| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Kazumasa Wakamatsu | + 2656 word(s) | 2656 | 2020-09-08 10:01:28 | | | |
| 2 | Vicky Zhou | -1336 word(s) | 1320 | 2020-09-10 11:40:01 | | | | |
| 3 | Vicky Zhou | -1301 word(s) | 1355 | 2020-09-10 11:45:57 | | | | |
| 4 | Vicky Zhou | Meta information modification | 1355 | 2020-09-10 11:51:41 | | | | |
| 5 | Vicky Zhou | -13 word(s) | 1342 | 2020-09-10 12:01:14 | | | | |
| 6 | Vicky Zhou | -13 word(s) | 1342 | 2020-09-10 12:06:34 | | | | |
| 7 | Vicky Zhou | Meta information modification | 1342 | 2020-10-27 09:40:42 | | | | |
| 8 | Lily Guo | Meta information modification | 1342 | 2021-02-07 04:50:09 | | |
Video Upload Options
Melanoma is a malignant tumor that originates from melanocytes. Although melanoma mainly occurs in the skin (cutaneous melanoma), it can also occur in the eyes (uveal melanoma), gastrointestinal tract, oral mucosa and genital tract (mucosal melanoma) [1][2][3][4][5].
1. Introduction
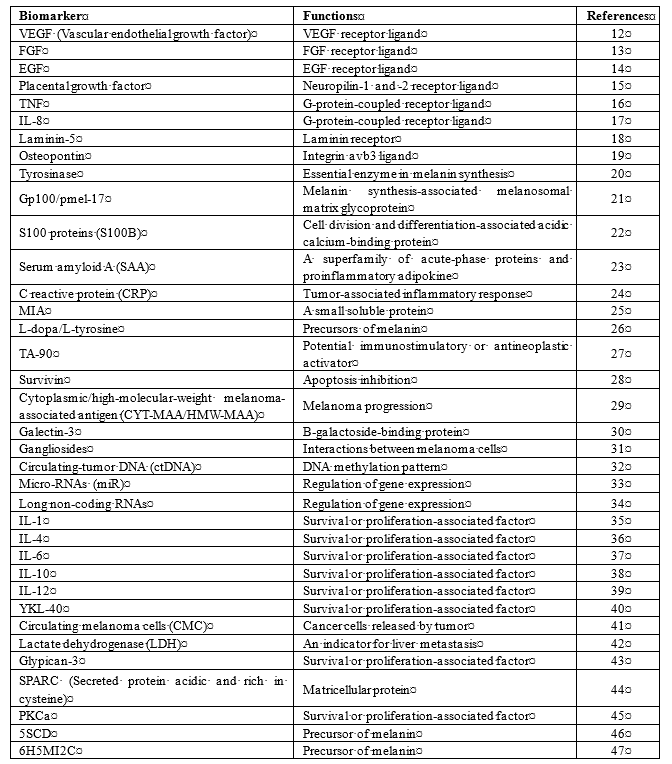
The incidence of melanoma has risen at an increasing global rate per year at 2–7% annually [6]. The risk of developing cutaneous melanoma is known to be higher in Caucasian than in Asian or African subjects, which indicates that the development of cutaneous melanoma is closely related to skin pigmentation since melanin, especially eumelanin, has been shown to have a protective function against UV-induced mutagenesis in the skin [7][8][9]. Several important risk factors that have been linked to the development of melanoma have been identified, including environmental factors, such as exposure to ultraviolet (UV) radiation, and host factors, such as family history [10][11]. As melanoma is one of the most serious and malignant cancers worldwide, efficient biomarkers are needed for early detection, efficient monitoring of the disease, and reliable prediction of survival and recurrence. A number of potentially useful melanoma biomarkers have been reported [12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47](Table 1). Among those, 5-S-cysteinyldopa (5SCD) is one of the most widely used and well-known melanoma biomarkers, especially in Japan.
