
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jonathan Hernandez | + 4336 word(s) | 4336 | 2020-12-08 07:58:00 | | | |
| 2 | Rita Xu | -887 word(s) | 3449 | 2020-12-15 04:56:22 | | | | |
| 3 | Rita Xu | Meta information modification | 3449 | 2020-12-15 06:45:55 | | |
Video Upload Options
Windstorm is one of the destructive natural disturbances, but the scale-link extent to which recurrent windstorms influenced forests ecosystems is poorly understood in a changing climate across regions. We reviewed the synergistic impacts of windstorms on forests and assessed research trends and methodological approaches from peer-reviewed articles published from 2000 to 2020 in tropical (TRF), subtropical (SUF), and temperate (TEF) forests/zones, based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Overall, the majority of the reviewed studies were conducted in TRF (i.e., 40%), intermediate in SUF (i.e., 34%), and the lowest in TEF (i.e., 26%). Among the four levels of biological organization, the species-population and community-ecosystem levels had the highest number of study cases, while the molecular-cellular-individual and landscape levels had the lowest study cases in all forest types. Most of the articles reviewed dealt largely on tree mortality/survival and regeneration/succession for TRF, tree mortality/survival and species composition/richness/diversity for SUF, and stem density, gap dynamics, and regeneration/succession for TEF. However, research on the effects of windstorms on mycorrhizal symbioses, population genetics, and physiological adaptation, element fluxes via litterfall, litter decomposition, belowground processes, biological invasion, and tree health are less common in all forest types. Further, most of the studies were conducted in permanent plots but these studies mostly used observational design, while controlled studies are obviously limited. Consequently, more observational and controlled studies are needed on the topic reviewed, particularly studies at the molecular-cellular-individual and landscape levels, to help inform forest management decision-making about developing sustainable and resilient forests amid climate change.
1. Introduction
Nearly all forest ecosystems throughout the world are shaped and influenced by natural disturbances, including cyclonic windstorms (hurricanes, cyclones, and tornadoes). Catastrophic storms cause immediate and long-term structural damage to individual trees, including massive defoliation and branch loss, a decline in stem density, basal area, diameter at breast height (DBH), and total height [1][2]. More specifically, the structure of the forest canopy can be molded by frequent wind disturbance, influencing the biophysical environment, tree physiology, atmospheric exchange, and biotic habitat [3][4]. Windstorms also shape the functional composition of forests by creating selection pressures via increased stem mortality, reduced reproduction, facilitated natural regeneration, and dominance of pioneer species characterized by less dense wood and enhanced plant functional traits (e.g., higher specific leaf area, low seed mass) [5][6]. The forest damage often varies in intensity of windstorms resulting in high variation in tree species and ecosystem damage, secondary succession, and trajectory of forest recovery [7][8].
The profound effects of windstorms on forests had long been studied, but most of these, if not limited to a particular area, had been focused primarily on short-term impacts on individual trees, such as defoliation, branch loss, and canopy disturbance [9][10][11]. Later on, much progress has been reported in explaining visible windstorms’ impacts on forest physical environment, tree regeneration dynamics, and forest recovery in temperate and tropical regions [12][13][14], resulting in the evolution of a contemporary view of windstorms as a multi-scale forest ecological disturbance [15][16][17]. Recently, a study of Lin [18] has shown a scale-link perspective of tropical cyclone ecology, in which the effects of cyclones at the community and ecosystem levels are linked to effects at the individual and species levels. However, the synergistic impacts of catastrophic windstorms on forests across levels of biological organizations has remained poorly understood and relatively unpredictable due to the unresolved issues relevant to the influence of climate change on windstorm characteristics. Windstorms have a significant role in characterizing ecosystem stability and dynamics, whose behavior and nature of damage change with climate change, with storms increasing in intensity and size [19][20]. Climate change affects not just the patterns of precipitation and temperature but also the frequency of cyclonic windstorms, which indirectly influences the forest carbon and emissions globally. The impacts are also aggravated by many interacting meteorological (e.g., wind speed, rainfall, and temperature), topographical (e.g., exposure), and biological factors (e.g., trees species and size), causing a complex alteration in forest function and dynamics across landscapes. Moreover, most of the understandings of tropical cyclones on forests were generally biased towards high latitudes regions, such as Canada, the USA, and New Zealand [21][22][23][24]. Some of these cited studies have also mentioned concerns regarding the methodological approaches for acquiring pre- and post-cyclone data across regions. This knowledge gap limits our understanding of the complex effects of windstorms, particularly on forests structure, species composition, carbon uptake and emissions, and forest productivity in the context of meeting emission targets for greenhouse gases and creating sustainable and resilient forests across geographical regions. Thus, further research about the impacts of windstorms on forests and ecosystems post-disturbance monitoring are needed to enhance our understanding of windstorm ecology amid climate change.
Here we define windstorms as the large-scale wind events, including hurricanes, typhoons, and cyclonic storms characterized by strong winds and heavy precipitation, which greatly influence many aspects of a forest from individual tree growth, tree regeneration, and community structure to ecosystem function [18][25]. Large scale analyses have constantly detected significant factors, including tree species, size, density, and shape, wood traits, soil characteristics, weather, topography, and altitude, influencing windstorm risk of trees and stands [26][27].
