater. Chem. Phys. 2020, 240, 122144.
- Tsuji, M.; Yamaguchi, D.; Matsunaga, M.; Ikedo, K. Epitaxial growth of core- shell nanocrystals prepared using a two-step reduction method. Cryst. Growth Des. 2011, 11, 1995–2005.
- Fan, F.-R.; Liu, D.-Y.; Wu, Y.-F.; Duan, S.; Xie, Z.-X.; Jiang, Z.-Y.; Tian, Z.-Q. Epitaxial growth of heterogeneous metal nanocrystals: From gold nano-octahedra to palladium and silver nanocubes. J. Am. Chem. Soc. 2008, 130, 6949–6951.
- Wu, Y.; Jiang, P.; Jiang, M.; Wang, T.-W.; Guo, C.-F.; Xie, S.-S.; Wang, Z.-L. The shape evolution of gold seeds and core–shell nanostructures. Nanotechnology 2009, 20, 305602.
- Tsuji, M.; Yamaguchi, D.; Matsunaga, M.; Alam, M.J. Epitaxial Growth of Core- Shell Nanocrystals Prepared Using the PVP-Assisted Polyol Reduction Method. Cryst. Growth Des. 2010, 10, 5129–5135.
- Habas, S.E.; Lee, H.; Radmilovic, V.; Somorjai, G.A.; Yang, P. Shaping binary metal nanocrystals through epitaxial seeded growth. Nat. Mater. 2007, 6, 692–697.
- Tsuji, M.; Hikino, S.; Tanabe, R.; Yamaguchi, D. Synthesis of Core–Shell Nanoparticles in High Yield Using a Polyol Method. Chem. Lett. 2010, 39, 334–336.
- Sharma, R.; Tiwari, S. Synthesis of fly ash based core–shell composites for use as functional pigment in paints. In Proceedings of the AIP Conference Proceedings 2016, Jaipur, Rajasthan, India, 24–25 October 2015; pp. 020083-1–020083-6.
- Zhang, J.; Li, X.; Shi, X.; Hua, M.; Zhou, X.; Wang, X. Synthesis of core–shell acrylic-polyurethane hybrid latex as binder of aqueous pigment inks for digital inkjet printing. Prog. Nat. Sci. Mater. Int. 2012, 22, 71–78.