Table 1. Serum and urinary biomarkers for melanoma
 2. 5SCD as a Marker of Melanoma Progression
2. 5SCD as a Marker of Melanoma Progression
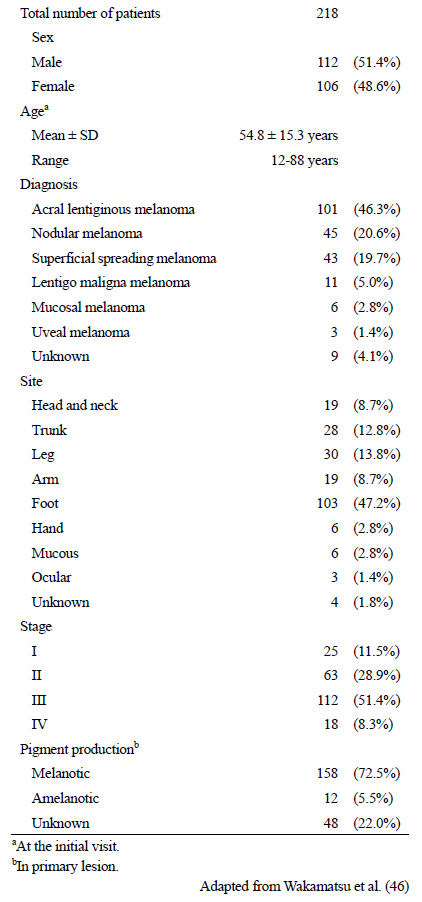
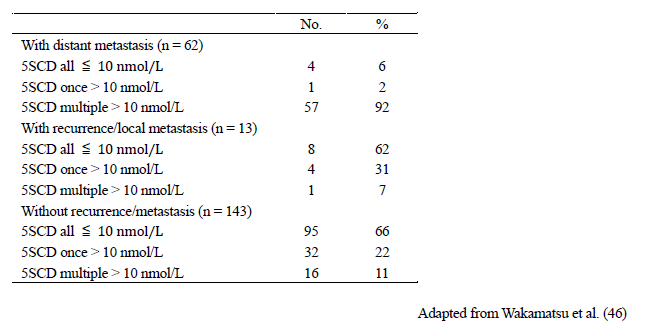
Serum levels of 5SCD were measured in 2648 samples taken from 218 Japanese melanoma patients in order to evaluate the usefulness of 5SCD as a progression marker of melanoma [46] (Tables 2 and 3). Levels of 5SCD were significantly elevated above the upper limit of the normal range (10 nmol/L) in stage IV melanoma patients, which suggested that elevated 5SCD values might be regarded as a sign of metastatic melanoma. This study included the largest number of patients and samples used to evaluate the clinical significance of serum levels of 5SCD, except for a report by Bánfalvi et al. in which 252 patients were examined [48]. The sensitivity of elevated serum 5SCD values (>10 nmol/L) to detect distant metastasis (stage IV) was 73% in 62 patients with metastatic melanoma, and the specificity in identifying the absence of distant metastasis was 98% in 156 patients with no apparent distant metastases. The sensitivity was improved to 77% when cases of amelanotic melanoma were excluded. Serum levels of 5SCD before surgical excision of tumors were determined for 141 patients [46]. The mean 5SCD value for those 141 patients was 15.7 nmol/L. The median 5SCD values for stage IV melanoma patients was significantly higher than healthy subjects and patients at melanoma stages I–III (p < 0.001). Serum levels of 5SCD were found to increase in a stage-dependent manner, especially for stage IV patients who had particularly high values. Stage IV patients with normal values tended to have amelanotic tumors, skin metastases only or hardly detectable metastases. According to an extensive study carried out in Sweden, the sensitivity of urinary levels of 5SCD to detect metastatic melanoma (stage IIIB and IV) was 60% in 161 patients, and the specificity was 98% in 410 patients. The sensitivity of serum levels of 5SCD to detect distant metastasis was reported to be 70% by Bánfalvi et al. [48]. These studies have shown that 5SCD levels often do not increase in patients with amelanotic melanoma [46][49]. On the other hand, it was known that serum 5SCD in mucosal and uveal melanoma show higher levels [49].
Table 2. Patient Characteristics
Table 3. Frequency of elevated levels of serum 5SCD according to the presence or absence of metastasis.
Follow-up of melanoma patients until the end stage revealed an exponential increase in the serum levels of 5SCD in most of those patients. The median value of 20.1 nmol/L at the time of visceral metastasis rose to 249 nmol/L shortly before death. Thus, 5SCD values appear to reflect the tumor burden right up to the end stage. The sharp rise in 5SCD levels during the progression of the disease appears to make 5SCD a particularly sensitive marker for monitoring the response to therapy. In summary, in 42 of the 49 melanoma patients (86%) with visceral metastases (stage IVB), an elevation of serum levels of 5SCD above 10 nmol/L was observed. In 19 of the 49 patients, serum 5SCD values were obtained at the onset of skin/lymph node metastasis (stage IIB to IVB) and 12 of those 19 patients (63%) had elevated 5SCD levels. The elevation of serum levels of 5SCD preceded clinical detection of visceral metastases in 16 of the 49 patients with progressive disease (33%), while the elevation coincided with the detection of visceral metastasis in 18 patients (37%). Thus, serum 5SCD values are as effective as conventional physical examinations and imaging techniques in detecting visceral metastases [46].
