
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Diego A. Bonilla | + 3291 word(s) | 3291 | 2021-01-14 10:12:59 | | | |
| 2 | Catherine Yang | Meta information modification | 3291 | 2021-01-25 04:25:32 | | |
Video Upload Options
A 4R’s approach to optimizing post-exercise recovery has been introduced: (i) Rehydration—a fundamental process that will depend on the athlete, environment and sports event; (ii) Refuel—the consumption of carbohydrates is not only important to replenish the glycogen reserves but also to contribute to the energy requirements for the immune system and tissue reparation. Several bioengineered carbohydrates were discussed but further research is needed; (iii) Repair—post-exercise ingestion of high-quality protein and creatine monohydrate benefit the tissue growth and repair; and (iv) Rest—pre-sleep nutrition has a restorative effect that facilitates the recovery of the musculoskeletal, endocrine, immune, and nervous systems. Nutritional consultancy based on the 4R’s is important for the wise stewardship of the hydration, feeding, and supplementation strategies to achieve a timely recovery.
1. Introduction
Recovery strategies to optimize post-exercise recovery depend to a large extent on the proximity of the next session, the degree of physiological stress, and the relevance of the next event. This determines how to rehydrate, replenish energy and consume the nutrients needed to improve tissue repair. To improve comprehension regarding the nutritional strategies that impact post-exercise recovery, a mnemonic entitled the 4R’s (Rehydrate, Refuel, Repair, and Rest) is introduced. This approach divides the nutrition intervention into four interrelated scenarios that follow the post-exercise time course in order to optimize the exercise-induced adaptations and recovery (Figure 1).
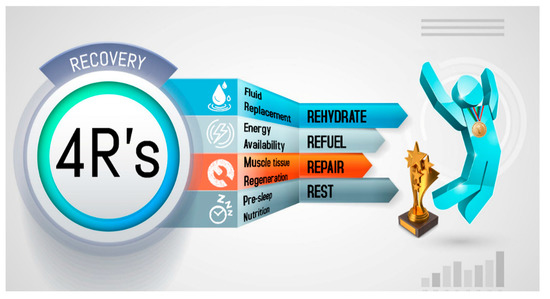
Figure 1. The 4R’s framework of nutritional strategies to optimize post-exercise recovery in athletes.
2. The 4R's
2.1. Rehydrate
One of the first goals during recovery is to replace any fluid and electrolyte deficits. Most physically active individuals sweat from 0.3 to 2.4 L·h−1, which depends on exercise intensity, duration, and environmental conditions such as altitude, heat, and humidity [1][2][3][4][5][6][7][18,19]. Moreover, individual characteristics (i.e., body mass, genetic predisposition, heat acclimatization state, physical fitness, and metabolic efficiency) might influence sweat rates for a given activity [3]. For instance, the highest sweat rate was registered at 3.7 L·h−1 for a world-class ultramarathon runner [4]. Thus, measuring pre and post-exercise body mass is a recommended practice to assess fluid status.
Rehydration is important, especially in team, endurance or ultra-endurance sports, where in many cases it is not possible to compensate for the loss of fluids and electrolytes that occur during exercise, particularly in hot and humid environments. As a general advise, for quick rehydration, it is recommended the consumption of 150% of the weight lost after exercise over a short recovery period (less than 4 h) [5][6], with a sodium concentration between 20 and 30 mEq·L−1 [1]. Athletes and practitioners should replenish three cups of fluid per pound of weight lost (~1.5 L·kg−1) and to make sure body mass is back up before the next training session. Furthermore, it has even been shown that consuming a sodium-containing drink between 40 and 60 mEq·L−1 can improve fluid retention and rehydration when there is little time between sessions or when there is moderate dehydration [7].
Rehydration can last between four and 24 h [8]. If recovery time and opportunities allow it, the consumption of sodium-rich foods, such as crackers, peanuts, bread, milk, cheese, ham, kabanos, and soups, may be sufficient to regain the state of euhydration. However, if the recovery time is less than 12 h, more aggressive rehydration strategies and the use of moisturizing beverages (e.g., glycerol) are required before the next training or competition [9]. One practical application to improve both the rate of rehydration and total fluid retention following exercise is the ingestion of glycerol [5][10]; however, professional advice is recommended to avoid potential gastrointestinal discomfort with any hyperhydration agent.
In the rehydration process, it has been found that neither the addition of potassium to the drink [11] nor the way of distributing the volume [12] nor the temperature influence the percentage of liquid preserved to be subsequently used by the body for rehydration [13]; however, it is believed that the delay in early rehydration after exercise is attributed to a reduction in sensations associated with thirst, and it is known that the taste and cool temperature of beverages can positively affect these sensations [14]. On the other hand, some drinks that can be used for rehydration and in turn help recovery has been successfully studied, such as chocolate milk [15][16][17].
Rehydration is a fundamental step in recovery, but what, how, when and how much will depend on the athlete and the particular event. We adhere to the position statement of the National Athletic Trainers’ Association to emphasize that education strategies for athletes should address personal sweat rates, hydration cues, and rehydration strategies that avoid both hypohydration and fluid overload [6].
