
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cristián A. Amador | + 1626 word(s) | 1626 | 2021-05-17 06:25:38 | | | |
| 2 | Karina Chen | Meta information modification | 1626 | 2021-05-31 05:49:29 | | |
Video Upload Options
Polymeric hydrogels (PolyHy) have been extensively explored for their applications in biomedicine as biosensors, in tissue engineering, diagnostic processes, and drug release. The physical and chemical properties of PolyHy indicate their potential use in regulating drug delivery.
1. Family of Hydrogels
Polymeric hydrogels can be classified based on different factors:
-
Types of bonds in the reticulated three-dimensional structure:
-
Physically cross-linked PolyHy: This hydrogel undergoes a transition from the three-dimensional state to the initial state of polymeric chains in solution and thus constitutes the so-called ‘reversible ones’. Cross-links are formed via attractive forces or interactions between the polymeric chains (Figure 1A), such as hydrogen bonding, hydrophobic and electrostatic interactions [1]. This group consists of multifunctional hydrogels based on the copolymers of polyacrylamide and tannic acid that are cross-linked through hydrogen bonds [2].
-
Chemically cross-linked PolyHy: This type of hydrogel is more stable in comparison with the physically cross-linked one due to the involvement of covalent bonds (Figure 1B). This indicates that they are irreversible, unless labile covalent bonds are intentionally introduced into the cross-linked structure. For instance, the chemical cross-linking of poly(vinyl alcohol) (PVA) hydrogels with citric acid has been successfully obtained to generate a controlled drug release system. Citric acid is a cheap and non-cytotoxic reagent compared to other crosslinkers that cannot be employed in biomedical applications due to their toxicity [3].
-
-
Stimuli-responsive hydrogels. The advances in chemical synthesis, availability of new precursors, and improvement in reaction mechanisms have resulted in the development of biomaterials that respond to environmental stimuli. This promotes the conversion of static hydrogels to dynamic ones that respond to generated changes or specific inputs in variables such as temperature and pH. Furthermore, the stimulus-sensitive PolyHy are known as ‘smart hydrogels’ because of their response to any slight changes in the environment, such as temperature, pH, ionic strength, light, electric field, and biomolecules, by changing the degree of swelling. The response of the smart hydrogels depends on the type of monomer or polymer used and/or structural modifications within the compound. The challenges in the development of smart hydrogels that exhibit a rapid response to the stimuli of the medium are primarily based on synthetic mechanisms [4][5][6][7][8][9].
-
PolyHy can also be classified based on their charge. Hydrogels are composed of molecular structures such as nonionic, cationic, anionic, amphoteric polyelectrolytes, or zwitterionic species. The development of zwitterionic hydrogels further confirmed their potential application in wound dressing [10][11]. The dual cross-linked networks composed of anionic and cationic monomers improve the mechanical properties of hydrogels and thus find application as injectable hydrogels [12]. On the other hand, new synthetic methodologies have been introduced in the field of cell adhesion to obtain composite hydrogels incorporating cationic groups in their structure to improve flexibility as well as cell adhesion [13].