2. Synergistic Effects of Catastrophic Windstorms on Forest Across Levels of Biological Organization
2.1. Individual-Level Effects
Generally, the effect of windstorms on forests may start from the effects on individual trees through defoliation, snapping, uprooting, and crown damage (Figure 1). These immediate effects can vary depending on the resistance properties and wood anatomical and mechanical traits of trees [28][29], stem biometrics [30], level of exposure, and tree health [18]. The wood density, for instance, has a strong correlation with tropical cyclone damage, such as defoliation, limb loss, snapping, and uprooting in tropical rainforests in Australia [31]. Wood density is related to the mechanical strength of a stem or wood stiffness; thus, tree species with higher wood density (e.g., >1.39 g cm−3) have shown lower cyclone damage in several countries such as Puerto Rico, Philippines, Taiwan, and Australia [31][32][33][34]. However, there was also evidence that trees with higher wood density (e.g., those with higher stem biomass costs) are less prone to snapping but more susceptible to uprooting, making wood density as an important factor determining windthrow resistance in both tropical and temperate forests [35][36]. This wood trait was also found to negatively correlated to the proportion of defoliated trees after Hurricane Jova impacted a dry tropical forest of the Mexican Pacific coast in 2011 [37]. These strong relationships between wood density and severity of damage observed in TRF and TEF are in contrast to the result reported for subtropical tree species, that is, trees with the highest wood densities of 0.83 g/cm had the lowest survival rate (i.e., 22%) and highest branch loss (i.e., 60%) in an urban forest [38]. This inconsistency may be due to the level of exposure of trees to windstorms in the urban area compared with the natural forest setting. The study of [39] also reported contrasting result, i.e., low-density wood exhibited high resistance to uprooting, which was attributed to less rigidity of trees and significant loss of larger branches. Larger DBH and longer stems of light-demanding pioneer species may be more susceptible to windstorm damage than slender stems of shade-tolerant or low light-demanding trees. To reach the canopy rapidly, pioneer species may invest more in diameter and height growth for short-term gains and competition, thereby investing less in wood tissues for mechanical support (and thus have low wood density and low cost-stems). In contrast, small and intermediate trees growing below the tallest and largest ones may require low light levels, resulting in reduced diameter growth and higher wood density. During windstorms, thinner and denser stems can, thus, reduce the damage by reducing the sail area during short gusts of wind and enabling them to bounce back after having been struck by falling branches [40]. Results of the previous study also stipulated that dense woods have a higher modulus of rupture, which allows wider crowns while maintaining mechanical stability compared with less dense woods [41]. Partly, this can explain the higher mortality rate of trees with > 60 cm DBH observed in most study cases in TRF, SUF, and TEF (Figure 2). Higher mortality rates were reported for pioneer tree species with low wood density in the Luquillo Experimental Forest (LEF) of Puerto Rico as a result of tropical cyclone Hugo [42].
2.2. Species and Population-Level Effects
Depending on the severity of the damage at the individual trees, the more serious effect can be seen at the species and population levels through windstorm-induced tree mortality and changes in stem density, causing a conspicuous influence on population demography or structure (Figure 1). This is because the removal of individuals in the forest area affects the spatial distribution of tree species. Increased tree mortality is one of the most evident effects of windstorms on forests in the form of tree suppression and mechanical damage, which predominantly depends on wind intensity and frequency [43] and tree size and species [44]. Here the majority of the reviewed studies indicated that the most-damaged tree species (both hardwoods and softwoods) decreased in stem density regardless of tree size due to tree mortality. Removal of trees in the forest can alter seed dispersal as influenced by tree distance and localized gap, resulting in fluctuations in stem regeneration rate, time, and stem abundance in the understory. Effects of tree mortality on population structure vary depending on which age, diameter, or height class and species survived or removed in the stand. When most of the small trees were damaged and killed by windstorms, for example, one potential effect is to retain the population of upper age, which tends to be more vulnerable to other causes of death (e.g., lightning, drought, fire, and pathogen attacks) due to old age.
Windstorm disturbance often leads to sudden increases in litterfall. Other reviewed studies attributed the massive litterfall deposition to decreased species diversity and stem abundance, by inhibiting seed germination through chemical and physical effects, such as covering seeds and killing of seedlings that are already present in the forest floor [45]. In this review, 26% of the study cases in TRF reported higher mortality in seedlings to saplings compared with the other DBH classes (Figure 5). Majority of the studies ascribed it not only to resource availability, susceptibility to windstorm damage, tree community and forest ecosystem attributes but also to the damage by falling emergent trees or massive litter deposition, particularly in tropical forests (e.g., [46][47][48][49]). The effects of massive litter accumulation and climatic factors on seedling recruitment were also investigated in a tropical forest, and results showed that, under the canopy, the removal of litter layer increased seedling emergence [50].
Several studies have also suggested that young secondary tropical forest in Puerto Rico experienced lesser damage from a hurricane than that of old secondary forests due to the low and uniform canopy and smaller-DBH trees [45]. Higher mortality in seedlings and saplings was also attributed to torrential rainfall with strong winds, which can wash away and suppress young regeneration in the understory (e.g., [51][52]). This implies that the generalities regarding the trajectory of forest structural changes caused by catastrophic windstorms may be impossible to date due to many interacting factors and, if not too few studies exist, the results of available studies have opposing conclusions. In temperate forests, for instance, the degrees of structural change vary significantly depending on wind intensities, rainfall amount, site biophysical conditions, and tree species characteristics [43][53]. The LEF and the Harvard Forest in Central Massachusetts, which both occasionally subject to the same catastrophic windstorms, are interesting examples of how windstorm damage varies depending on many biotic and abiotic factors in tropical and temperate forests. The LEF experienced greater damage than that of Harvard Forest due to higher susceptibility to wind damage, and that susceptibility was attributed to early leaf senescence, higher topographic exposure, and year-round warmer temperatures and higher precipitation [54].
Moreover, decrease and/or increase in stem density may also alter population genetics (Figure 1) through some indirect processes, including the removal of genetically superior and/or inferior trees [18][55] and spread of fast-growing invasive species [56]. These two processes may determine the number and quality of seedlings that can be recruited into a given area. Removal or decrease in stem density of inferior trees (e.g., poor health due to pathogen infection, abiotic stresses) may lead to the replacement of these trees with fitter ones that are already present at a given site or by genotype migration [57]. A study of [58] reported positive correlations among population size, its fitness and within-population diversity.