2.2. Refuel
At the end of the exercise, there are several strategies to maximize muscle and liver glycogen replenishment, especially when two or more sessions are performed on the same day or when competing on consecutive days. For planning, it is necessary to consider the state of training, schedules, and the magnitude of the depletion of reserves, besides the type of exercise [18]. In this sense, the amount of carbohydrates is determined by the need to replenish muscle glycogen stores, and according to Jeukendrup (2017) [19], this is closely related to:
-
Time to next training session or competition,
-
Nutrition periodization to achieve adaptations,
-
Need for muscle repair and growth,
-
The amount consumed before and after as part of global requirements.
Although certain general recommendations can be given, the carbohydrate intake must be fine-tuned based on individual features, total energy daily expenditure, exercise training requirements, and the respective feedback from training performance in daily recovery [20]. In athletes with high body mass (e.g., basketball and rugby) or players under a weight loss program it might be better to reduce the energy intake to the needs of the previous category [20]. Additionally, resistance/power athletes do not need much carbohydrates as endurance athletes to maintain optimal liver and muscle glycogen; therefore, based on the exercise and sports nutrition review update of the International Society of Sports Nutrition [21], daily carbohydrate needs might be ranked as follows:
-
Moderate duration/low-intensity training (e.g., 2–3 h per day of intense exercise performed 5–6 times per week): 5–8 g·kg−1 body mass·day−1
-
Moderate to heavy endurance training (e.g., 3–6 h per day of intense training in 1–2 daily workouts for 5–6 days per week): 8–10 g·kg−1 body mass·day−1
-
Extreme exercise programs or competition (+6 h per day or high competition frequency during the week): 10–12 + g·kg−1 body mass·day−1
Moreover, in the post-exercise period, it takes about four hours for carbohydrates to be digested and absorbed into muscle and liver tissues to be incorporated as glycogen. Hence, if rapid recovery is required due to a limited time period available, the priority should be to consume large amounts of daily carbohydrates (>8 g·kg−1 body mass·day−1) and to eat a high carbohydrate meal within two hours following exercise with at least 1.2 g·kg−1·h−1 for the first four hours of recovery [22]. Ingestion of a glucose polymer or the combination of glucose and fructose (sucrose) results in a fast replenishment of muscle glycogen stores whilst also minimizing gastrointestinal distress [23], and there is no need for protein and/or amino acid ingestion in order to enhance the insulin levels if sufficient carbohydrates are consumed (1.2 g·kg−1·h−1). In fact, higher insulin concentrations do not further increase the rate of muscle glycogen synthesis when carbohydrate intake is sufficient [19]. Slightly less carbohydrate plus protein (e.g., 1 g carbohydrate·kg−1 and 0.5 g protein·kg−1) within 30 min after exercise or carbohydrates along with caffeine may also be used to aid rapid glycogen resynthesis [21]. Additionally, compared to carbohydrate ingestion alone, multiday supplementation with creatine monohydrate along with an adequate amount of carbohydrates has been reported to have a higher positive impact on muscle glycogen synthesis [24].
The consumption of carbohydrates is not only important to replenish the reserves, but also to contribute to cover the energy requirements that are fundamental to help the competition of the immune system and the repair of the tissues [25]. Sports nutritionists, coaches and athletes should be cautious with the potential physiological implications of the relative energy deficiency in sport, which includes impaired metabolic rate, hormonal disruptions, menstrual dysfunction, reduced bone health, immunity, protein synthesis, and cardiovascular health [26][27].
Considering the potential impact of the type of carbohydrates on performance and recovery, some bioengineered formulations with different physicochemical characteristics are described in the next lines. These bioengineering processes refer to the application of theoretical and experimental methods of the basic sciences to produce new scientific knowledge with practical applications, such as the modification of the chemical structure of a molecule by means of biotechnological procedures that use microorganisms (e.g., bacteria) in order to produce substances with different metabolic responses since they are not found regularly in food. We recommend the reader to visit the Carbohydrate Structure Database (CSDB, http://csdb.glycoscience.ru) [28] and the database of Chemical Entities of Biological Interest (ChEBI) [29] to know in depth about advances, structures and different applications of the new generation of carbohydrates produced through different processes and that are of biological interest.
2.3. Repair
Scientific research has demonstrated that muscle protein synthesis (MPS) can be stimulated by either a physical allostatic challenge (e.g., resistance exercise stimulus) or by the ingestion of dietary protein, with synergistic responses when protein is consumed before and immediately after resistance exercise training [30]. According to the International Society of Sports Nutrition position stand on nutrient timing, post-exercise ingestion (immediately to 2 h) of high-quality protein food represents a robust stimulus that impacts positively on MPS; however, similar increases in MPS have been found when high-quality proteins are ingested immediately before exercise [31]. Indeed, taking into account the sport-specific daily needs, if an insufficient amount of protein is consumed, the athletes will develop and maintain a negative nitrogen balance, which is an indicator of protein catabolism and negatively affect recovery. Over time, this can lead to muscle wasting, injury, disease and intolerance to training [21]. Thus, the peri-exercise ingestion of insulinotropic protein and/or essential amino acid mixtures might stimulate post-exercise net muscle protein anabolism, and this might contribute to faster tissue growth and repair [18]. Similarly, recent findings have provided evidence that marathon runners that consume moderate amounts of protein post-exercise can have recovery benefits [32][33]. With a good grade of evidence, compared to ingestion of carbohydrate alone, co-ingestion of carbohydrate plus protein together during the recovery period have resulted in no difference in the rate of muscle glycogen synthesis but it improves net protein balance [1].