-
Another classification involves natural and synthetic hydrogels. Due to the biocompatible, biodegradable, and non-toxic nature, natural hydrogels find potential biomedical applications. Based on the type of natural polymer, there are three biomaterials, namely protein-based, polysaccharide-based, and natural polyester-based hydrogels [14][15][16][17]. In this review, we are interested in focusing on the biomedical applications of hydrogels based on polysaccharides, mainly. Therefore, later, we dedicate a section to this type of biomaterial. On the other hand, synthetic hydrogels have favorable properties for industrial applications, such as superior mechanical properties compared to natural hydrogels. However, hybrid hydrogels with improved and intermediate properties between natural and synthetic hydrogels have been manufactured. Biocompatible hydrogels functionalized with carbon nanotubes have been successfully synthesized to stabilize hydroxyapatite. The cytotoxicity results of these materials show promising insights for their application in bone tissue engineering [18]. In addition, there is a group of biodegradable synthetic hydrogels, which have been widely used for biomedical applications. Principally, in this group we can mention polymers, such as poly(caprolactone), poly(glycolic acid), poly(lactic acid), poly(D,L-lactide-co-glycolide), and polyurethane [19]. The studies on fucoidan-modified PVA hydrogels have revealed substantial improvement in cell adhesion and hemocompatibility, suggesting the utility of these hybrid materials as implants or vascular devices [20]. In cell microenvironment engineering, these materials provide very thin platforms that are utilized to seed the endothelial cells. An attractive biocompatible scaffold based on modified gelatin and a biodegradable polyester of poly(D,L-lactic acid) (PDLLA) was developed. The combination of these two biomaterials provides the ideal environment for cytocompatibility and cellular interaction exhibited by gelatin. In addition, PDLLA provides appropriate mechanical resistance for the graft tissue in corneal endothelial transplant [21].
-
Based on their size, hydrogels are classified into those containing macroscopic structures and those containing networks of smaller dimensions, such as microgels or nanogels. The conformation of hydrogels with micrometric precision at the level of individual cells has allowed their application as artificial cells [22][23], drug and cell carrier systems [24][25], and assembled elemental units in artificial tissue construction [26][27]. Polysaccharide-based microgels are one of the most commonly used hydrogels. Recent studies have focused on microgels that can stabilize and release lipophilic particles. Microgels were prepared from corn starch via an oxidized-annealing process. Further, the adsorption/release capacity of lysozyme by the microgels was evaluated. Studies showed that the oxidized-annealed microgels exhibit high charge and release capacities over a wide range of pH and ionic strength values. This is attributed to the electrostatic forces between the carboxyl groups generated by the oxidation-annealing process of the microgel and lysozyme [28]. The hydrogels embedded in microgels to form a hydrogel/microgel system have been utilized as wound dressings. The carboxymethyl chitosan- and oxidized carboxymethyl cellulose-based microgels are drug-loaded via the Schiff base reaction. The gel time, morphology, swelling ratio, weight loss ratio, mechanical properties, release profiles of pH-sensitive drugs, and antibacterial activities were analyzed. The addition of microgels was found to offer stability, improved mechanical performance, and sensitivity to drug release at different pH conditions for the hydrogels. The addition of Ag compounds to the hydrogel/microgel composites demonstrated desirable antibacterial properties, which ensured their possible application in wound dressing [9].
On the other hand, nanogels, which are also known as nano-sized hydrogels or hydrogel nanoparticles, are nanostructured three-dimensional networks composed of functional polymers, whose sizes are in the sub-micrometer region, typically from 20 to 200 nm. Hydrogel nanoparticles are formed by physical as well as chemical cross-linking methods. Nanogels offer several advantages, such as biocompatibility, high swelling capacity, and high-water solubility. In addition, they are classified as stimuli-responsive and nonresponsive hydrogels. Nanogels respond to fluctuations in environmental factors, such as temperature, pH, pressure, electric and magnetic fields, molecular species, ionic strength, or a combination of different factors. Therefore, nanogels are exceptional candidates for application in biomedicine, such as biosensors, drug release, tissue engineering, diagnosis, etc. [20][29][30].
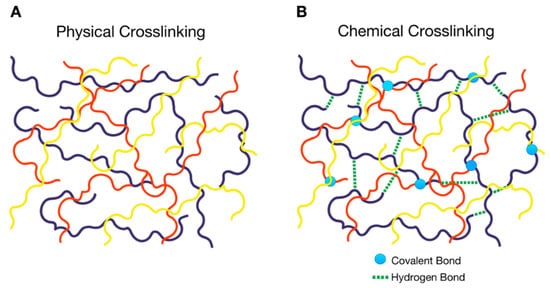
Figure 1. Schematic representation of the types of cross-linking in a polymer hydrogel (PolyHy); (A) physical cross-linking of different types of polymeric chains denoted by different colors, (B) chemical cross-linking consisting of covalent bonds between the polymeric chains denoted by circles and non-binding interactions such as hydrogen bonds represented by dashed lines.