2.3. Community and Ecosystem-Level Effects
The effects of catastrophic windstorms at the lower level of biological organization have the potential to affect forest successional patterns, which may also influence the forest species composition and community structure dynamics (Figure 1). Most of the studies reviewed, particularly those conducted at the LEF, reported that plant community establishment in windstorm-disturbed site begins with significant crown damage of adult trees and creation of gaps, followed by early emergence of pioneer species and gradual replacement by shade-tolerant species [5][59][60]. Field and remote sensing data have also shown a positive and strong correlation among windstorm disturbance measures (e.g., gap characteristics and tree mortality) and a fraction of resprouters, floristic composition, and species diversity four years after the catastrophic windstorm in central Amazon forest [61]. Similarly, high stem recruitment in both new and older gaps was reported for a temperate forest due to increased light levels compared with areas under closed canopy [62]. The frequency of occurrence of many understory species also significantly increased in both pine and oak forests, but the frequency of disturbance specialist species was higher in the pine forest 14 years after catastrophic windthrow [63]. A similar observation was observed three years after cyclone Hudah struck Masoala forest in Madagascar, i.e., an increase in the frequency of woody pioneer species (e.g., Trema orientalis Linn. Blume) [64]. When large trees, which are often the mother trees, are removed, the offspring growing in their vicinity may experience a substantial increase in abundance during the first few years following windstorm disturbance based on gap-phase regeneration theory [65][66][67]. The high-light conditions created by fallen trees are considered a critical determinant of seedling establishment and community structure dynamics [68][69]. However, not all windstorm disturbances can cause significant variation in light availability between gaps and non-gaps forest understory due to varying frequency and intensity of windstorms [65]. This can be exemplified in the study of [70] in which the species richness and other diversity indicators have no significant difference before and after the disturbance of hurricane Beta, and was consistent with the study of Imbert and Portecop [71]. Based on the review of Xi et al. [43], we can say that such results may be due to the lower intensity of the hurricane (i.e., Category one) compared with the intensity reported in the other reviewed studies, with categories ranging from three to five. Moreover, species richness decreased in all secondary forests in Puerto Rico, especially shrub species, based on short-term (4–5 years) response data [46]. The author attributed the result to the limitation of pioneer species recruitment in young secondary forest. This is because the changes in species richness in such a forest depend more on the composition of the seedling bank, rather than immediate recruitment of pioneers following wind disturbance [45]. In a young secondary tropical forest, the loss of trees, particularly those under small size classes, may result in the loss of important species that cannot be compensated by the regeneration of early successional ones. However, decrease in stem density due to tree mortality can also stimulate germination of seeds from both external sources and soil seed banks [72], depending on the age, life-history characteristics, land-use history, physical environment, and storm’s timing, speed, and intensity [73]. A recent review, for instance, reported that the regeneration and survival of species after disturbance depend on a species’ life-history traits [74].
Severe defoliation, snapping off of branches, and uprooting also pose significant alterations and/or fluctuations in the carbon and nutrient fluxes due to increased litter inputs in the forest floor (Figure 1). The process begins at improved litter decomposition rate in windstorm-hit areas as a response to a substantial increase in gap size and conspicuous increase in understory light levels [43][75]. Several studies have shown that areas exposed to strong winds, intense rainfall, and high air temperature increased litter decomposition rate (e.g., [76][77]). Coarse woody debris after windstorm disturbance can also provide additional habitat and food for microorganisms, detritivores, and soil insect communities, thereby increasing the rate of litter decomposition. Thus, the massive litter production associated with the effects of windstorms may significantly improve C and nutrient pools in the soil, and nutrient resorption [78][79]. However, massive litterfall does not always result in improved nutrient fluxes as it can also, in some instances, cause inaccessibility and immobilization of nutrients [14][80][81]. This is because plant species in communities differ in wood and leaf traits; hence, they also differ in many aspects including responses to windstorms and rates of decay. Different communities of decomposers (e.g., bacteria) also have contrasting feeding preferences depending on litter quality [82]. Hurricane-induced depositions of litter in Costa Rica’s primary forests have shown a significant reduction in the richness of decomposers of certain genera while selecting only the dominant genera possibly better suited to decompose the litter material [83]. Further, decay rates of dead standing and downed trees depend on many interacting factors, such as log size, local climate, and whether logs are in contact with the ground or saturated [84].
2.4. Landscape-Level Effects
Few studies reported the apparent effect of windstorms on atmospheric carbon dynamics through either biomass destruction or lost carbon sequestration capacity of forested landscapes (Figure 1). Generally, reviewed studies have indicated that severe storms can enhance, increase, or maintain sequestration capacity of forested landscapes via changes in site conditions that are relevant to tree growth (e.g., nutrient inputs, species composition, and rates of recruitments) [23][85][86][87]. This is because forest fragmentation and vegetation cover change caused by windstorms can affect the floristic and functional attributes of tree communities via edge effects, with possible effects on forest landscapes’ total biomass. In this review, a high percentage (30–50%) of the studies reported that trees with DBH larger than 60 cm exhibited high mortality rate and crown deterioration in all forest types (Figure 2). The loss of large trees, being the most vulnerable to wind disturbance and microclimatic changes at forest edges, can negatively affect carbon stock and forests’ sequestration capacity [88][89][90], despite the high proliferation rate of pioneer species at edges [91]. Because these pioneer species are usually short-lived and small-sized, they cannot compensate for the biomass loss of larger trees with a much faster decay rate compared with the time required to gain equivalent biomass from tree growth in tropical and subtropical forests [73][89][92]. In the temperate forest, a few studies have shown that edge creation substantially increased forest biomass due to novel microenvironment conditions near or at forest edges (e.g., [93][94][95]), although some studies have also reported contrasting results (e.g., [87][96]). In cyclonic-and hurricane-zones where major wind disturbance is more frequent, however, some tree communities may increase the abundance of disturbance-resistant and-resilient species which are more adapted to edge effects [86][97], suggesting that the effects on biomass and forest growth may be dependent on the prevailing species composition and species life-history traits.
Studies have estimated the forest biomass loss due to the decrease in NDVI, uprooting of trees, snagging and breaking of tree boles and branches, defoliation, and reduction in leaf area [67][98]. A recent study reported that NDVI in exotic monoculture plantations decreased by >25% and only 12% of the landscape was unaffected by the typhoon, indicating a significant reduction in the green biomass in the landscape [50]. Several similar studies also reported a sudden significant drop in NDVI values after windstorms in the tropical, subtropical and temperate zones (e.g., [99][100][101][102]). The authors attributed it to the absence of native tree seedlings, high fire susceptibility and frequency due to dead trees and poor understory vegetation, and poor resistance to mechanical damage of exotic trees. Field experiments across geographical zones have already shown moderate to strong and significant correlations among green biomass, NDVI values, and percent groundcover [103][104][105].
Moreover, most of the reviewed studies also explained that massive and unsalvaged debris (particularly those calorific logs) can also heighten fire risk and insect outbreak, potentially increasing carbon losses and greenhouse gas emissions at the landscape level. This suggests that carbon sequestration in a windstorm-disturbed landscape may also depend on whether the windstorm debris is burned, completely decomposed, or consumed by insects.
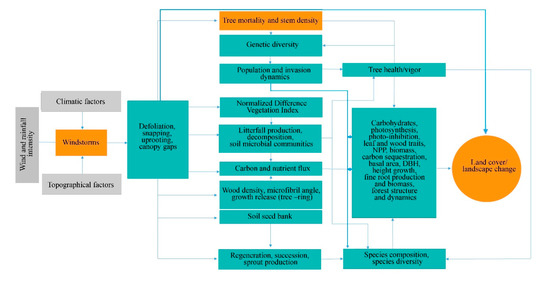
Figure 1. A conceptual diagram of the synergistic effects of windstorms on forest structure, composition, function, and dynamics.