The International Society of Sports Nutrition position stand [30] about proteins for recovery is:
-
Optimal dose of protein for athletes to enhance MPS are dependent upon age, energy intake (higher amount is needed under energy restriction), and recent resistance exercise stimuli. Post-exercise recommendations are 0.5 g of a high-quality protein per kilogram of body mass, or an absolute dose of 40 g. Protein per meal should be between 0.25 and 0.40 g of protein per kg of body mass, or absolute values of 20 g.
-
Given the observed benefits of pre- and post-exercise protein ingestion, athletes’ tolerance should be assessed to determine the optimal time period during which to ingest protein. Notwithstanding, in spite of the anabolic effect of exercise is long-lasting (at least 24 h), athletes can take advantage of the higher muscle sensitivity to nutrient uptake after exercise due to the likely diminishment over time.
On the other hand, a large body of evidence suggests that creatine monohydrate supplementation (0.1 g·kg−1·day−1) not only optimize exercise adaptations and increase performance but also may reduce muscle damage and/or enhance recovery from intense exercise [34]. These effects are partially due to the optimization of the creatine kinase (CK) system which not only serves as a spatial/temporal buffer of ATP regeneration but also leads to positive regulation of anabolic signaling pathways (such as IGF-I and MAPK) and, hence, might promote faster tissue repair and recovery [35]. Additionally, it has been shown that chronic creatine supplementation prior to an exhaustive exercise bout and glycogen loading promotes greater glycogen resynthesis than just carbohydrate loading alone [36].
The role of antioxidants and anti-inflammatory substances are also highlighted in the post-exercise repair process. An increased antioxidant status leads to the reduction of oxidative stress caused by the production of reactive oxygen species during the inflammatory process [37]; therefore, the use of antioxidants can reduce muscle soreness and help with recovery in the short term, but high doses have also been linked to reduced training benefits in the long term. For example, Levers et al. (2015) found significant beneficial effects on serum markers of muscle catabolism, physiological stress, and inflammatory mechanisms after a short-term supplementation with 480 mg·day−1 of Montmorency powdered tart cherries surrounding a single bout of resistance exercise [38]. More recently, Brown et al. (2019) also reported that supplementation with Montmorency cherry concentrate can be considered as a practical nutritional intervention to reduce symptoms of muscle damage and improve post-exercise recovery on subsequent days in females [39]. It appears that secondary metabolites with antioxidant properties that are found in tart cherry extract might attenuate muscle soreness, strength decrement during recovery, and markers of muscle catabolism in resistance-trained individuals. In this sense, a systematic review and meta-analysis about the effects of antioxidants consumption showed that short-term polyphenol supplementation (e.g., quercetin) may boost athletic performance in predominately-trained males with an average intervention dose of 688 ± 478 mg·day−1 [40]. This has been reinforced by more recent research showing that acute and chronic supplementation with >1000 mg fruit-derived polyphenols per day will enhance recovery following muscle damage via antioxidant and anti-inflammatory mechanisms [41]; which suggest that fruit supplements could be used as part of the post-exercise recovery strategy although the need to educate and encourage athletes to consume more fruits and vegetables in the diet should be associated not only with recovery but also with health [42]. Similarly, curcumin is rich in polyphenols and has shown anti-inflammatory and antioxidant properties. The supplementation with 6 g of curcumin and 60 mg of piperine each day between 48 h before and 48 h after exercise-induced muscle damage have resulted in positive effects on recovery of the muscle function at 24 h and 48 h after the exercise [43].
The use of beetroot juice has been investigated in the management of hypertension [44][45], for the improvement of physical performance [46][47], and also in post-exercise recovery [48]. It has been found that acute supplementation of beetroot juice (250 mL) ×3 servings, two serving 24 h and 48 h following completion of 100-drop jumps attenuated muscle soreness and decrements in countermovement jump performance induced by eccentric exercise, while apparently having no effect on maximal isometric voluntary contractions, CK and some inflammatory markers (IL-6, TNF-alpha and IL-8) [49]. Interestingly, a betalain-rich concentrate of beetroots has shown to improve performance in competitive male and female triathletes and attenuated the increase of CK and fatigue suggesting an increase in recovery [50]. Other herbal and mushroom supplements that have promising effects to improve post-exercise recovery are Zingiber officinale [51], Zingiber officinale + Bixa orellana L. [52], Rhodiola rosea [53], Cordyceps militaris [54], and Rhodiola rosea + Cordyceps sinensis [55]. A root extract that warrants special attention is Withania somnifera (most known as ashwagandha) given some research have found ergogenic effects in athletic [56][57] and physically active individuals [58]. Moreover, the adaptogenic, anti-inflammatory, and antioxidant properties of ashwagandha [59][60][61] turns this herbal extract into a potential strategy to optimize recovery and promote exercise-induced adaptations. Although more research is required, dosages are between 300 and 500 mg of aqueous root extract twice per day.