2. Tissue Engineering Applications of Natural Polymeric Hydrogels
The hydrogels consisting of natural polymers have been recognized as prime candidates for designing tissue scaffolds. This is attributed to the excellent characteristics such as cost efficiency, high compatibility, and biodegradability exhibited by this type of hydrogels. Natural polymeric hydrogels, such as polysaccharides and proteins, were similar to the natural extracellular matrix. Thus, the hydrogels are endowed with magnificent properties, such as cellular proliferation and survival, to overcome certain challenges in tissue engineering [31][32][33]. Recently, a microbial polysaccharide-based macroporous hydrogel was manufactured from salecan and gellan gum by a one-step method [31]. These were obtained via the association of polysaccharide chains by means of Van der Waals forces, hydrogen bonds, and hydrophobic interactions. It should be noted that the manufacturing methodologies did not require crosslinkers or toxic monomers. Instead, the salecan/gellan gum hydrogel that exhibits excellent biocompatibility and properties to mimic the native tissues were utilized. In this context, cardiac tissue engineering has emerged as a branch of tissue engineering, which uses combinations of cells, biological and/or synthetic materials, growth factors, differentiation factors, proangiogenic factors, and monitoring, as a promising and challenging approach to induce the regeneration of heart tissue [34]. This field of research gave rise to the study of biocompatible hybrid systems [35], such as polysaccharide-based PolyHy, for application in tissue engineering [36][37].
Alginate (Alg) and chitosan (CS) are natural polysaccharides that are widely used in biomedicine, as well as food and pharmaceutical industries [38][39]. This is attributed to the high biocompatibility and biodegradability of polysaccharides [15][40]. A recent study on the improvement of the mechanical tensile strength of decellularized extracellular cardiac matrix (ECM) using Alg/CS hydrogels [41] showed a high swelling capacity, good mechanical resistance, moderate biodegradable properties, and excellent cell viability. The results of the study reveal that CS- and Alg-based platforms find potential applications in cardiac tissue engineering. In addition, other natural polymers, such as collagen, gelatin (Gel), and fibrin, have been studied for myocardial tissue engineering. The collagen/CS composite scaffold has been widely used for myocardial tissue engineering [36]. Since collagen, which is the main component of the ECM within the myocardium, has a fast degradation rate and poor mechanical properties [42][43], it has been combined with other polysaccharides to improve the characteristics of the composite PolyHy.
The research in tissue engineering aims to manufacture materials that mimic native tissues of the human organs. The biomimetic materials capable of mimicking the structure and composition of the native extracellular matrices are essential for successful organ tissue repair and transplantation in humans. Similarly, fibrin-agarose tissue-like hydrogels (FATLHs) have allowed successful biofabrication of different biological substitutes, and thus, delivered promising ex vivo and in vivo results [44]. These fibrin-agarose-based materials biodegrade and integrate into implanted areas and vital organs without any histopathological alteration. Thus, FATLHs exhibit potential clinical uses in engineering applications due to their biosecurity and biocompatibility. Furthermore, these artificial tissues combine to be stronger, and thus offer a stable mechanical response along with favorable cytocompatibility [45].
References
- Lin, Y.-H.; Liang, H.-F.; Chung, C.-K.; Chen, M.-C.; Sung, H.-W. Physically crosslinked alginate/N,O-carboxymethyl chitosan hydrogels with calcium for oral delivery of protein drugs. Biomaterials 2005, 26, 2105–2113.
- Li, X.; Peng, X.; Li, R.; Zhang, Y.; Liu, Z.; Huang, Y.; Long, S.; Li, H. Multiple Hydrogen Bonds–Reinforced Hydrogels with High Strength, Shape Memory, and Adsorption Anti-Inflammatory Molecules. Macromol. Rapid Commun. 2020, 2000202.