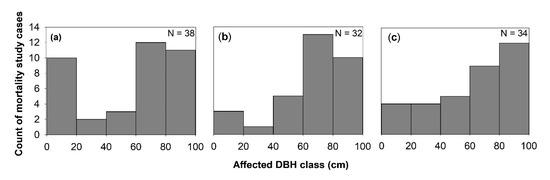
Figure 2. Windstorms effects on mortality across diameter at breast height (DBH) classes in (a) TRF, (b) SUF, and (c) TEF. N is the total number of articles that belong to each forest zone, and only studies that used % mortality were considered.
References
- Henkel, T.K.; Chambers, J.Q.; Baker, D.A. Delayed tree mortality and Chinese tallow (Triadica sebifera) population explosion in a Louisiana bottomland hardwood forest following Hurricane Katrina. Forest Ecol. Manag. 2016, 378, 222–232, doi:10.1016/j.foreco.2016.07.036.
- Vozmishcheva, A.S.; Bondarchuk, S.N.; Gromyko, M.N.; Kislov, D.E.; Pimenova, E.A.; Salo, M.A.; Korznikov, K.A. Strong Disturbance Impact of Tropical Cyclone Lionrock (2016) on Korean Pine-Broadleaved Forest in the Middle Sikhote-Alin Mountain Range, Russian Far East. Forests 2019, 10, 1017, doi:10.3390/f10111017.
- Frazer, G.W.; Wulder, M.A.; Niemann, K.O. Simulation and quantification of the fine-scale spatial pattern and heterogeneity of forest canopy structure: A lacunarity-based method designed for analysis of continuous canopy heights. Forest Ecol. Manag. 2005, 214, 65–90, doi:10.1016/j.foreco.2005.03.056.
- Weishampel, J.F.; Drake, J.B.; Cooper, A.; Blair, J.B.; Hofton, M. Forest canopy recovery from the 1938 hurricane and subsequent salvage damage measured with airborne LiDAR. Remote Sens. Environ. 2007, 109, 142–153, doi:10.1016/j.rse.2006.12.016.
- Scalley, T.H.; Scatena, F.N.; Lugo, A.E.; Moya, S.; Ruiz, C.R.E. Changes in Structure, Composition, and Nutrients During 15 Yr of Hurricane-Induced Succession in a Subtropical Wet Forest in Puerto Rico. Biotropica 2010, 42, 455–463, doi:10.1111/j.1744-7429.2009.00609.x.
- Webb, E.L.; Bult, M.V.D.; Fa’aumu, S.; Webb, R.C.; Tualaulelei, A.; Carrasco, L.R. Factors Affecting Tropical Tree Damage and Survival after Catastrophic Wind Disturbance. Biotropica 2013, 46, 32–41, doi:10.1111/btp.12067.
- Ostertag, R.; Silver, W.L.; Lugo, A.E. Factors affecting mortality and resistance to damage following hurricanes in a rehabilitated subtropical moist forest. Biotropica 2005, 37, 16–24, doi:10.1111/j.1744-7429.2005.04052.x.
- Uriarte, M.; Canham, C.D.; Thompson, J.; Zimmerman, J.K.; Murphy, L.; Sabat, A.M.; Fetcher, N.; Haines, B.L. Natural disturbance and human land use as determinants of tropical forest dynamics: Results from a forest simulator. Monogr. 2009, 79, 423–443, doi:10.1890/08-0707.1.
- Unwin, G.L.; Applegate, G.B.; Stocker, G.C.; Nicholson, D.L. Initial effects of tropical cyclone “Winifred” on forests in north Queensland. Ecol. Soc. Aust. 1988, 15, 283–296.
- Vasquez-Yanes, C.; Orozco-Segovia, A. Seed germination of a tropical rain forest pioneer tree (Heliocarpus donnell-smithii) in response to diurnal fluctuation of temperature. Plant. 1982, 56, 295–298, doi:10.1111/j.1399-3054.1982.tb00341.x.
- Walker, L.R. Tree Damage and Recovery from Hurricane Hugo in Luquillo Experimental Forest, Puerto-Rico. Biotropica 1991, 23, 379–385, doi:10.2307/2388255.
- Tzeng, H.-Y.; Wang, W.; Tseng, Y.-H.; Chiu, C.-A.; Kuo, C.-C.; Tsai, S.-T. Tree mortality in response to typhoon-induced floods and mudslides is determined by tree species, size, and position in a riparian Formosan gum forest in subtropical Taiwan. PLoS ONE 2018, 13, e0190832, doi:10.1371/journal.pone.0190832.
- Xi, W.M.; Peet, R.K.; Decoster, J.K.; Urban, D.L. Tree damage risk factors associated with large, infrequent wind disturbances of Carolina forests. Forestry 2008, 81, 317–334, doi:10.1093/forestry/cpn020.
- Lugo, A.E. Visible and invisible effects of hurricanes on forest ecosystems: An international review. Austral Ecol. 2008, 33, 368–398.
- Ibanez, T.; Keppel, G.; Menkes, C.; Gillespie, T.W.; Lengaigne, M.; Mangeas, M.; Rivas-Torres, G.; Birnbaum, P. Globally consistent impact of tropical cyclones on the structure of tropical and subtropical forests. Ecol. 2019, 107, 279–292, doi:10.1111/1365-2745.13039.
- Lin, K.C.; Hamburg, S.P.; Wang, L.X.; Duh, C.T.; Huang, C.M.; Chang, C.T.; Lin, T.C. Impacts of increasing typhoons on the structure and function of a subtropical forest: Reflections of a changing climate. Rep. 2017, 7, 4911, doi:10.1038/s41598-017-05288-y.
- Peereman, J.; Hogan, J.A.; Lin, T.C. Landscape Representation by a Permanent Forest Plot and Alternative Plot Designs in a Typhoon Hotspot, Fushan, Taiwan. Remote Sens. 2020, 12, 660, doi:10.3390/rs12040660.
- Lin, T.C.; Hogan, J.A.; Chang, C.T. Tropical Cyclone Ecology: A Scale-Link Perspective. Trends Ecol. Evol. 2020, 35, 594–604, doi:10.1016/j.tree.2020.02.012.
- Bender, M.A.; Knutson, T.R.; Tuleya, R.E.; Sirutis, J.J.; Vecchi, G.A.; Garner, S.T.; Held, I.M. Modeled Impact of Anthropogenic Warming on the Frequency of Intense Atlantic Hurricanes. Science 2010, 327, 454–458, doi:10.1126/science.1180568.