On the other hand, it appears that supplementation with branched-chain amino acids (BCAA) after high-intensity exercise may favor a hormonal environment that contributes to attenuate the loss of strength, reduce muscle damage, and generate an anabolic environment [62]. Indeed, several systematic reviews and meta-analysis have concluded that BCAA supplementation (>200 mg·kg−1·day−1) may optimize recovery and mitigate muscle soreness following muscle-damaging exercise [63][64][65][66]. Notwithstanding, in resistance-trained males, the attenuation of muscular performance decrements and the observed decrease in plasma CK levels after BCAA supplementation is likely negligible when consumed with a diet consisting of ~1.2 g·kg−1·day−1 of protein[67]. The leucine-derived compound HMB has shown to improve work capacity recovery after high-intensity exercise [68], and may attenuate markers of muscle damage, augment acute immune and endocrine responses while preventing loss of lean body mass in catabolic situations [69]. A recent systematic review and meta-analysis by Rahimi et al. (2018) concluded that HMB may be seen as a recovery agent following exercise-induced muscle damage [70], but more research on recovery from injury that includes periods of extreme inactivity is needed given it does not consistently increase strength and/or lean mass or reduce markers of muscle damage [71]. Finally, it has been recently reviewed systematically if other foods, such as Pomegranate [72], cow’s milk [73], or Chocolate milk [74], might potentially improve exercise-performance and post-exercise recovery but further research is needed to extract definitive conclusions.
2.4. Rest
There is no doubt that sleep is an absolutely vital physiological function and one of the most important factors in post-exercise recovery [75]. It has been emphasized that naps, sleep extension, and sleep-hygiene practices seem to be advantageous to the performance by optimizing recovery [76]. In spite of the above, von Rosen et al. (2017) reported that the recommended amount of sleep during weekdays (8 h) was not obtained by 19% of 340 Swedish adolescent elite athletes of several disciplines during the autumn semester. Moreover, athletes sleeping more than eight hours and reached the recommended nutrition intake reduced the odds of suffering a new injury [77]. Portuguese elite female gymnasts have also found to have poor sleep habits with consequences on daytime sleepiness, sleep quality, and low energy availability associated with macro and micronutrients’ deficiencies [78]. In fact, according to a recent systematic review by Gupta et al. [79], athletes show a high overall prevalence of insomnia symptoms characterized by increased sleep latency, sleep fragmentation, non-restorative sleep, and excessive daytime fatigue. Currently, there is a lack of evidence and future research should focus on conducting sleep interventions among different athlete populations to address their specific sleep demands and disturbances [80].
It is known that eating the right combination of foods before going to sleep and what foods to avoid in the evening may be beneficial in enhancing sleep [81]. That is the rationale for the pre-sleep nutrition strategies, considering that several nutrients have been shown to improve sleep such as carbohydrates (high-glycemic index dinners), melatonin, tryptophan-rich protein, antioxidant-rich fruits (e.g., tart cherry juice and kiwi), and micronutrients [82]. Casein proteins, a type of secreted calcium (phosphate)-binding phosphoproteins, are among the most common nutrients used for pre-sleep nutrition given they are considered a high-quality protein source with high digestibility and bioavailability but with a slower digestion rate in comparison to whey [83]. Thus, the timing of nutrient intake is as important as the composition to fulfill the nutrition needs of the athletes [31]. Res et al. [84] reported for the first time that casein protein ingestion immediately before sleep was not only effectively digested and absorbed but also increased MPS and net protein balance in healthy young males that performed a resistance-training bout in the evening. Moreover, it has been demonstrated that pre-sleep casein protein ingestion augments the muscle adaptive response in terms of muscle mass and strength after a 12-week resistance exercise training program in young men in comparison to placebo [85]. Therefore, the extended window of opportunity as a result of the additive effects of resistance exercise training and protein ingestion on MPS makes the pre-sleep casein protein supplementation an effective nutrient timing strategy to optimize muscle conditioning and recovery [86] with no need to add extra leucine [87]. Although the positive effects of pre-sleep nutrition have been found particularly in resistance-type exercise training [88], more research is needed in endurance-trained athletes considering recent findings showed no improvement [89]. The available evidence and recommendations under this new paradigm of pre-sleep nutrition are:
-
The consumption of 40–48 g of casein approximately 30 min before sleep improves post-exercise recovery and positively affect acute protein metabolism during an overnight period in healthy young adults [83][90].
-
Ashwagandha supplementation (>150 mg aqueous root extract quaque hora somni) seems to be an effective nutritional strategy to improve sleep quality in healthy male and female subjects [91]; consequently, it should be also considered before sleep.
References
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528.
- Kreider, R.B. Essentials of Exercise & Sport Nutrition: Science to Practice; Lulu Press, Inc.: Morrisville, CA, USA, 2019.
- American College of Sports Medicine; Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S. Exercise and Fluid Replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390.
- Kreider, R.B. Physiological Considerations of Ultraendurance Performance. Int. J. Sport Nutr. 1991, 1, 3–27.
- McCubbin, A.J.; Allanson, B.A.; Caldwell Odgers, J.N.; Cort, M.M.; Costa, R.J.S.; Cox, G.R.; Crawshay, S.T.; Desbrow, B.; Freney, E.G.; Gaskell, S.K.; et al. Sports Dietitians Australia Position Statement: Nutrition for Exercise in Hot Environments. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 83–98.
- Roberts, W.O.; O'Connor, F.G.; Kenney, W.L.; Cooper, L.; Cheuvront, S.N.; Casa, D.J.; Armstrong, L.E.; Anderson, S.A.; McDermott, B.P. National Athletic Trainers' Association Position Statement: Fluid Replacement for the Physically Active. J. Athl. Train. 2017, 52, 877–895.
- Merson, S.J.; Maughan, R.J.; Shirreffs, S.M. Rehydration with drinks differing in sodium concentration and recovery from moderate exercise-induced hypohydration in man. Eur. J. Appl. Physiol. 2008, 103, 585–594.
