| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dan Blendea | + 2048 word(s) | 2048 | 2021-08-09 13:21:18 | | | |
| 2 | Beatrix Zheng | Meta information modification | 2048 | 2021-08-26 03:47:25 | | |
Video Upload Options
The baseline electrocardiogram (ECG) is less informative in neurally mediated syncope (NMS) than in arrhythmic syncope. However, some of the ECG patterns present in NMS can have diagnostic and prognostic value in such patients. This work reviews known ECG findings related to the cardioinhibitory reflex, as well as recently studied ECG patterns present in patients with NMS, such as the isolated very low QRS voltage.
1. Introduction
Given that syncope is an entity frequently seen in the emergency department, it is essential for clinicians to distinguish between neurally mediated syncope (NMS) and other causes of transient loss of consciousness (TLOC), in order to establish an accurate diagnosis and provide appropriate medical treatment. While determining the cause of syncope relies significantly on clinical findings, the electrocardiogram (ECG) can add valuable information.
In the 2017 American College of Cardiology/American Heart Association/ Heart Rhythm Society (ACC/AHA/HRS) syncope guideline, the ECG has a class I recommendation, together with history and physical exam as part of the initial evaluation, during which it can help identifying an underlying arrhythmogenic substrate for the syncopal episode [1].
The baseline ECG is perhaps less informative in NMS than in arrhythmic syncope. Specific ECG patterns in NMS are primarily related to the cardioinhibitory (CI) reflex. In addition, there are other ECG findings such as QRS voltage patterns that could that have clinical importance in patients with NMS.
The purpose of this work is to review the different ECG patterns that can occur in patients with NMS and to describe the value of the ECG in the management of such patients.
2. Pathophysiology of Neurally Mediated Syncope
Syncope is defined as a sudden, short-duration TLOC, which occurs in the context of inadequate cerebral perfusion [2]. The main mechanism in NMS is represented by an abnormal autonomic response initiated by diverse triggers, such as prolonged standing, strong emotions, cough, micturition, or defecation [3]. The triggers activate peripheral or central receptors and the vagal central sites, which have a pivotal role in the reflex mechanism of NMS, responding to stimulus with an efferent CI and/or a vasodepressor component [4][5][6]. The response to these triggers is more likely to occur or to be more severe when hypovolemia, hypoxemia, or thermal stress are present [2]. Knowing that cerebral perfusion is mainly dependent on systemic blood pressure (BP), and that BP depends on stroke volume, heart rate (HR), and peripheral vascular resistance, syncope can appear whenever one of these parameters decreases, without being efficiently compensated by an increase of any of the other two [3][7].
The afferent neural signals are mainly transmitted to the nucleus solitarius, which has multiple connections with the dorsal and ambiguous nuclei of the vagus nerve, usually triggering two types of responses. The first one results in generating a defense reaction that includes a sympathetic neural activation. On the opposite hand, the second one is characterized by sympathetic withdrawal and parasympathetic activation. The overactivation of the latter mechanism is responsible for vasodilation, bradycardia, and consequent syncope [8]. It is known that the mechanism of NMS also involves disturbances in different serum neurohormonal levels. These include adrenaline, noradrenaline, serotonin, nitric oxide, and pancreatic polypeptide [3]. The interaction between neural activation and humoral factors influences the manner in which a syncopal event becomes manifest.
In addition, studies on postural syncope revealed a potential involvement of the cardiac mechanoreceptors in mediating the vagal response [7]. Normally, due to blood pooling in the lower extremities while standing, the circulating volume drops and BP decreases. Signals are further transmitted to the central sites in order to generate a compensatory reaction and to avoid cerebral hypoperfusion. Therefore, sympathetic stimulation leads to an increase of the peripheral resistance along with positive chronotropism and inotropism [7]. As people with NMS tend to have exaggerated venous pooling while standing, a forceful heart contraction on an underfilled ventricle will stimulate the cardiac mechanoreceptors with vagal afferent pathway and will trigger an inhibitory response (Bezold–Jarisch reflex), with consequent bradycardia, vasodilatation, hypotension, and TLOC. This concept is known as the ventricular theory, and it is widely accepted [7].
The described hemodynamic mechanisms vary with different factors, one of them being age, with younger patients being more prone to becoming asystolic during vagal overstimulation [9].
