
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giorgia Ailuno | + 2219 word(s) | 2219 | 2021-09-10 09:55:01 | | | |
| 2 | Catherine Yang | -56 word(s) | 2163 | 2021-09-13 04:53:58 | | |
Video Upload Options
Many modern therapeutic approaches are based on precise diagnostic evidence, where imaging procedures play an essential role. To date, in the diagnostic field, a plethora of agents have been investigated to increase the selectivity and sensitivity of diagnosis. However, the most common drawbacks of conventional imaging agents reside in their non-specificity, short imaging time, instability, and toxicity. Moreover, routinely used diagnostic agents have low molecular weights and consequently a rapid clearance and renal excretion, and this represents a limitation if long-lasting imaging analyses are to be conducted. Thus, the development of new agents for in vivo diagnostics requires not only a deep knowledge of the physical principles of the imaging techniques and of the physiopathological aspects of the disease but also of the relative pharmaceutical and biopharmaceutical requirements. In this scenario, skills in pharmaceutical technology have become highly indispensable in order to respond to these needs.
1. Introduction
In the past, medical imaging was usually subdivided into anatomic and functional techniques, depending on their acquisition modalities and type of information produced. Anatomic imaging includes diagnostic techniques able to locate and delineate structural changes, whereas functional imaging involves imaging modalities that provide information on the functionality and metabolic processes of organs and tissues. Improved knowledge of disease mechanisms through genomics, proteomics, and animal modeling, along with advances in chemistry and molecular biology, has led to the introduction of a new field of imaging, known as molecular imaging. This type of medical imaging is defined as the ability to visualize and quantitatively measure the function of biological and cellular processes in vivo, including information on the receptor number, signal transduction aberrations, and enzymatic activities. Molecular imaging methods generally exploit radiolabeled agents with specific physical and chemical properties to detect biochemical markers or enzymatic reactions. While anatomical imaging plays a major role for diagnosis, surgical guidance, treatment monitoring, and follow up, molecular imaging aims to achieve earlier diagnosis and patient-specific therapeutic treatment (personalized medicine).
All the imaging techniques described in the previous section are affected by different limitations. For example, optical imaging has an associated high sensitivity and spatial resolution, but it suffers from limited tissue depth penetration (typically less than 1 cm of tissue); PET is a highly sensitive radionuclide-based imaging technique, but it is spatially limited in resolution; MRI and CT are comparatively insensitive methods of imaging but are convenient techniques and are characterized by high spatial resolution. Moreover, most of the molecular imaging agents currently used in clinical practice have a very short blood circulation time (<30 min) due to their small size and non-specific biodistribution. Hence, the development of high-contrast targeted imaging agents, with controllable circulation and biodistribution characteristics, is of fundamental importance to overcome these limitations.
In order to overcome the shortcomings of individual imaging modalities, multimodal imaging techniques have been developed [1]. Fusing the molecular information related to the disease with anatomical or functional information provides accurate knowledge of pathophysiology, combined with higher sensitivity and specificity [2]. PET/MRI, PET/CT, SPECT/CT, and MRI/optical fluorescence are examples of this type of multimodal imaging.
2. Main Technological Strategies and Examples of Imaging Agents Developed for Improving Diagnostic Efficiency
To improve diagnostic efficiency, several strategies, such as chemical modification, the development of prodrugs/bio-precursors, and the development of conjugates with molecules/antibodies/proteins/peptides/nanoparticles, have been explored.
Radiotracers are faced with challenges to preserve their stability in vivo, cross biological barriers, and reach the target organ, tissue, cell, or subcellular organelles in order to avoid false-positive in terms of imaging and give a correct diagnosis of a disease.