Cumulative survival curves for melanoma patients who underwent surgical resection according to their 5SCD levels pre- and post-operatively were examined [46]. In patients with elevated 5SCD values pre- and post-operatively (n = 5), the 50% survival time was 10 months and the cumulative survival was 20%. In patients with 5SCD values above 10 nmol/L pre-operatively and below 10 nmol/L post-operatively (n = 15), the 50% survival time was 18 months and the cumulative survival was 23%. In patients with 5SCD values below 10 nmol/L pre- and post-operatively (n = 108), the 5 years survival was 76%. Serum levels of 5SCD are also useful as a prognostic marker; melanoma patients with elevated 5SCD values before or after excision of their tumors had significantly shorter survival times compared with those who had normal values. It is interesting to note that pre-operative values of 5SCD exceeding 10 nmol/L were associated with a poor prognosis, even when post-operative values were below the upper limit. Patients without metastases rarely had a 5SCD value exceeding 30 nmol/L. After the 5SCD value rose above 30 nmol/L, patients with distant metastases survived an average of 6.3 months. These results show that 5SCD levels reflect tumor burden sensitively, and that higher 5SCD values might indicate more widespread dissemination of melanoma metastases.
Kärnell et al. reported urinary 5SCD and 6H5MI2C in 92 patients with melanoma during chemotherapy [50]. The sensitivity of 5SCD for the detection of stage III–IV melanoma was 83%, while the corresponding sensitivity of 6H5MI2C was 52%. A significant correlation was found between 5SCD decrease and clinical regression (p < 0.001) and between 5SCD increase and clinical progression (p < 0.001). Corresponding correlations were not found for 6H5MI2C. Thus, the use of 5SCD was recommended as a valuable, reliable and simple biochemical markers to use in the clinical follow-up of melanoma patients with advanced disease. Johansson et al. showed the use of reverse transcription polymerase chain reaction (RT-PCR) analysis of melanoma specific transcripts for the identification of circulating melanoma cells [51]. Pigment-related and S100 calcium-binding protein B (S100B) transcripts were quantified in 12 different melanoma cell lines and related to the 5SCD levels, pigment and S100B. Tyrosinase, tyrosinase-related protein-1, and tyrosinase-related protein-2 mRNA correlated significantly with 5SCD levels. The amount of S100B mRNA correlated significantly with the amount of S100B (p < 0.001). The measurement of S100B mRNA was more sensitive, but the use of this transcript was hampered by its presence in the blood cells.
3. Conclusions
Melanoma is one of the most lethal cancers worldwide, but still there are not efficient serum biomarkers to conduct an early detection and an efficient monitoring of the disease. Therefore, it is of utmost importance to discover novel circulating markers in order to implement diagnosis, prognosis and treatment of this malignancy. 5SCD is an excellent candidate due to its potential informative power about important tumor features, the theoretical possibility to use it as an early-stage melanoma marker and the advantages of the detection method compared to biopsies. The serum level of 5SCD is a sensitive and specific marker for predicting the presence of distant melanoma metastases when analyzed regularly. The elevation of serum levels of 5SCD to >10 nmol/L often precedes the clinical detection of visceral metastases. This method is therefore useful in following up outpatients with suspected metastatic melanoma when combined with physical examinations and the routine use of clinical laboratory tests. Serum levels of 5SCD could be used to accurately assess therapy responses in future clinical trials for the treatment of advanced melanoma.
References
- Landreville, S.; Agapova, O.A.; Harbour, J.W. Emerging insights into the molecular pathogenesis of uveal melanoma. Future Oncol. 2008, 4, 629–636.
- Akaraviputh, T.; Arunakul, S.; Lohsiriwat, V.; Iramaneerat, C.; Trakarnsanga, A. Surgery for gastrointestinal malignant melanoma: Experience from surgical training center. World J. Gastroenterol. 2010, 16, 745–748.