3. Cardioinhibitory Response
Cardioinhibitory response was previously referred to as sinoatrial syncope or malignant vasovagal syndrome [10]. Cardioinhibitory syncope encompasses a relatively small group of patients with NMS. The clinical relevance lies within its trend to affect predominantly young people, presenting with atypical recurrent syncope and less pronounced prodromal symptoms. ECG manifestations of the CI response can be detected incidentally on telemetry in the emergency department, or with aid of inpatient or outpatient heart rhythm monitoring devices.
Common expressions of sinus node (SN) inhibition are sinus bradycardia and sinus arrest [11][12]. The atrioventricular (AV) node depression in NMS has heterogeneous presentation, including several subtypes of AV block, such as second-degree AV block type I or type II, 2:1 AV block, high-grade AV block, and third-degree AV block, or, more important, the association of two or more subtypes [13].
Sinus arrest or sinoatrial block in NMS is more frequent than AV block, either because of a higher sensibility to vagal stimulation than the AV node or because vagal stimulation of the SN with marked sinus bradycardia and pauses does not allow the AV block to manifest itself on the ECG due to lack of any atrial electrical activity [13].
The CI response due to a reflex mechanism is typically a diagnosis of exclusion, after the anatomical involvement of the SN and AV node was ruled out. A concordant depression of both SN and AV node function is suggestive of a vagally mediated mechanism [13][14](Figure 1).
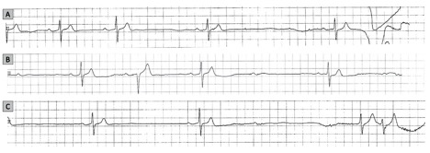
Induced CI response is less frequent than the spontaneous response, being present in only 28% of the tilt-table test (TTT) induced NMS, while spontaneous CI was encountered in 53% of cases [11][12][13]. The differences are apparent when comparing data on induced syncope observed during TTT in the study of Zysco et al. [12] and during spontaneous syncope diagnosed as likely neurally mediated with data documented by implantable loop recorder (ILR) in the ISSUE 2 study [11][13] and the study of Brignole et al. [15] sinus arrest alone occurred in 23% of patients with induced vs. 30% of patients with spontaneous syncope. SN inhibition with sinus arrest during spontaneous syncope seems to be present equally in young patients and in the elderly. In contrast, induced syncope with sinus arrest during TTT tends to affect younger patients more than older patients [11]. Another distinct pattern is that of AV block with sinus bradycardia or arrest, which occurred in 5% of induced and 9% of spontaneous syncope. Atrioventricular block alone was found to be extremely rare in patients with induced syncope and in approximately 15% of patients with spontaneous syncope [11][12][13].
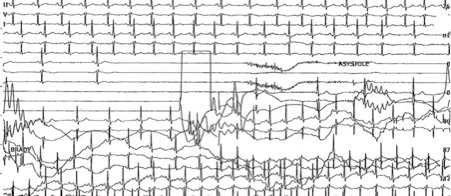
It is worth noting the uncommon presentation forms of NMS, such as atrial fibrillation and supraventricular tachycardia. A few studies mention a minority of patients who had atrial tachyarrhythmia after asystole [10], or atrial flutter with AV block during TTT followed by syncope [16]. Although it is more common for sinus tachycardia to succeed the asystolic episode during syncope (Figure 2), there have been cases in which it preceded the asystolic episode, accompanied by a significant drop in the mean BP, suggesting a vasodepressive component [17][18].
Although CI response during NMS may have dramatic consequences, the reaction is usually self-limited and not life-threatening.
There are some cases which may benefit from cardiac pacing, such as recurrent and severe NMS and confirmed asystolic episode, in spite of conservative management, according to ACC/AHA/HRS 2017 guidelines [19]. Current European guidelines recommend pacing due to asystole, AV block or a combination of the two, in patients over 40 years of age presenting spontaneous symptomatic pause longer than 3 seconds or asymptomatic pause longer than 6 seconds [2][19]. However, there is no data supporting cardiac pacing in NMS patients younger than 40. It is worth mentioning that the implantation of a pacemaker won't be beneficial in the absence of a documented bradycardia-induced syncope due to vasovagal reflex [19][20][21].