A widely investigated strategy to improve the outcomes of imaging procedures is active targeting. For example, The group of Caviglioli at the University of Genoa developed two novel VCAM-1 targeted radiopharmaceuticals [3], based on the VCAM-1-binding peptide having the sequence VHPKQHRGGSKGC, which was previously discovered by in vivo phage display selection [4]. The novel imaging agents, which are able to specifically and selectively bind this adhesion protein, might be exploited for the early diagnosis of a number of inflammation-related pathologies such as atherosclerosis, some neurodegenerative diseases (such as multiple sclerosis and Alzheimer’s disease), and several types of cancer. The first radiopharmaceutical synthesized by the group, named MacroP, is based on a maleimidomonoamide DOTA derivative, having a maleimide moiety exploitable for conjugation
reactions with the thiol groups of peptides and proteins: indeed, this molecule was directly conjugated with the thiol group of the cysteine of the aforementioned VCAM-1 binding peptide. The second radiopharmaceutical, named NAMP, was conceived to be exploited in a flexible three-step pre-targeting system based on the avidin/biotin high-affinity complex [5]. In the three-step pre-targeting procedure, NAMP, a biotin derivative of the same VCAM-1-binding peptide mentioned above, would be injected first; the second step would involve the administration of avidin or its derivatives streptavidin or NeutrAvidinTM, strongly binding NAMP biotin moiety; finally, a biotinylated radiotracer able to bind avidin would be injected. Both MacroP and NAMP were thoroughly characterized and identified by mass spectrometry, and, after labeling with 68Ga, preliminary in vitro tests were performed on TNF- activated human umbilical vein endothelial cells (HUVEC), evidencing that both radiopharmaceuticals retained the VCAM-1 binding ability, with higher radiosignal uptake from TNF- activated cells compared with non-activated control cells. However, even if both compounds resulted in promising PET radiotracers for VCAM-1 imaging, NAMP exhibited its superiority in the labeling procedure yield and PET sensitivity.
Different types of NPs are emerging as powerful tools for molecular imaging.To overcome the limitations of therapies currently used in clinics to treat HER2-positive breast cancer, Rainone and his group [6] explored the potential theranostic use of fluorescent SiNP-TZ directly labeled with 99m Tc on the histidine residues of Hc-TZ and loaded with the anticancer drug doxorubicin (Dox). The authors prepared two formulations characterized by SiNP:Hc-TZ ratios of 1:2 and 1:8, investigating the effect of the different targeting agent density on the NP surface; radiolabeling was performed by incubating [99m Tc(H 2O) 3(CO) 3] + with a suspension of SiNP-TZ, obtaining an RCP of 19.8% by HPLC analysis. An ex vivo biodistribution study on a HER2 + mouse model employing 99m Tc-SiNP-TZ with different NP:targeting agent ratios revealed that the 1:8 ratio led to higher tumor uptake, as confirmed by fluorescent microscopy. Moreover, an in vivo preliminary study was performed on SK-BR-3 tumor-bearing mice to evaluate the distribution kinetics of 99m Tc-SiNP-TZ (1:8) through SPECT, showing rapid radioactivity accumulation in the tumor after 1 h, remarkably increasing at 4 h. Regarding the therapeutic purpose, Dox was externally grafted on the surface of non-radiolabeled SiNP-TZ by exploiting an IPTES-Dox complex [7], obtaining a loading of 1.5–1.6%, assayed by UV-Vis spectrophotometry. The NP were characterized for their size by DLS, evidencing an increase of both the mean hydrodynamic diameter and PDI, compared to SiNP-TZ (83.4 ± 17.3 nm and 76.97 ± 4.97 nm, respectively; 0.114 ± 0.020 and 0.384 ± 0.009, respectively). SK-BR-3 cells incubated with Dox-SiNP-TZ exhibited higher drug uptake compared to the controls (HER2 - cells and non-targeted Dox-loaded SiNP). In vivo optical imaging observation of Dox-SiNP-TZ injected in breast cancer xenografted mice evidenced a Dox accumulation in the tumor comparable to Caelyx ® , which is a commercially available liposomal formulation of Dox. Moreover, the authors observed higher treatment efficacy in vivo, which was measured as tumor volume regression and tumor growth inhibition, in mice receiving Dox- SiNP-TZ compared to Caelyx ® . Finally, investigations on the proteomic profiling and treatment response suggested a potential benefit of DOX-SiNP-TZ for therapeutic application in HER2 + breast cancer.