- Burke, L. Nutricion en el Deporte/Nutrition in Sport: Un Enfoque Práctico/a Practical Approach; Editorial Médica Panamericana S.A.: Madrid, Spain, 2010.
- Kavouras, S.A.; Armstrong, L.E.; Maresh, C.M.; Casa, D.J.; Herrera-Soto, J.A.; Scheett, T.P.; Stoppani, J.; Mack, G.W.; Kraemer, W. Rehydration with glycerol: endocrine, cardiovascular, and thermoregulatory responses during exercise in the heat. J. Appl. Physiol. 2005, 100, 442–450.
- Van Rosendal, S.P.; Coombes, J.S. Glycerol Use in Hyperhydration and Rehydration: Scientific Update. In Acute Topics in Sport Nutrition; Karger AG: Basel, Switzerland, 2012; pp. 104–112.
- Perez-Idarraga, A.; Aragon-Vargas, L.F. Postexercise rehydration: Potassium-rich drinks versus water and a sports drink. Appl. Physiol. Nutr. Metab. = Physiol. Appl. Nutr. Metab. 2014, 39, 1167–1174.
- Aragón-Vargas, L.F.; Pérez Idárraga, A. Rehidratación posejercicio: La forma de distribuir la ingesta de un volumen constante de líquido no altera su conservación. Pensar En Mov. Rev. Cienc. Ejerc. Salud 2011, 9, 12–21.
- Aragón-Vargas, L.F.; Pérez-Idárraga, A. Post-Exercise Rehydration: No Change in Diuresis from Water Ingested at Different Temperatures. Med. Sport. 2010, 14, 77–82.
- Sandick, B.L.; Engell, D.B.; Maller, O. Perception of drinking water temperature and effects for humans after exercise. Physiol. Behav. 1984, 32, 851–855.
- Karp, J.R.; Johnston, J.D.; Tecklenburg, S.; Mickleborough, T.D.; Fly, A.D.; Stager, J.M. Chocolate milk as a post-exercise recovery aid. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 78–91.
- Roy, B.D. Milk: The new sports drink? A Review. J. Int. Soc. Sports Nutr. 2008, 5, 15.
- Shirreffs, S.M.; Watson, P.; Maughan, R.J. Milk as an effective post-exercise rehydration drink. Br. J. Nutr. 2007, 98, 173–180.
- Jentjens, R.; Jeukendrup, A. Determinants of post-exercise glycogen synthesis during short-term recovery. Sports Med. 2003, 33, 117–144.
- Jeukendrup, A.E. Periodized Nutrition for Athletes. Sports Med. 2017, 47, 51–63.
- Mujika, I.; Burke, L.M. Nutrition in Team Sports. Ann. Nutr. Metab. 2010, 57, 26–35.
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15.
- Burke, L.M.; Hawley, J.A.; Wong, S.H.; Jeukendrup, A.E. Carbohydrates for training and competition. J. Sports Sci. 2011, 29, S17–S27.
- Gonzalez, J.; Fuchs, C.; Betts, J.; van Loon, L. Glucose Plus Fructose Ingestion for Post-Exercise Recovery—Greater than the Sum of Its Parts? Nutrients 2017, 9, 344.
- Murray, B.; Rosenbloom, C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr. Rev. 2018, 76, 243–259.
- Burke, L.M.; Loucks, A.B.; Broad, N. Energy and carbohydrate for training and recovery. J. Sports Sci. 2006, 24, 675–685.
- Varðardóttir, B.; Guðmundsdóttir, S.L.; Ólafsdóttir, A.S. Þegar orkuna skortir–áhrif hlutfallslegs orkuskorts í íþróttum (RED-s) á heilsu og árangur. Læknablaðið 2020, 106, 406–413.
- Mountjoy, M.; Sundgot-Borgen, J.; Burke, L.; Ackerman, K.E.; Blauwet, C.; Constantini, N.; Lebrun, C.; Lundy, B.; Melin, A.; Meyer, N.; et al. International Olympic Committee (IOC) Consensus Statement on Relative Energy Deficiency in Sport (RED-S): 2018 Update. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 316–331.
- Toukach, P.V.; Egorova, K.S. Carbohydrate structure database merged from bacterial, archaeal, plant and fungal parts. Nucleic Acids Res. 2016, 44, D1229–D1236.
- Degtyarenko, K.; de Matos, P.; Ennis, M.; Hastings, J.; Zbinden, M.; McNaught, A.; Alcantara, R.; Darsow, M.; Guedj, M.; Ashburner, M. ChEBI: A database and ontology for chemical entities of biological interest. Nucleic Acids Res. 2007, 36, D344–D350.
- Jager, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20.
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14.
- Saunders, M.J.; Luden, N.D.; DeWitt, C.R.; Gross, M.C.; Dillon Rios, A. Protein Supplementation During or Following a Marathon Run Influences Post-Exercise Recovery. Nutrients 2018, 10, 333.
- Churchward-Venne, T.A.; Pinckaers, P.J.M.; Smeets, J.S.J.; Betz, M.W.; Senden, J.M.; Goessens, J.P.B.; Gijsen, A.P.; Rollo, I.; Verdijk, L.B.; van Loon, L.J.C. Dose-response effects of dietary protein on muscle protein synthesis during recovery from endurance exercise in young men: A double-blind randomized trial. Am. J. Clin. Nutr. 2020, 112, 303–317.