4. QRS Voltage
A recently studied ECG pattern related to NMS is the presence of isolated very low voltage (VLV) QRS complexes. VLV was defined as a voltage of ≤ 0.3mV in frontal leads and of ≤ 0.7 mV in precordial leads. This ECG pattern appears different from the usual low voltage in all frontal or precordial leads [22][23] (Figure 3).
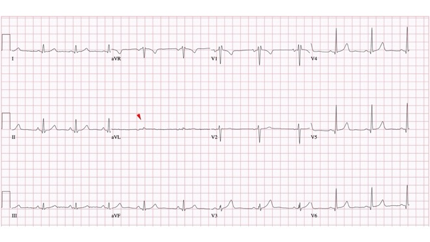
In a study that included 216 patients with suspected NMS and who underwent a TTT, as part of their diagnostic workup, the lowest QRS amplitude present in the frontal leads was most frequently encountered in lead aVL. The lead displaying VLV was close to perpendicular to the QRS axis. The voltage in frontal leads was found to be significantly lower in the TTT-positive group of patients in comparison with the TTT-negative group [22].
The mechanism for this isolated QRS voltage reduction is not known. However, given the fact that there was a significant correlation between the QRS with lowest voltage and left ventricle (LV) end-diastolic diameter, the authors hypothesized that the presence of isolated VLV is due at least in part to a specific LV geometry encountered in patients with NMS [22].
Patients with NMS have reductions in voltage not only in frontal leads, but also in the precordial leads [22][24]. VLV in precordial leads, defined as a QRS voltage of ≤ 0.7 mV, was present in 36% of the 135 patients with NMS enrolled in a prospective study, with lead V1 displaying this pattern in most of the cases. After a median follow-up of 15 months, syncope recurrence rate was 26% in patients with VLV in precordial leads and 21% in patients without VLV. The precordial VLV was found to be predictive independently of VLV in frontal leads [25].
The proposed alteration of ventricular activation, geometry and associated isolated VLV, was further studied by vectorcardiography, thus providing a better spatial display of the magnitude and direction of the activation vectors.
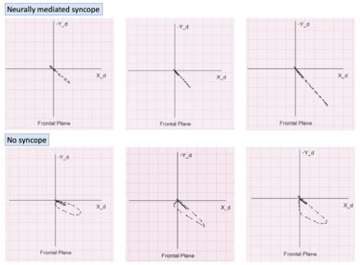
The QRS loop area and width-to-length ratio in NMS patients were significantly lower that in syncope-free population [26] (Figure 4). In a prospective study of 190 patients, followed for a median of 10 months, the presence of flat QRS loops in frontal plane leads was found to predict recurrence of NMS independent of isolated VLV and LV end-diastolic diameter [27].
Figure 4. Examples of flat vectorcardiographic loops in the frontal plane in three patients with neurally mediated syncope – top panel; Vectorcardiographic loops in the frontal plane in three subjects with no history of syncope – bottom panel.
Given that these ECG and vectorcardiogram (VCG) parameters were strongly predictive of recurrent syncope, an attempt was made to incorporate them in a predictive model. History of ≥ 2 syncopal events, LV end-diastolic diameter of < 39 mm by echocardiography, isolated VLV QRS in frontal leads and flat QRS VCG loops in frontal plane were identified as independent predictors for NMS recurrence. Three points were assigned for history of isolated VLV in frontal leads and ≥ 2 syncopal events, 2 points for flat QRS VCG loops in frontal plane and 1 point for LV end-diastolic diameter of < 39 mm measured by echocardiography. The total risk score included three categories: low risk (0-2), intermediate risk (3-5) and high risk (≥ 6). The actuarial total syncope recurrence rate at 1 year was 54.6% in the high-risk score category, 25.3% in the intermediate risk category, and 6.2% in the low-risk category. The ROC curve showed an AUC of 0.77 for the predictive value of the total risk score [28].
To summarize, the presence of isolated VLV in frontal leads is a parameter that could be applied in the workup of patients with syncope: it could help in the process of clarifying the diagnosis when the clinical variables do not offer sufficient clarity, and most importantly it could help stratify the risk of recurrence of patients with NMS. The latter could potentially be used in the process of selecting patients for pacemaker implantation. However, this would require validation in randomized prospective trials.