- Bakalian, S.; Marshall, J.C.; Logan, P.; Faingold, D.; Maloney, S.; Di Cesare, S.; Martins, C.; Fernandes, B.F.; Burnier, M.N., Jr. Molecular pathways mediating liver metastasis in patients with uveal melanoma. Clin. Cancer Res. 2008, 14, 951–956.
- Rigel, D.S.; Russak, J.; Friedman, R. The evolution of melanoma diagnosis: 25 years beyond the ABCDs. CA A Cancer J. Clin. 2010, 60, 301–316.
- Seetharamu, N.; Ott, P.A.; Pavlick, A.C. Mucosal melanoma: A case-based review of the literature. Oncologist 2010, 15, 772–781.
- Gladfelter, P.; Darwish, N.H.E.; Mousa, S.A. Current status and future direction in the management of malignant melanoma. Melanoma Res. 2017, 27, 403–410.
- Lens, M.B.; Dawes, M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br. J. Dermatol. 2004, 150, 179–185.
- Hu, D.N.; Yu, G.; McCormick, S.A.; Finger, P.T. Population-based incidence of conjunctival melanoma in various races and ethnic groups and comparison with other melanoma. Am. J. Ophthalmol. 2008, 145, 418–423.
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300.
- Markovic, S.N.; Erickson, L.A.; Rao, R.D.; Weenig, R.H.; Pockaj, B.A.; Bardia, A.; Vachon, C.M.; Schild, S.E.; McWilliams, R.R.; Hand, J.L.; et al. Melanoma Study Group of the Mayo Clinic Cancer Center. Malignant melanoma in the 21st century, part 1: Epidemiology, risk factors, screening, prevention, and diagnosis. Mayo Clin. Proc. 2007, 82, 364–380.
- Schulman, J.M.; Fisher, D.E. Indoor ultraviolet tanning and skin cancer: Health risks and opportunities. Curr. Opin. Oncol. 2009, 21, 144–149.
- Alessi, C.; Scapulatempo Neto, C.; Viana, C.R.; Vazquez, V.L. PD-1/PD-L1 and VEGF-A/VEGF-C expression in lymph node microenvironment and association with melanoma metastasis and survival. Melanoma Res. 2017, 27, 565–572.
- Kurniawan, F.; Kartasasmita, R.E.; Yoshioka, N.; Mutalib, A.; Tjahjono, D.H. Computational Study of Imidazolylporphyrin Derivatives as a Radiopharmaceutical Ligand for Melanoma. Curr. Comput. Alded Drug Des. 2018, 14, 191–199.
- Matsumoto, K.; Masataka Umitsu, M.; De Silva, D.M.; Arpita Roy, A.; Bottaro, D.P. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017, 108, 296–307.
- Graziani, G.; Pedro, M.; Lacal, P.M. Neuropilin-1 as therapeutic target for malignant melanoma. Front. Oncol. 2015, 5, 125.
- Campos, L.S.; Rodriguez, Y.I.; Leopoldino, A.M.; Hait, N.C.; Bergami, P.L.; Castro, M.G.; Sanchez, E.S.; Maceyka, M.; Spiegel, S.; Alvareza, S.E. Filamin A expression negatively regulates sphingosine-1-phosphate-induced NF-κB activation in melanoma cells by inhibition of Akt signaling. Mol. Cell. Biol. 2016, 36, 320–329.
- Jenkins, M.H.; Brinckerhoff, C.E.; David, W.; Mullins, D.W. CXCR3 signaling in BRAFWT melanoma increases IL-8 expression and tumorigenicity. PLoS ONE 2015, 10, e0121140.
- Tsuji, T.; Kawada, Y.; Kai-Murozono, M.; Komatsu, S.; Han, S.A.; Takeuchi, K.; Mizushima, H.; Miyazaki, K.; Irimura, T. Regulation of melanoma cell migration and invasion by laminin-5 and alpha3beta1 integrin (VLA-3). Clin. Exp. Metastasis 2002, 19, 127–134.
- Kiss, T.; Ecsedi, S.; Vizkeleti, L.; Koroknai, V.; Emri, G.; Kovács, N.; Adany, R.; Balazs, M. The role of osteopontin expression in melanoma progression. Tumor Biol. 2015, 36, 7841–7847.
- Esmeralda Carrillo, E.; Prados, J.; Marchal, J.A.; Boulaiz, H.; Martínez, A.; Rodríguez-Serrano, F.; Caba, O.; Serrano, S.; Aránega, A. Prognostic value of RT-PCR tyrosinase detection in peripheral blood of melanoma patients. Dis. Markers 2006, 22, 175–181.