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14.
- Bonilla, D.A.; Moreno, Y. Molecular and metabolic insights of creatine supplementation on resistance training. Rev. Colomb. Química 2015, 44, 11–18.
- Roberts, P.A.; Fox, J.; Peirce, N.; Jones, S.W.; Casey, A.; Greenhaff, P.L. Creatine ingestion augments dietary carbohydrate mediated muscle glycogen supercompensation during the initial 24 h of recovery following prolonged exhaustive exercise in humans. Amino Acids 2016, 48, 1831–1842.
- Myburgh, K.H. Polyphenol supplementation: Benefits for exercise performance or oxidative stress? Sports Med. 2014, 44, S57–S70.
- Levers, K.; Dalton, R.; Galvan, E.; O'Connor, A.; Goodenough, C.; Simbo, S.; Mertens-Talcott, S.U.; Rasmussen, C.; Greenwood, M.; Riechman, S.; et al. Effects of powdered Montmorency tart cherry supplementation on acute endurance exercise performance in aerobically trained individuals. J. Int. Soc. Sports Nutr. 2015, 13, 22.
- Brown, M.A.; Stevenson, E.J.; Howatson, G. Montmorency tart cherry (Prunus cerasus L.) supplementation accelerates recovery from exercise-induced muscle damage in females. Eur. J. Sport Sci. 2018, 19, 95–102.
- Somerville, V.; Bringans, C.; Braakhuis, A. Polyphenols and Performance: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 1589–1599.
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, 3–23.
- Doma, K.; Gahreman, D.; Connor, J. Fruit supplementation reduces indices of exercise-induced muscle damage: A systematic review and meta-analysis. Eur. J. Sport Sci. 2020, 1–18.
- Delecroix, B.; Abaidia, A.E.; Leduc, C.; Dawson, B.; Dupont, G. Curcumin and Piperine Supplementation and Recovery Following Exercise Induced Muscle Damage: A Randomized Controlled Trial. J. Sports Sci. Med. 2017, 16, 147–153.
- Bonilla, D.A.; Paipilla, A.F.; Marin, E.; Vargas-Molina, S.; Petro, J.L.; Perez-Idarraga, A. Dietary Nitrate from Beetroot Juice for Hypertension: A Systematic Review. Biomolecules 2018, 8, 134.
- Siervo, M.; Lara, J.; Ogbonmwan, I.; Mathers, J.C. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: A systematic review and meta-analysis. J. Nutr. 2013, 143, 818–826.
- Cuenca, E.; Jodra, P.; Perez-Lopez, A.; Gonzalez-Rodriguez, L.G.; Fernandes da Silva, S.; Veiga-Herreros, P.; Dominguez, R. Effects of Beetroot Juice Supplementation on Performance and Fatigue in a 30-s All-Out Sprint Exercise: A Randomized, Double-Blind Cross-Over Study. Nutrients 2018, 10, 1222.
- Mosher, S.L.; Sparks, S.A.; Williams, E.L.; Bentley, D.J.; Mc Naughton, L.R. Ingestion of a Nitric Oxide Enhancing Supplement Improves Resistance Exercise Performance. J. Strength Cond. Res. 2016, 30, 3520–3524.
- Clifford, T.; Berntzen, B.; Davison, G.W.; West, D.J.; Howatson, G.; Stevenson, E.J. Effects of Beetroot Juice on Recovery of Muscle Function and Performance between Bouts of Repeated Sprint Exercise. Nutrients 2016, 8, 506.
- Clifford, T.; Bell, O.; West, D.J.; Howatson, G.; Stevenson, E.J. The effects of beetroot juice supplementation on indices of muscle damage following eccentric exercise. Eur. J. Appl. Physiol. 2015, 116, 353–362.
- Montenegro, C.F.; Kwong, D.A.; Minow, Z.A.; Davis, B.A.; Lozada, C.F.; Casazza, G.A. Betalain-rich concentrate supplementation improves exercise performance and recovery in competitive triathletes. Appl. Physiol. Nutr. Metab. 2017, 42, 166–172.
- Wilson, P.B. A Randomized Double-Blind Trial of Ginger Root for Reducing Muscle Soreness and Improving Physical Performance Recovery among Experienced Recreational Distance Runners. J. Diet. Suppl. 2018, 17, 121–132.
- Dominguez-Balmaseda, D.; Diez-Vega, I.; Larrosa, M.; San Juan, A.F.; Issaly, N.; Moreno-Pérez, D.; Burgos, S.; Sillero-Quintana, M.; Gonzalez, C.; Bas, A.; et al. Effect of a Blend of Zingiber officinale Roscoe and Bixa orellana L. Herbal Supplement on the Recovery of Delayed-Onset Muscle Soreness Induced by Unaccustomed Eccentric Resistance Training: A Randomized, Triple-Blind, Placebo-Controlled Trial. Front. Physiol. 2020, 11.
- Pu, W.-l.; Zhang, M.-y.; Bai, R.-y.; Sun, L.-k.; Li, W.-h.; Yu, Y.-l.; Zhang, Y.; Song, L.; Wang, Z.-x.; Peng, Y.-f.; et al. Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomed. Pharmacother. 2020, 121.