References
- Shen, W.K.; Sheldon, R.S.; Benditt, D.G.; Cohen, M.I.; Forman, D.E.; Goldberger, Z.D.; Grubb, B.P.; Hamdan, M.H.; Krahn, A.D.; Link, M.S.; et al. 2017 ACC/AHA/HRS Guideline for the Evaluation and Management of Patients With Syncope: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2017, 70, e39-e110, doi:10.1016/j.jacc.2017.03.003.
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martin, A.; et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 2018, 39, 1883-1948, doi:10.1093/eurheartj/ehy037.
- van Dijk, J.G.; van Rossum, I.A.; Thijs, R.D. Timing of Circulatory and Neurological Events in Syncope. Front Cardiovasc Med 2020, 7, 36, doi:10.3389/fcvm.2020.00036.
- Rocha, B.M.L.; Gomes, R.V.; Cunha, G.J.L.; Silva, B.M.V.; Pocinho, R.; Morais, R.; Araujo, I.; Fonseca, C. Diagnostic and therapeutic approach to cardioinhibitory reflex syncope: A complex and controversial issue. Rev Port Cardiol 2019, 38, 661-673, doi:10.1016/j.repc.2018.11.007.
- Jardine, D.L.; Wieling, W.; Brignole, M.; Lenders, J.W.M.; Sutton, R.; Stewart, J. The pathophysiology of the vasovagal response. Heart Rhythm 2018, 15, 921-929, doi:10.1016/j.hrthm.2017.12.013.
- Wieling, W.; Jardine, D.L.; de Lange, F.J.; Brignole, M.; Nielsen, H.B.; Stewart, J.; Sutton, R. Cardiac output and vasodilation in the vasovagal response: An analysis of the classic papers. Heart Rhythm 2016, 13, 798-805, doi:10.1016/j.hrthm.2015.11.023.
- Mosqueda-Garcia, R.; Furlan, R.; Tank, J.; Fernandez-Violante, R. The elusive pathophysiology of neurally mediated syncope. Circulation 2000, 102, 2898-2906, doi:10.1161/01.cir.102.23.2898.
- Benditt, D.G. Neurally mediated syncopal syndromes: pathophysiological concepts and clinical evaluation. Pacing Clin Electrophysiol 1997, 20, 572-584, doi:10.1111/j.1540-8159.1997.tb06211.x.
- Stewart, J.M.; Medow, M.S.; Sutton, R.; Visintainer, P.; Jardine, D.L.; Wieling, W. Mechanisms of Vasovagal Syncope in the Young: Reduced Systemic Vascular Resistance Versus Reduced Cardiac Output. J Am Heart Assoc 2017, 6, doi:10.1161/JAHA.116.004417.
- Mehlsen, J.; Kaijer, M.N.; Mehlsen, A.B. Autonomic and electrocardiographic changes in cardioinhibitory syncope. Europace 2008, 10, 91-95, doi:10.1093/europace/eum237.
- Brignole, M.; Sutton, R.; Menozzi, C.; Garcia-Civera, R.; Moya, A.; Wieling, W.; Andresen, D.; Benditt, D.G.; Grovale, N.; De Santo, T.; et al. Lack of correlation between the responses to tilt testing and adenosine triphosphate test and the mechanism of spontaneous neurally mediated syncope. Eur Heart J 2006, 27, 2232-2239, doi:10.1093/eurheartj/ehl164.
- Zysko, D.; Gajek, J.; Kozluk, E.; Mazurek, W. Electrocardiographic characteristics of atrioventricular block induced by tilt testing. Europace 2009, 11, 225-230, doi:10.1093/europace/eun299.
- Brignole, M. Different electrocardiographic manifestations of the cardioinhibitory vasovagal reflex. Europace 2009, 11, 144-146, doi:10.1093/europace/eun390.
- Alboni, P.; Holz, A.; Brignole, M. Vagally mediated atrioventricular block: pathophysiology and diagnosis. Heart 2013, 99, 904-908, doi:10.1136/heartjnl-2012-303220.
- Brignole, M.; Gaggioli, G.; Menozzi, C.; Del Rosso, A.; Costa, S.; Bartoletti, A.; Bottoni, N.; Lolli, G. Clinical features of adenosine sensitive syncope and tilt induced vasovagal syncope. Heart 2000, 83, 24-28, doi:10.1136/heart.83.1.24.