- Altman, J.; Dolezal, J.; Cerny, T.; Song, J.S. Forest response to increasing typhoon activity on the Korean peninsula: Evidence from oak tree-rings. Change Biol. 2013, 19, 498–504, doi:10.1111/gcb.12067.
- Fisk, J.P.; Hurtt, G.C.; Chambers, J.Q.; Zeng, H.; Dolan, K.A.; Negron-Juarez, R.I. The impacts of tropical cyclones on the net carbon balance of eastern US forests (1851–2000). Res. Lett. 2013, 8, 045017.
- Kurz, W.A.; Stinson, G.; Rampley, G.J.; Dymond, C.C.; Neilson, E.T. Risk of natural disturbances makes future contribution of Canada’s forests to the global carbon cycle highly uncertain. Natl. Acad. Sci. USA 2008, 105, 1551–1555.
- Uriarte, M.; Papaik, M. Hurricane impacts on dynamics, structure and carbon sequestration potential of forest ecosystems in Southern New England, USA. Tellus A 2007, 59, 19–28, doi:10.3402/tellusa.v59i4.15022.
- Dolan, K.A.; Hurtt, G.C.; Chambers, J.Q.; Dubayah, R.O.; Frolking, S.; Masek, J.G. Using ICESat’s Geoscience Laser Altimeter System (GLAS) to assess large-scale forest disturbance caused by hurricane Katrina. Remote Sens. Environ. 2011, 115, 86–96, doi:10.1016/j.rse.2010.08.007.
- Xi, W.M.; Peet, R.K.; Urban, D.L. Changes in forest structure, species diversity and spatial pattern following hurricane disturbance in a Piedmont North Carolina forest, USA. Plant. Ecol. 2008, 1, 43–57.
- Mayer, P.; Brang, P.; Dobbertin, M.; Hallenbarter, D.; Renaud, J.P.; Walthert, L.; Zimmermann, S. Forest storm damage is more frequent on acidic soils. Forest Sci. 2005, 62, 303–311.
- Peterson, C.J. Within-stand variation in windthrow in southern boreal forests of Minnesota: Is it predictable? J. For. Res. 2004, 34, 365–375, doi:10.1139/x03-257.
- Butler, D.W.; Gleason, S.M.; Davidson, I.; Onoda, Y.; Westoby, M. Safety and streamlining of woody shoots in wind: An empirical study across 39 species in tropical Australia. New Phytol. 2012, 193, 137–149, doi:10.1111/j.1469-8137.2011.03887.x.
- Piermattei, A.; von Arx, G.; Avanzi, C.; Fonti, P.; Gartner, H.; Piotti, A.; Urbinati, C.; Vendramin, G.G.; Buntgen, U.; Crivellaro, A. Functional Relationships of Wood Anatomical Traits in Norway Spruce. Front. Plant Sci. 2020, 11, 683, doi:10.3389/fpls.2020.00683.
- Meyer, F.D.; Paulsen, J.; Korner, C. Windthrow damage in Picea abies is associated with physical and chemical stem wood properties. Trees-Struct. Funct. 2008, 22, 463–473, doi:10.1007/s00468-007-0206-3.
- Curran, T.J.; Brown, R.L.; Edwards, E.; Hopkins, K.; Kelley, C.; McCarthy, E.; Pounds, E.; Solan, R.; Wolf, J. Plant functional traits explain interspecific differences in immediate cyclone damage to trees of an endangered rainforest community in north Queensland. Austral Ecol. 2008, 33, 451–461, doi:10.1111/j.1442-9993.2008.01900.x.
- Uriarte, M.; Thompson, J.; Zimmerman, J.K. Hurricane Maria tripled stem breaks and doubled tree mortality relative to other major storms. Nat. Commun. 2019, 10, 1362, doi:10.1038/s41467-019-09319-2.
- Hogan, J.A.; Zimmerman, J.K.; Thompson, J.; Uriarte, M.; Swenson, N.G.; Condit, R.; Hubbell, S.; Johnson, D.J.; Sun, I.F.; Chang-Yang, C.H.; et al. The Frequency of Cyclonic Wind Storms Shapes Tropical Forest Dynamism and Functional Trait Dispersion. Forests 2018, 9, 404, doi:10.3390/f9070404.
- Gelder, H.A.V.; Poorter, L.; Sterck, F.J. Wood mechanics, allometry, and life-history variation in a tropical rain forest tree community. New Phytol. 2006, 171, 367–378, doi:10.1111/j.1469-8137.2006.01757.x.
- Iida, Y.; Poorter, L.; Sterck, F.J.; Kassim, A.R.; Kubo, T.; Potts, M.D.; Kohyama, T.S. Wood density explains architectural differentiation across 145 co-occurring tropical tree species. Funct. Ecol. 2011, 26, 274–282, doi:10.1111/j.1365-2435.2011.01921.x.
- Ribeiro, G.H.P.M.; Chambers, J.Q.; Peterson, C.J.; Trumbore, S.E.; Marra, D.M.; Wirth, C.; Cannon, J.B.; Negron-Juarez, R.I.; Lima, A.J.N.; de Paula, E.V.C.M.; et al. Mechanical vulnerability and resistance to snapping and uprooting for Central Amazon tree species. For. Ecol. Manag. 2016, 380, 1–10, doi:10.1016/j.foreco.2016.08.039.
- Paz, H.; Vega-Ramos, F.; Arreola-Villa, F. Understanding hurricane resistance and resilience in tropical dry forest trees: A functional traits approach. For. Ecol. Manag. 2018, 426, 115–122, doi:10.1016/j.foreco.2018.03.052.
- Duryea, M.L.; Kampf, E.; Littell, R.C.; Rodriguez-Pedraza, C.D. Hurricanes and the urban forest: Effects on tropical and subtropical tree species. Arboric Urban For. 2007, 33, 98–112.
- Canham, C.D.; Papaik, M.J.; Latty, E.F. Interspecific variation in susceptibility to windthrow as a function of tree size and storm severity for northern temperate tree species. Can. J. For. Res. 2001, 31, 1–10, doi:10.1139/cjfr-31-1-1.
- Siliprandi, N.C.; Nogueira, E.M.; Toledo, J.J.; Fearnside, P.M.; Nascimento, H.E.M. Inter-site variation in allometry and wood density of Goupia glabra Aubl. in Amazonia. Braz. J. Biol. 2016, 76, 268–276, doi:10.1590/1519-6984.22514.