- Maeng, H.; Terabe, M.; Berzofsky, J.A. Cancer Vaccines: Translation from mice to human clinical trials. Curr. Opin. Immunol. 2018, 51, 111–122.
- Bresnick, A.R.; Weber, D.J.; Zimmer, D.B. S100 proteins in cancer. Nat. Rev. Cancer 2015, 15, 96–109.
- Zhou, J.; Sheng, J.; Yong Fan, Y.; Zhu, X.; Tao, Q.; He, Y.; Wang, S. Association between serum amyloid A levels and cancers: A systematic review and meta-analysis. Postgrad. Med. J. 2018, 94, 499–507.
- Fang, S.; Wang, Y.; Sui, D.; Liu, H.; Ross, M.I.; Gershenwald, J.E.; Cormier, J.N.; Royal, R.E.; Lucci, A.; Schacherer, C.W.; et al. C-reactive protein as a marker of melanoma progression. J. Clin. Oncol. 2015, 33, 1389–1396.
- Song, J.; Merbs, S.L.; Sokoll, L.J.; Chan, D.W.; Zhang, Z. A multiplex immunoassay of serum biomarkers for the detection of uveal melanoma. Clin. Proteom. 2019, 16, 10.
- Utikal, J.; Schadendorf, D.; Ugurel, S. Serologic and immunohistochemical prognostic biomarkers of cutaneous malignancies. Arch. Dermatol. Res. 2007, 298, 469–477.
- Kelley, M.C.; Jones, R.C.; Gupta, R.K.; Yee, R.; Stern, S.; Wanek, L.; Morton, D.L. Tumor associated antigen TA-90 immune complex assay predicts subclinical metastasis and survival for patients with early stage melanoma. Cancer 1998, 83, 1355–1361.
- Samarkos, M.; Papaxoinis, G.; Athanasoula, K.; Benopoulou, O.; Bouros, S.; Anastasopoulou, A.; Diamantopoulos, P.; Gogas, H.; Mantzourani, M. Significance of survivin mRNA blood levels in patients with melanoma. J. BU ON 2018, 23, 96–103.
- Vergilis, I.J.; Szarek, M.; Ferrone, S.; Reynolds, S.R. Presence and prognostic significance of melanoma-associated antigens CYT-MAA and HMW-MAA in serum of patients with melanoma. J. Investig. Dermatol. 2005, 125, 526–531.
- Nangia-Makker, P.; Hogan, V.; Raz, A. Galectin-3 and cancer stemness. Glycobiology 2018, 28, 172–181.
- Ohmi, Y.; Kambe, M.; Ohkawa, Y.; Hamamura, K.; Tajima, O.; Takeuchi, R.; Furukawa, K.; Furukawa, K. Differential roles of gangliosides in malignant properties of melanomas. PLoS ONE 2018, 13, e0206881.
- Tuaeva, N.O.; Falzone, L.; Porozov, Y.B.; Nosyrev, A.E.; Trukhan, V.M.; Kovatsi, L.; Spandidos, D.A.; Drakoulis, N.; Kalogeraki, A.; Mamoulakis, C.; et al. Translational Application of Circulating DNA in Oncology: Review of the Last Decades Achievements. Cells 2019, 8, 1251.
- Mastroianni, J.; Stickel, N.; Andrlova, H.; Hanke, K.; Melchinger, W.; Duquesne, S.; Schmidt, D.; Falk, M.; Andrieux, G.; Pfeifer, D.; et al. miR-146a controls immune response in the melanoma microenvironment. Cancer Res. 2019, 79, 183–195.
- Richtig, G.; Ehall, B.; Richtig, E.; Aigelsreiter, A.; Tony Gutschner, T.; Pichler, M. Function and clinical implications of long non-coding RNAs in melanoma. Int. J. Mol. Sci. 2017, 18, 715.
- Mantovani, A.; Barajon, I.; Garlanda, C. IL-1 and IL-1 Regulatory Pathways in Cancer Progression and Therapy. Immunol. Rev. 2018, 281, 57–61.
- Jia, Y.; Xie, X.; Shi, X.; Li, S. Associations of common IL-4 gene polymorphisms with cancer risk: A meta-analysis. Mol. Med. Rep. 2017, 16, 1927–1945.