- Tan, L.; Song, X.; Ren, Y.; Wang, M.; Guo, C.; Guo, D.; Gu, Y.; Li, Y.; Cao, Z.; Deng, Y. Anti-inflammatory effects of cordycepin: A review. Phytother. Res. 2020.
- Tsai, P.-H.; Lin, F.-C.; Huang, C.-C.; Hou, C.-W.; Cheng, I.-S. Effects of Rhodiola rosea-Cordyceps sinensis Supplementation on Glycogen Synthesis in Exercised Human Skeletal Muscle. Sports Exerc. Res. 2019, 21, 375–386.
- Choudhary, B.; Shetty, A.; Langade, D.G. Efficacy of Ashwagandha (Withania somnifera [L.] Dunal) in improving cardiorespiratory endurance in healthy athletic adults. Ayu (Int. Q. J. Res. Ayurveda) 2015, 36, 63.
- Wankhede, S.; Langade, D.; Joshi, K.; Sinha, S.R.; Bhattacharyya, S. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2015, 12.
- Ziegenfuss, T.; Kedia, A.; Sandrock, J.; Raub, B.; Kerksick, C.; Lopez, H. Effects of an Aqueous Extract of Withania somnifera on Strength Training Adaptations and Recovery: The STAR Trial. Nutrients 2018, 10, 1807.
- Langade, D.; Kanchi, S.; Salve, J.; Debnath, K.; Ambegaokar, D. Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract in Insomnia and Anxiety: A Double-blind, Randomized, Placebo-controlled Study. Cureus 2019.
- Salve, J.; Pate, S.; Debnath, K.; Langade, D. Adaptogenic and Anxiolytic Effects of Ashwagandha Root Extract in Healthy Adults: A Double-blind, Randomized, Placebo-controlled Clinical Study. Cureus 2019.
- Singh, N.; Bhalla, M.; De Jager, P.; Gilca, M. An Overview on Ashwagandha: A Rasayana (Rejuvenator) of Ayurveda. Afr. J. Tradit. Complementary Altern. Med. 2011, 8.
- Kraemer, W.J.; Ratamess, N.A.; Volek, J.S.; Häkkinen, K.; Rubin, M.R.; French, D.N.; Gómez, A.L.; McGuigan, M.R.; Scheett, T.P.; Newton, R.U.; et al. The effects of amino acid supplementation on hormonal responses to resistance training overreaching. Metab. Clin. Exp. 2006, 55, 282–291.
- Fouré, A.; Bendahan, D. Is Branched-Chain Amino Acids Supplementation an Efficient Nutritional Strategy to Alleviate Skeletal Muscle Damage? A Systematic Review. Nutrients 2017, 9, 1047.
- Rahimi, M.H.; Shab-Bidar, S.; Mollahosseini, M.; Djafarian, K. Branched-chain amino acid supplementation and exercise-induced muscle damage in exercise recovery: A meta-analysis of randomized clinical trials. Nutrition 2017, 42, 30–36.
- Hormoznejad, R.; Zare Javid, A.; Mansoori, A. Effect of BCAA supplementation on central fatigue, energy metabolism substrate and muscle damage to the exercise: A systematic review with meta-analysis. Sport Sci. Health 2019, 15, 265–279.
- Fedewa, M.V.; Spencer, S.O.; Williams, T.D.; Becker, Z.E.; Fuqua, C.A. Effect of branched-Chain Amino Acid Supplementation on Muscle Soreness following Exercise: A Meta-Analysis. Int. J. Vitam. Nutr. Res. 2019, 89, 348–356.
- VanDusseldorp, T.A.; Escobar, K.A.; Johnson, K.E.; Stratton, M.T.; Moriarty, T.; Cole, N.; McCormick, J.J.; Kerksick, C.M.; Vaughan, R.A.; Dokladny, K.; et al. Effect of Branched-Chain Amino Acid Supplementation on Recovery Following Acute Eccentric Exercise. Nutrients 2018, 10, 1389.
- Correia, A.L.M.; de Lima, F.D.; Bottaro, M.; Vieira, A.; da Fonseca, A.C.; Lima, R.M. Pre-exercise β-hydroxy-β-methylbutyrate free-acid supplementation improves work capacity recovery: A randomized, double-blinded, placebo-controlled study. Appl. Physiol. Nutr. Metab. 2018, 43, 691–696.
- Silva, V.R.; Belozo, F.L.; Micheletti, T.O.; Conrado, M.; Stout, J.R.; Pimentel, G.D.; Gonzalez, A.M. β-hydroxy-β-methylbutyrate free acid supplementation may improve recovery and muscle adaptations after resistance training: A systematic review. Nutr. Res. 2017, 45, 1–9.
- Rahimi, M.H.; Mohammadi, H.; Eshaghi, H.; Askari, G.; Miraghajani, M. The Effects of Beta-Hydroxy-Beta-Methylbutyrate Supplementation on Recovery Following Exercise-Induced Muscle Damage: A Systematic Review and Meta-Analysis. J. Am. Coll. Nutr. 2018, 37, 640–649.
- Rawson, E.S.; Miles, M.P.; Larson-Meyer, D.E. Dietary Supplements for Health, Adaptation, and Recovery in Athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 188–199.
- Ammar, A.; Bailey, S.J.; Chtourou, H.; Trabelsi, K.; Turki, M.; Hökelmann, A.; Souissi, N. Effects of pomegranate supplementation on exercise performance and post-exercise recovery in healthy adults: A systematic review. Br. J. Nutr. 2018, 120, 1201–1216.