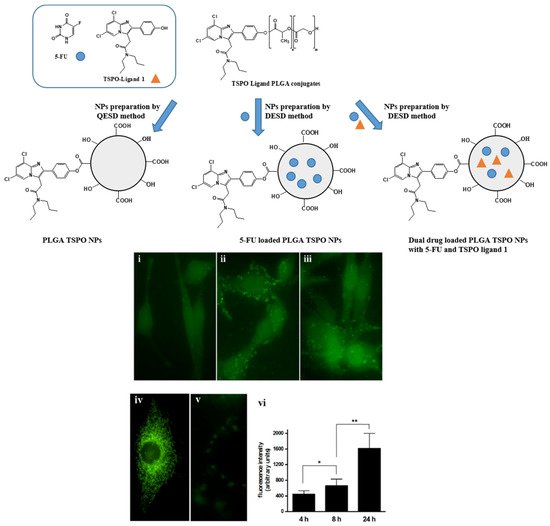
Laquintana et al. [7] described the preparation of NPs employing a TSPO ligand−PLGA conjugated (PLGA−TSPO) polymer. Specifically, the PLGA–TSPO NPs ( Figure 1 ) were prepared by a quasi-emulsion solvent diffusion procedure (QESD). In order to demonstrate the ability of TSPO targeted NPs to be internalized by cells in vitro, PLGA hydroxylic end groups were conjugated to the fluorescent probe FITC, obtaining fluorescent FITC−PLGA NPs and FITC−TSPO-PLGA−NPs. The internalization of FITC−PLGA−TSPO NPs into rat C6 glioma cells was investigated by fluorescence microscopy. As shown in Figure 1 (panels i−iii, vi), cells treated with 0.2 μM FITC−PLGA−TSPO NPs (concentration is referred to FITC) revealed a time-dependent increase of internalized green fluorescence that widely diffused in the cytosol of the cells. By contrast, no green fluorescence was imaged when cells were incubated in the presence of FITC (7 μM) (panel v), thus demonstrating that the internalized fluorescence of the cells was exclusively due to the cellular uptake of the FITC-conjugated probe. In addition, by comparing uptake studies conducted with FITC−TSPO NPs, FITC NPs, or FITC−TSPO NPs in combination with TSPO ligand 1, no significant differences were observed (data not shown), proving that, as expected, the TSPO moiety does not influence the uptake of the NPs inside cells. Since TSPO is a subcellular target, once ligands are internalized by the cells, they can bind the mitochondrial protein, inducing apoptosis.
Similarly, Lopalco et al. [8] prepared nanogels (NGs) made with TSPO ligand dextran conjugates (TSPO-Dex) and demonstrated their utility as potential delivery systems of two TSPO ligands as apoptotic agents. The internalization of TSPO ligand–dextran NGs into C6 glioma cells was studied in vitro by fluorescence microscopy. On this purpose, fluorescent TSPO ligand–dextran NGs were prepared using FITC–Dextran and TSPO–FITC–Dex NGs. Cells incubated with 5 μM TSPO–FITC– Dex NGs showed a widely diffused green fluorescence in the cytosol after 24 h. The fluorescence in cells was exclusively due to the cellular uptake of TSPO–FITC–Dex NGs because no green fluorescence was imaged when cells were incubated in the presence of 5 μM FITC–Dextran.
3. Translational Research
A key role in the development of new diagnostic tools is played by the translational research. The translational medical research includes all the studies aiming to bridge the gap between the laboratory discoveries and the clinical application, speeding up the transfer of scientific advances into clinical practice [9], “from the bench to bedside”. Indeed, translational medicine is a precision medicine that is capable of bringing the right treatment to the right patient.
Currently, an ambitious Italian project, the ACC-GerSom project of the Fondazione Policlinico Universitario “A. Gemelli”, has been proposed as a new model of translational development [9]. The project, coordinating the work of laboratories of different scientific institutes for research, hospitalization, and healthcare, is finalized to genomic diagnostics of different types of oncology disease and, in particular, it is addressed to patients with breast, ovarian, and colon cancer [10].
Another pivotal Italian research institution, IIT—European Institute of Oncology, has launched a wide translational research program focused on the development of new diagnostic tools for cancer. The Novel Diagnostics Program aims at exploiting the plentiful data stemming from “omics” projects to identify and develop new clinical markers for diagnosis and therapy response prediction in breast, prostate, bladder, and lung cancer patients. Recent results have been published by Dama et al. [11].
The overmentioned examples support the idea that the integrated work involving experts of different fields of the medical area will be decisive in the translation of the formulations described throughout this review into clinically used diagnostic tools.
4. Conclusions
Novel strategies to improve the imaging outcomes, leading to more precise and safe diagnostic procedures, are currently needed, and many Italian research groups offer a wide variety of examples in the field of imaging innovation.
Considering the works discussed in this review, it is evident that among the various applications, most attention is dedicated to the development of tools for cancer diagnosis.
Nanoparticulate systems seem to be among the most investigated because of their versatility and the possibility of being exploited for developing theranostic agents. Indeed, Italian research groups developed NPs as imaging agents detectable through different imaging techniques: in particular, nanosystems for PET detection (such as 68Ga-labeled carboxymethylcellulose NPs, 18F-labeled TSPO ligands, albumin and curcumin derivatives labeled with different radionuclides) are among the most studied, due to the high sensitivity characterizing this technique.