- Mohapatra, P.; Prasad, C.P.; Andersson, T. Combination therapy targeting the elevated interleukin-6 level reduces invasive migration of BRAF inhibitor-resistant melanoma cells. Mol. Oncol. 2019, 13, 480–494. [Google Scholar] [CrossRef]
- Trifunović, J.; Miller, L.; Debeljak, Z.; Horvat, V. Pathologic patterns of interleukin 10 expression—A review. Biochem. Med. 2015, 25, 36–48.
- Lasek, W.; Zagożdżon, R.; Jakobisiak, M. Interleukin 12: Still a promising candidate for tumor immunotherapy? Cancer Immunol. Immunother. 2014, 63, 419–435.
- Lugowska, I.; Maria Kowalska, M.; Fuksiewicz, M.; Kotowicz, B.; Mierzejewska, E.; Koseła-Paterczyk, H.; Szamotulska, K.; Rutkowski, P. Serum markers in early-stage and locally advanced melanoma. Tumor Biol. 2015, 36, 8277–8285.
- Mumford, B.; Robertson, G.P. Circulating Melanoma Cells in the Diagnosis and Monitoring of Melanoma: An Appraisal of Clinical Potential. Mol. Diagn. Ther. 2014, 18, 175–183.
- Wagner, N.B.; Forschner, A.; Leiter, U.; Garbe, C.; Eigentler, T.K. S100B and LDH as early prognostic markers for response and overall survival in melanoma patients treated with anti-PD-1 or combined anti-PD-1 plus anti-CTLA-4 antibodies. Br. J. Cancer 2018, 119, 339–346.
- Li, Y.; Li, M.; Shats, I.; Krahn, J.M.; Flake, G.P.; Umbach, D.M.; Li, X.; Li, L. Glypican 6 is a putative biomarker for metastatic progression of cutaneous melanoma. PLoS ONE 2019, 14, e0218067.
- Salvatierra, E.; Alvarez, M.J.; Leishman, C.C.; Baquero, E.R.; Lutzky, V.P.; Chuluyan, H.E.; Podhajcer, O.L. SPARC Controls Melanoma Cell Plasticity through Rac1. PLoS ONE 2015, 10, e0134714.
- Chalkiadaki, G.; Nikitovic, D.; Katonis, P.; Berdiaki, A.; Tsatsakis, A.; Kotsikogianni, I.; Karamanos, N.K.; Tzanakakis, G.N. Low molecular weight heparin inhibits melanoma cell adhesion and migration through a PKCa/JNK signaling pathway inducing actin cytoskeleton changes. Cancer Lett. 2011, 312, 235–244.
- Wakamatsu, K.; Kageshita, T.; Furue, M.; Hatta, N.; Kiyohara, Y.; Nakayama, J.; Ono, T.; Saida, T.; Tanaka, M.; Tsuchida, T.; et al. Evaluation of 5-S-cysteinyldopa as a marker of melanoma progression: 10 years’ experience. Melanoma Res. 2002, 12, 245–253.
- Kärnell, R.; von Schoultz, E.; Hansson, L.O.; Nilsson, B.; Arstrand, K.; Kågedal, B. S100B protein, 5-S-cysteinyldopa and 6-hydroxy-5-methoxyindole-2-carboxylic acid as biochemical markers for survival prognosis in patients with malignant melanoma. Melanoma Res. 1997, 7, 393–399.
- Bánfalvi, T.; Gilde, K.; Boldizsar, M.; Kremmer, T.; Otto, S. Serum levels of S100 protein and 5-S-cysteinyldopa as markers of melanoma progression. Pathol. Oncol. Res. 1999, 5, 218–223.
- Horikoshi, T.; Ito, S.; Wakamatsu, K.; Onodera, H.; Eguchi, H. Evaluation of melanin-related metabolites as markers of melanoma progression. Cancer 1994, 73, 629–636.
- Kärnell, R.; Kågedal, B.; Lindholm, C.; Nilsson, B.; Arstrand, K.; Ringborg, U. The value of cysteinyldopa in the follow-up of disseminated malignant melanoma. Melanoma Res. 2000, 10, 363–369.
- Johansson, M.; Takasaki, A.; Lenner, L.; Arstrand, K.; Kågedal, B. Quantitative relationship between pigment-related mRNA and biochemical melanoma markers in melanoma cell lines. Melanoma Res. 2002, 12, 193–200.