- Alcantara, J.M.A.; Sanchez-Delgado, G.; Martinez-Tellez, B.; Labayen, I.; Ruiz, J.R. Impact of cow’s milk intake on exercise performance and recovery of muscle function: A systematic review. J. Int. Soc. Sports Nutr. 2019, 16.
- Amiri, M.; Ghiasvand, R.; Kaviani, M.; Forbes, S.C.; Salehi-Abargouei, A. Chocolate milk for recovery from exercise: A systematic review and meta-analysis of controlled clinical trials. Eur. J. Clin. Nutr. 2018, 73, 835–849.
- Vitale, K.C.; Owens, R.; Hopkins, S.R.; Malhotra, A. Sleep Hygiene for Optimizing Recovery in Athletes: Review and Recommendations. Int. J. Sports Med. 2019, 40, 535–543.
- Fullagar, H.H.K.; Duffield, R.; Skorski, S.; Coutts, A.J.; Julian, R.; Meyer, T. Sleep and Recovery in Team Sport: Current Sleep-Related Issues Facing Professional Team-Sport Athletes. Int. J. Sports Physiol. Perform. 2015, 10, 950–957.
- von Rosen, P.; Frohm, A.; Kottorp, A.; Fridén, C.; Heijne, A. Too little sleep and an unhealthy diet could increase the risk of sustaining a new injury in adolescent elite athletes. Scand. J. Med. Sci. Sports 2017, 27, 1364–1371.
- Silva, M.R.G.; Paiva, T. Poor precompetitive sleep habits, nutrients’ deficiencies, inappropriate body composition and athletic performance in elite gymnasts. Eur. J. Sport Sci. 2015, 16, 726–735.
- Gupta, L.; Morgan, K.; Gilchrist, S. Does Elite Sport Degrade Sleep Quality? A Systematic Review. Sports Med. 2016, 47, 1317–1333.
- Bonnar, D.; Bartel, K.; Kakoschke, N.; Lang, C. Sleep Interventions Designed to Improve Athletic Performance and Recovery: A Systematic Review of Current Approaches. Sports Med. 2018, 48, 683–703.
- Peuhkuri, K.; Sihvola, N.; Korpela, R. Diet promotes sleep duration and quality. Nutr. Res. 2012, 32, 309–319.
- Doherty, R.; Madigan, S.; Warrington, G.; Ellis, J. Sleep and Nutrition Interactions: Implications for Athletes. Nutrients 2019, 11, 822.
- Kim, J. Pre-sleep casein protein ingestion: New paradigm in post-exercise recovery nutrition. Phys. Act. Nutr. 2020, 24, 6–10.
- Res, P.T.; Groen, B.; Pennings, B.; Beelen, M.; Wallis, G.A.; Gijsen, A.P.; Senden, J.M.G.; Van Loon, L.J.C. Protein Ingestion before Sleep Improves Postexercise Overnight Recovery. Med. Sci. Sports Exerc. 2012, 44, 1560–1569.
- Snijders, T.; Res, P.T.; Smeets, J.S.J.; van Vliet, S.; van Kranenburg, J.; Maase, K.; Kies, A.K.; Verdijk, L.B.; van Loon, L.J.C. Protein Ingestion before Sleep Increases Muscle Mass and Strength Gains during Prolonged Resistance-Type Exercise Training in Healthy Young Men. J. Nutr. 2015, 145, 1178–1184.
- Wall, B.T.; Burd, N.A.; Franssen, R.; Gorissen, S.H.M.; Snijders, T.; Senden, J.M.; Gijsen, A.P.; van Loon, L.J.C. Presleep protein ingestion does not compromise the muscle protein synthetic response to protein ingested the following morning. Am. J. Physiol.-Endocrinol. Metab. 2016, 311, E964–E973.
- Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Snijders, T.; Halson, S.L.; Rollo, I.; Verdijk, L.B.; van Loon, L.J.C. Presleep dietary protein-derived amino acids are incorporated in myofibrillar protein during postexercise overnight recovery. Am. J. Physiol.-Endocrinol. Metab. 2018, 314, E457–E467.
- Snijders, T.; Trommelen, J.; Kouw, I.W.K.; Holwerda, A.M.; Verdijk, L.B.; van Loon, L.J.C. The Impact of Pre-sleep Protein Ingestion on the Skeletal Muscle Adaptive Response to Exercise in Humans: An Update. Front. Nutr. 2019, 6.
- Larsen, M.S.; Clausen, D.; Jørgensen, A.A.; Mikkelsen, U.R.; Hansen, M. Presleep Protein Supplementation Does Not Improve Recovery during Consecutive Days of Intense Endurance Training: A Randomized Controlled Trial. Int. J. Sport Nutr. Exerc. Metab. 2019, 1–9.
- Reis, C.E.G.; Loureiro, L.M.R.; Roschel, H.; da Costa, T.H.M. Effects of pre-sleep protein consumption on muscle-related outcomes—A systematic review. J. Sci. Med. Sport 2020.
- Deshpande, A.; Irani, N.; Balkrishnan, R.; Benny, I.R. A randomized, double blind, placebo controlled study to evaluate the effects of ashwagandha (Withania somnifera) extract on sleep quality in healthy adults. Sleep Med. 2020, 72, 28–36.