Moreover, combined imaging tools for multimodal imaging, such as the iron oxide NPs, SiNPs, and Fe-Au nanoalloys reported in the review, seem the most promising for future development. In fact, these multimodal systems, allowing to overcome and compensate for the limitations characterizing the clinically available detection techniques, might greatly increase the efficiency and quality of diagnostic outcomes. Indeed, the field of multimodal imaging represents an attractive area of research for many Italian groups.
References
- Torresan, V.; Forrer, D.; Guadagnini, A.; Badocco, D.; Pastore, P.; Casarin, M.; Selloni, A.; Coral, D.; Ceolin, M.; Fernández van Raap, M.B.; et al. 4D Multimodal Nanomedicines Made of Nonequilibrium Au–Fe Alloy Nanoparticles. ACS Nano 2020, 14, 12840–12853.
- Huang, W.Y.; Davis, J.J. Multimodality and nanoparticles in medical imaging. Dalt. Trans. 2011, 40, 6087–6103.
- Pastorino, S.; Baldassari, S.; Ailuno, G.; Zuccari, G.; Drava, G.; Petretto, A.; Cossu, V.; Marini, C.; Alfei, S.; Florio, T.; et al. Two Novel PET Radiopharmaceuticals for Endothelial Vascular Cell Adhesion Molecule-1 (VCAM-1) Targeting. Pharmaceutics 2021, 13, 1025.
- Kelly, K.A.; Nahrendorf, M.; Yu, A.M.; Reynolds, F.; Weissleder, R. In vivo phage display selection yields atherosclerotic plaque targeted peptides for imaging. Mol. Imaging Biol. 2006, 8, 201–207.
- Caviglioli, G.; Baldassari, S.; Zuccari, G.; Pastorino, S.; Florio, T.; Sambuceti, M.; Ailuno, G. Compounds and Methods for Detecting Early Atherosclerotic Lesions in Blood. Vessels. Patent WO2019175019A1, 19 September 2019.
- Rainone, P.; De Palma, A.; Sudati, F.; Roffia, V.; Rigamonti, V.; Salvioni, L.; Colombo, M.; Ripamonti, M.; Spinelli, A.E.; Mazza, D.; et al. 99mTc-radiolabeled silica nanocarriers for targeted detection and treatment of HER2-positive breast cancer. Int. J. Nanomed. 2021, 16, 1943–1960.
- Riva, B.; Bellini, M.; Corvi, E.; Verderio, P.; Rozek, E.; Colzani, B.; Avvakumova, S.; Radeghieri, A.; Rizzuto, M.A.; Morasso, C.; et al. Impact of the strategy adopted for drug loading in nonporous silica nanoparticles on the drug release and cytotoxic activity. J. Colloid Interface Sci. 2018, 519, 18–26.
- Laquintana, V.; Denora, N.; Lopalco, A.; Lopedota, A.; Cutrignelli, A.; Lasorsa, F.M.; Agostino, G.; Franco, M. Translocator Protein Ligand–PLGA Conjugated Nanoparticles for 5-Fluorouracil Delivery to Glioma Cancer Cells. Mol. Pharm. 2014, 11, 859–871.
- Lopalco, A.; Cutrignelli, A.; Denora, N.; Perrone, M.; Iacobazzi, R.M.; Fanizza, E.; Lopedota, A.; Depalo, N.; De Candia, M.; Franco, M.; et al. Delivery of proapoptotic agents in glioma cell lines by TSPO ligand–dextran nanogels. Int. J. Mol. Sci. 2018, 19, 1155.
- De Maria Marchiano, R.; Di Sante, G.; Piro, G.; Carbone, C.; Tortora, G.; Boldrini, L.; Pietragalla, A.; Daniele, G.; Tredicine, M.; Cesario, A.; et al. Translational research in the era of precision medicine: Where we are and where we will go. J. Pers. Med. 2021, 11, 216.
- Laghi, L.; Ricciardiello, L. The changing approach for identifying hereditary colorectal cancer syndromes. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 593–594.
- Rainone, P.; De Palma, A.; Sudati, F.; Roffia, V.; Rigamonti, V.; Salvioni, L.; Colombo, M.; Ripamonti, M.; Spinelli, A.E.; Mazza, D.; et al. 99mTc-radiolabeled silica nanocarriers for targeted detection and treatment of HER2-positive breast cancer. Int. J. Nanomed. 2021, 16, 1943–1960.