- Niklas, K.J. Plant Biomechanics: An Engineering Approach to Plant Form and Function; University of Chicago: Illinois, IL, USA, 1992.
- Zimmerman, J.K.; Everham, E.M.; Waide, R.B.; Lodge, D.J.; Taylor, C.M.; Brokaw, N.V.L. Responses of Tree Species to Hurricane Winds in Subtropical Wet Forest in Puerto-Rico—Implications for Tropical Tree Life-Histories. J. Ecol. 1994, 82, 911–922, doi:10.2307/2261454.
- Xi, W.M. Synergistic effects of tropical cyclones on forest ecosystems: A global synthesis. J. For. Res. 2015, 26, 1–21, doi:10.1007/s11676-015-0018-z.
- Shibuya, M.; Ishibashi, S. Stand-level windthrow patterns and long-term dynamics of surviving trees in natural secondary stands after a stand-replacing windthrow event. Forestry 2019, 92, 473–480, doi:10.1093/forestry/cpz015.
- Lomascolo, T.; Aide, T.M. Seed and seedling bank dynamics in secondary forests following hurricane Georges in Puerto Rico. Caribb. J. Sci. 2001, 37, 259–270.
- Pascarella, J.B.; Aide, T.M.; Zimmerman, J.K. Short-term response of secondary forests to hurricane disturbance in Puerto Rico, USA. For. Ecol. Manag. 2004, 199, 379–393.
- Fontes, C.G.; Chambers, J.Q.; Higuchi, N. Revealing the causes and temporal distribution of tree mortality in Central Amazonia. For. Ecol. Manag. 2018, 424, 177–183, doi:10.1016/j.foreco.2018.05.002.
- Metcalfe, D.J.; Bradford, M.G.; Ford, A.J. Cyclone damage to tropical rain forests: Species- and community-level impacts. Austral Ecol. 2008, 33, 432–441, doi:10.1111/j.1442-9993.2008.01898.x.
- Sherman, R.E.; Fahey, T.J.; Martinez, P. Hurricane impacts on a mangrove forest in the Dominican Republic: Damage patterns and early recovery. Biotropica 2001, 33, 393–408, doi:10.1646/0006-3606(2001)033[0393:Hioamf]2.0.Co;2.
- Santos, S.L.D.; Válio, I.F. Litter accumulation and its effect on seedling recruitment in a Southeast Brazilian Tropical Forest. Braz. J. Bot. 2002, 25, 89–92, doi:10.1590/s0100-84042002000100011.
- Abbas, S.; Nichol, J.E.; Fischer, G.A.; Wong, M.S.; Irteza, S.M. Impact assessment of a super-typhoon on Hong Kong’s secondary vegetation and recommendations for restoration of resilience in the forest succession. Agr. For. Meteorol. 2020, 280, 107784, doi:10.1016/j.agrformet.2019.107784.
- Wang, Q.; Yu, D.; Li, Z.Q.; Wang, L.G. The Effect of Typhoons on the Diversity and Distribution Pattern of Aquatic Plants on Hainan Island, South China. Biotropica 2008, 40, 692–699, doi:10.1111/j.1744-7429.2008.00430.x.
- Fath, B. Encyclopedia of Ecology; Elsevier: Amsterdam, Netherlands, 2019.
- Boose, E.R.; Serrano, M.I.; Foster, D.R. Landscape and regional impacts of hurricanes in Puerto Rico. Ecol. Monogr. 2004, 74, 335–352, doi:10.1890/02-4057.
- Verbylaite, R.; Pliura, A.; Lygis, V.; Suchockas, V.; Jankauskiene, J.; Labokas, J. Genetic Diversity and Its Spatial Distribution in Self-Regenerating Norway Spruce and Scots Pine Stands. Forests 2017, 8, 470, doi:10.3390/f8120470.
- Murphy, H.T.; Metcalfe, D.J.; Bradford, M.G.; Ford, A.F.; Galway, K.E.; Sydes, T.A.; Westcott, D.J. Recruitment dynamics of invasive species in rainforest habitats following Cyclone Larry. Austral Ecol. 2008, 33, 495–502, doi:10.1111/j.1442-9993.2008.01904.x.
- Alfaro, R.I.; Fady, B.; Vendramin, G.G.; Dawson, I.K.; Fleming, R.A.; Saenz-Romero, C.; Lindig-Cisneros, R.A.; Murdock, T.; Vinceti, B.; Navarro, C.M.; et al. The role of forest genetic resources in responding to biotic and abiotic factors in the context of anthropogenic climate change. For. Ecol. Manag. 2014, 333, 76–87, doi:10.1016/j.foreco.2014.04.006.
- Leimu, R.; Mutikainen, P.; Koricheva, J.; Fischer, M. How general are positive relationships between plant population size, fitness and genetic variation? J. Ecol. 2006, 94, 942–952, doi:10.1111/j.1365-2745.2006.01150.x.
- Monoy, C.C.; Tomlinson, K.W.; Iida, Y.; Swenson, N.G.; Slik, J.W.F. Temporal Changes in Tree Species and Trait Composition in a Cyclone-prone Pacific Dipterocarp Forest. Ecosystems 2016, 19, 1013–1022, doi:10.1007/s10021-016-9983-0.
- Swenson, N.G.; Stegen, J.C.; Davies, S.J.; Erickson, D.L.; Forero-Montana, J.; Hurlbert, A.H.; Kress, W.J.; Thompson, J.; Uriarte, M.; Wright, S.J.; et al. Temporal turnover in the composition of tropical tree communities: Functional determinism and phylogenetic stochasticity. Ecology 2012, 93, 490–499, doi:10.1890/11-1180.1.
- Marra, D.M.; Chambers, J.Q.; Higuchi, N.; Trumbore, S.E.; Ribeiro, G.H.P.M.; dos Santos, J.; Negron-Juarez, R.I.; Reu, B.; Wirth, C. Large-Scale Wind Disturbances Promote Tree Diversity in a Central Amazon Forest. PLoS ONE 2014, 9, 103711, doi:10.1371/journal.pone.0103711.
- Miura, M.; Manabe, T.; Nishimura, N.; Yamamoto, S.I. Forest canopy and community dynamics in a temperate old-growth evergreen broad-leaved forest, south-western Japan: A 7-year study of a 4-ha plot. J. Ecol. 2001, 89, 841–849, doi:10.1046/j.0022-0477.2001.00603.x.