- Chou, H.H.; Lin, K.H.; Luqman, N.; Kuo, C.T. Prolonged ventricular asystole, sinus arrest, and paroxysmal atrial flutter-fibrillation: an uncommon presentation of vasovagal syncope. Pacing Clin Electrophysiol 2003, 26, 914-917, doi:10.1046/j.1460-9592.2003.t01-1-00159.x.
- Leitch, J.W.; Klein, G.J.; Yee, R.; Leather, R.A.; Kim, Y.H. Syncope associated with supraventricular tachycardia. An expression of tachycardia rate or vasomotor response? Circulation 1992, 85, 1064-1071, doi:10.1161/01.cir.85.3.1064.
- Moya, A.; Brignole, M.; Menozzi, C.; Garcia-Civera, R.; Tognarini, S.; Mont, L.; Botto, G.; Giada, F.; Cornacchia, D.; International Study on Syncope of Uncertain Etiology, I. Mechanism of syncope in patients with isolated syncope and in patients with tilt-positive syncope. Circulation 2001, 104, 1261-1267, doi:10.1161/hc3601.095708.
- Akella, K.; Olshansky, B.; Lakkireddy, D.; Gopinathannair, R. Pacing Therapies for Vasovagal Syncope. J Atr Fibrillation 2020, 13, 2406, doi:10.4022/jafib.2406.
- Baron-Esquivias, G.; Morillo, C.A.; Moya-Mitjans, A.; Martinez-Alday, J.; Ruiz-Granell, R.; Lacunza-Ruiz, J.; Garcia-Civera, R.; Gutierrez-Carretero, E.; Romero-Garrido, R. Dual-Chamber Pacing With Closed Loop Stimulation in Recurrent Reflex Vasovagal Syncope: The SPAIN Study. J Am Coll Cardiol 2017, 70, 1720-1728, doi:10.1016/j.jacc.2017.08.026.
- Olshansky, B. Vasovagal Syncope: To Pace or Not to Pace. J Am Coll Cardiol 2017, 70, 1729-1731, doi:10.1016/j.jacc.2017.08.025.
- Blendea, D.; McPherson, C.A.; Pop, S.; Anton, F.P.; Crisan, S.; Ruskin, J.N. Isolated very low QRS voltage predicts response to tilt-table testing in patients with neurally mediated syncope. Pacing Clin Electrophysiol 2019, 42, 1558-1565, doi:10.1111/pace.13815.
- Madias, J.E. Low QRS voltage and its causes. Journal of electrocardiology 2008, 41, 498-500, doi:10.1016/j.jelectrocard.2008.06.021.
- Blendea, D.; McPherson, C.A.; Pop, S.; Ruskin, J.N. Isolated very low QRS voltage in the frontal leads predicts recurrence of neurally mediated syncope. Heart Rhythm 2019, doi:10.1016/j.hrthm.2019.06.006.
- Blendea, D.; Cimpeanu, M.; Jelnean, M.; Chiorescu, R.; Crisan, S.; Pop, S. QRS voltage in precordial leads in patients with neurally mediated syncope. European heart journal 2020, 41, doi:10.1093/ehjci/ehaa946.0705.
- Blendea, D.; Ruskin, J.N.; McPherson, C.A. Vectorcardiographic QRS loop geometry in patients with neurally mediated syncope. Eur Heart J 2018, 39, ehy566.P6632 [Abstract].
- Blendea, D.; McPherson, C.; Pop, S.; Blachon, A.; Cimpeanu, M.; Ruskin, J.N. Vectorcardiographic QRS loop geometry predicts recurrence of neurally mediated syncope. J Am Coll Cardiol 2020, 75, 329-329, doi:doi:10.1016/S0735-1097(20)30956-6.
- Tentea, C.-P.; Chiorescu, R.; Crisan, S.; Pop, S.; Ruskin, J.N.; Blendea, D. A Risk Score That Predicts Recurrence of Neurally Mediated Syncope Using Electrocardiographic and Vectorcardiographic Parameters. Circulation 2020, 142, A16337-A16337, doi:doi:10.1161/circ.142.suppl_3.16337.