- Palmer, M.W.; McAlister, S.D.; Arevalo, J.R.; DeCoster, J.K. Changes in the understory during 14 years following catastrophic windthrow in two Minnesota forests. J. Veg. Sci. 2000, 11, 841–854, doi:10.2307/3236554.
- Birkinshaw, C.; Randrianjanahary, M. The Effects of Cyclone Hudah on the Forest of Masoala Peninsula, Madagascar. Madag. Conserv. Dev. 2007, 2, doi:10.4314/mcd.v2i1.44125.
- Yao, A.-W.; Chiang, J.-M.; Mcewan, R.; Lin, T.-C. The effect of typhoon-related defoliation on the ecology of gap dynamics in a subtropical rain forest of Taiwan. J. Veg. Sci. 2014, 26, 145–154, doi:10.1111/jvs.12217.
- Kern, C.C.; Montgomery, R.A.; Reich, P.B.; Strong, T.F. Canopy gap size influences niche partitioning of the ground-layer plant community in a northern temperate forest. J. Plant Ecol. 2012, 6, 101–112, doi:10.1093/jpe/rts016.
- Lin, T.C.; Hamburg, S.P.; Lin, K.C.; Wang, L.J.; Chang, C.T.; Hsia, Y.J.; Vadeboncoeur, M.A.; McMullen, C.M.M.; Liu, C.P. Typhoon Disturbance and Forest Dynamics: Lessons from a Northwest Pacific Subtropical Forest. Ecosystems 2011, 14, 127–143.
- Namikawa, K.; Matsui, T.; Kobayashi, M.; Goto, R.; Kuramoto, S. Initial establishment and regeneration processes of an outlying isolated Fagus crenata Blume forest stand in the northernmost boundary of its range in Hokkaido, northern Japan. Plant Ecol. 2010, 207, 161–174.
- Yang, H.; Liu, S.R.; Cao, K.F.; Wang, J.X.; Li, Y.D.; Xu, H. Characteristics of typhoon disturbed gaps in an old-growth tropical montane rainforest in Hainan Island, China. J. For. Res. 2017, 28, 1231–1239.
- Ruiz, J.; Fandino, M.C. The Impact of Hurricane Beta on the Forests of Providencia Island, Colombia, Southwest Caribbean. Caldasia 2010, 32, 425–434.
- Imbert, D.; Portecop, J. Hurricane disturbance and forest resilience: Assessing structural vs. functional changes in a Caribbean dry forest. For. Ecol. Manag. 2008, 255, 3494–3501.
- Shang, K.-K.; Zhang, Q.-P.; Da, L.-J.; Hara, K.; Yang, Y.-C.; Fujihara, M.; Tomita, M.; Zhao, Y. Effects of natural and artificial disturbance on landscape and forest structure in Tiantong National Forest Park, East China. Landsc. Ecol. Eng. 2011, 10, 163–172, doi:10.1007/s11355-010-0148-6.
- Chapman, E.L.; Chambers, J.Q.; Ribbeck, K.F.; Baker, D.B.; Tobler, M.A.; Zeng, H.C.; White, D.A. Hurricane Katrina impacts on forest trees of Louisiana’s Pearl River basin. For. Ecol. Manag. 2008, 256, 883–889, doi:10.1016/j.foreco.2008.05.057.
- Petrokas, R.; Baliuckas, V.; Manton, M. Successional Categorization of European Hemi-boreal Forest Tree Species. Plants 2020, 9, 1381, doi:10.3390/plants9101381.
- Bellingham, P.J.; Tanner, E.V.J.; Healey, J.R. Hurricane disturbance accelerates invasion by the alien tree Pittosporum undulatum in Jamaican montane rain forests. J. Veg. Sci. 2005, 16, 675–684.
- Salinas, N.; Malhi, Y.; Meir, P.; Silman, M.; Cuesta, R.R.; Huaman, J.; Salinas, D.; Farfan, F. The sensitivity of tropical leaf litter decomposition to temperature: Results from a large-scale leaf translocation experiment along an elevation gradient in Peruvian forests. New Phytol. 2010, 189, 967–977, doi:10.1111/j.1469-8137.2010.03521.x.
- Park, B.B.; Rahman, A.; Han, S.H.; Youn, W.B.; Hyun, H.J.; Hernandez, J.; An, J.Y. Carbon and Nutrient Inputs by Litterfall in Evergreen and Deciduous Forests in Korea. Forests 2020, 11, 143, doi:10.3390/f11020143.
- Martinez-Yrizar, A.; Jaramillo, V.J.; Maass, M.; Burquez, A.; Parker, G.; Alvarez-Yepiz, J.C.; Araiza, S.; Verduzco, A.; Sarukhan, J. Resilience of tropical dry forest productivity to two hurricanes of different intensity in western Mexico. For. Ecol. Manag. 2018, 426, 53–60, doi:10.1016/j.foreco.2018.02.024.
- Bloem, S.J.V.; Murphy, P.G.; Lugo, A.E.; Ostertag, R.; Costa, M.R.; Bernard, I.R.; Colon, S.M.; Mora, M.C. The Influence of Hurricane Winds on Caribbean Dry Forest Structure and Nutrient Pools1. Biotropica 2005, 37, 571–583, doi:10.1111/j.1744-7429.2005.00074.x.
- Cordova, S.C.; Olk, D.C.; Dietzel, R.N.; Mueller, K.E.; Archontouilis, S.V.; Castellano, M.J. Plant litter quality affects the accumulation rate, composition, and stability of mineral-associated soil organic matter. Soil Biol. Biochem. 2018, 125, 115–124, doi:10.1016/j.soilbio.2018.07.010.
- Beard, K.H.; Vogt, K.A.; Vogt, D.J.; Scatena, F.N.; Covich, A.P.; Sigurdardottir, R.; Siccama, T.G.; Crowl, T.A. Structural and functional responses of a subtropical forest to 10 years of hurricanes and droughts. Ecol. Monogr. 2005, 75, 345–361, doi:10.1890/04-1114.
- Bonanomi, G.; Cesarano, G.; Lombardi, N.; Motti, R.; Scala, F.; Mazzoleni, S.; Incerti, G. Litter chemistry explains contrasting feeding preferences of bacteria, fungi, and higher plants. Sci. Rep. 2017, 7, 9208, doi:10.1038/s41598-017-09145-w.
- Eaton, W.D.; McGee, K.M.; Alderfer, K.; Jimenez, A.R.; Hajibabaei, M. Increase in abundance and decrease in richness of soil microbes following Hurricane Otto in three primary forest types in the Northern Zone of Costa Rica. PLoS ONE 2020, 15, doi:10.1371/journal.pone.0231187.
- Vanderwel, M.C.; Malcolm, J.R.; Smith, S.M. An integrated model for snag and downed woody debris decay class transitions. For. Ecol. Manag. 2006, 234, 48–59, doi:10.1016/j.foreco.2006.06.020.
- McNulty, S.G. Hurricane impacts on US forest carbon sequestration. Environ. Pollut. 2002, 116, S17–S24, doi:10.1016/S0269-7491(01)00242-1.
- Laurance, W.F.; Curran, T.J. Impacts of wind disturbance on fragmented tropical forests: A review and synthesis. Austral Ecol. 2008, 33, 399–408, doi:10.1111/j.1442-9993.2008.01895.x.
- Smith, I.A.; Hutyra, L.R.; Reinmann, A.B.; Marrs, J.K.; Thompson, J.R. Piecing together the fragments: Elucidating edge effects on forest carbon dynamics. Front. Ecol. Environ. 2018, 16, 213–221, doi:10.1002/fee.1793.
- Fauset, S.; Johnson, M.O.; Gloor, M.; Baker, T.R.; Monteagudo, A.; Brienen, R.J.W.; Feldpausch, T.R.; Lopez-Gonzalez, G.; Malhi, Y.; ter Steege, H.; et al. Hyperdominance in Amazonian forest carbon cycling. Nat. Commun. 2015, 6, 6857, doi:10.1038/ncomms7857.
- Laurance, W.F.; Nascimento, H.E.; Laurance, S.G.; Andrade, A.C.; Fearnside, P.M.; Ribeiro, J.E.; Capretz, R.L. Rain forest fragmentation and the proliferation of successional trees. Ecology 2006, 87, 469–482, doi:10.1890/05-0064.
- Melito, M.; Metzger, J.P.; Oliveira, A.A. Landscape-level effects on aboveground biomass of tropical forests: A conceptual framework. Glob. Change Biol. 2017, 24, 597–607, doi:10.1111/gcb.13970.
- Michalski, F.; Nishi, I.; Peres, C.A. Disturbance-mediated drift in tree functional groups in Amazonian forest fragments. Biotropica 2007, 39, 691–701, doi:10.1111/j.1744-7429.2007.00318.x.
- Chambers, J.Q.; Fisher, J.I.; Zeng, H.C.; Chapman, E.L.; Baker, D.B.; Hurtt, G.C. Hurricane Katrina’s carbon footprint on U. S. Gulf Coast forests. Science 2007, 318, 1107–1107, doi:10.1126/science.1148913.
- Briber, B.M.; Hutyra, L.R.; Reinmann, A.B.; Raciti, S.M.; Dearborn, V.K.; Holden, C.E.; Dunn, A.L. Tree Productivity Enhanced with Conversion from Forest to Urban Land Covers. PLoS ONE 2015, 10, e0136237, doi:10.1371/journal.pone.0136237.
- Reinmann, A.B.; Hutyra, L.R. Edge effects enhance carbon uptake and its vulnerability to climate change in temperate broadleaf forests. Proc. Natl. Acad. Sci. USA 2017, 114, 107–112, doi:10.1073/pnas.1612369114.
- Remy, E.; Wuyts, K.; Boeckx, P.; Gundersen, P.; Verheyen, K. Edge effects in temperate forests subjected to high nitrogen deposition. Proc. Natl. Acad. Sci. USA 2017, 114, E7032, doi:10.1073/pnas.1709099114.
- Chen, J.Q.; Franklin, J.F.; Spies, T.A. Vegetation Responses to Edge Environments in Old-Growth Douglas-Fir Forests. Ecol. Appl. 1992, 2, 387–396, doi:10.2307/1941873.
- Moran, C.; Catterall, C.P.; Green, R.J.; Olsen, M.F. Functional variation among frugivorous birds: Implications for rainforest seed dispersal in a fragmented subtropical landscape. Oecologia 2004, 141, 584–595, doi:10.1007/s00442-004-1685-1.
- Lee, M.F.; Lin, T.C.; Vadeboncoeur, M.A.; Hwong, J.L. Remote sensing assessment of forest damage in relation to the 1996 strong typhoon Herb at Lienhuachi Experimental Forest, Taiwan. For. Ecol. Manag. 2008, 255, 3297–3306, doi:10.1016/j.foreco.2008.02.010.
- Hu, T.G.; Smith, R.B. The Impact of Hurricane Maria on the Vegetation of Dominica and Puerto Rico Using Multispectral Remote Sensing. Remote Sens. 2018, 10, 827, doi:10.3390/rs10060827.
- Battles, J.J.; Cleavitt, N.L.; Saah, D.S.; Poling, B.T.; Fahey, T.J. Ecological impact of a microburst windstorm in a northern hardwood forest. Can. J. For. Res. 2017, 47, 1695–1701, doi:10.1139/cjfr-2017-0206.
- Bhowmik, A.K.; Cabral, P. Cyclone Sidr Impacts on the Sundarbans Floristic Diversity. Earth Sci. Res. 2013, 2, 62, doi:10.5539/esr.v2n2p62.
- Bianchette, T.A.; Liu, K.B.; Lam, N.S.N.; Kiage, L.M. Ecological Impacts of Hurricane Ivan on the Gulf Coast of Alabama: A Remote Sensing Study. J. Coast. Res. 2009, 2, 1622–1626.
- Prabhakara, K.; Hively, W.D.; McCarty, G.W. Evaluating the relationship between biomass, percent groundcover and remote sensing indices across six winter cover crop fields in Maryland, United States. Int. J. Appl. Earth Obs. 2015, 39, 88–102, doi:10.1016/j.jag.2015.03.002.
- Rahetlah, V.B.; Andrianarisoa, B.; Salgado, P.; Tillard, T.; Razafindrazaka, H.; Le Mézo, L.; Ramalanjaona, V.L. Relationship between normalized difference vegetation index (NDVI) and forage biomass yield in the Vakinankaratra region, Madagascar. Lives Res. Rural Dev. 2014, 26, 11.
- Lewis, R.J.; Bannar-Martin, K.H. The Impact of Cyclone Fanele on a Tropical Dry Forest in Madagascar. Biotropica 2011, 44, 135–140, doi:10.1111/j.1744-7429.2011.00799.x.