- Sabzi, M.; Afshari, M.J.; Babaahmadi, M.; Shafagh, N. pH-dependent swelling and antibiotic release from citric acid crosslinked poly(vinyl alcohol) (PVA)/nano silver hydrogels. Colloids Surf. B Biointerfaces 2020, 188, 110757.
- Liu, J.; Fan, X.; Tao, Y.; Deng, C.; Yu, K.; Zhang, W.; Deng, L.; Xiong, W. Two-Step Freezing Polymerization Method for Efficient Synthesis of High-Performance Stimuli-Responsive Hydrogels. ACS Omega 2020, 5, 5921–5930.
- Huang, J.; Jiang, X. Injectable and Degradable pH-Responsive Hydrogels via Spontaneous Amino–Yne Click Reaction. ACS Appl. Mater. Interfaces 2018, 10, 361–370.
- Rajan, R.; Pangkom, N.; Matsumura, K. Design of Stimuli-Responsive Polyampholytes and Their Transformation into Micro-Hydrogels for Drug Delivery. In Polymers in Therapeutic Delivery; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2020; Volume 1350, pp. 47–62. ISBN 978-0-8412-3814-5.
- Liu, Q.; Liu, L. Novel Light-Responsive Hydrogels with Antimicrobial and Antifouling Capabilities. Langmuir 2019, 35, 1450–1457.
- Xiang, T.; Lu, T.; Zhao, W.-F.; Zhao, C.-S. Ionic-Strength Responsive Zwitterionic Copolymer Hydrogels with Tunable Swelling and Adsorption Behaviors. Langmuir 2019, 35, 1146–1155.
- Huang, K.-T.; Ishihara, K.; Huang, C.-J. Polyelectrolyte and Antipolyelectrolyte Effects for Dual Salt-Responsive Interpenetrating Network Hydrogels. Biomacromolecules 2019, 20, 3524–3534.
- He, H.; Xuan, X.; Zhang, C.; Song, Y.; Chen, S.; Gong, X.; Ren, B.; Zheng, J.; Wu, J. Simple Thermal Pretreatment Strategy to Tune Mechanical and Antifouling Properties of Zwitterionic Hydrogels. Langmuir 2019, 35, 1828–1836.
- GhavamiNejad, A.; Park, C.H.; Kim, C.S. In Situ Synthesis of Antimicrobial Silver Nanoparticles within Antifouling Zwitterionic Hydrogels by Catecholic Redox Chemistry for Wound Healing Application. Biomacromolecules 2016, 17, 1213–1223.
- Bakaic, E.; Smeets, N.M.B.; Badv, M.; Dodd, M.; Barrigar, O.; Siebers, E.; Lawlor, M.; Sheardown, H.; Hoare, T. Injectable and Degradable Poly(Oligoethylene glycol methacrylate) Hydrogels with Tunable Charge Densities as Adhesive Peptide-Free Cell Scaffolds. ACS Biomater. Sci. Eng. 2018, 4, 3713–3725.
- Goto, K.; Teramoto, Y. Development of chitinous nanofiber-based flexible composite hydrogels capable of cell adhesion and detachment. Polym. J. 2020, 52, 959–967.
- Singh, M.R.; Patel, S.; Singh, D. Chapter 9—Natural Polymer-based Hydrogels as Scaffolds for Tissue Engineering. In Nanobiomaterials in Soft Tissue Engineering; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 231–260. ISBN 978-0-323-42865-1.
- Hsu, S.; Hung, K.-C.; Chen, C.-W. Biodegradable polymer scaffolds. J. Mater. Chem. B 2016, 4, 7493–7505.
- Kouhi, M.; Varshosaz, J.; Hashemibeni, B.; Sarmadi, A. Injectable gellan gum/lignocellulose nanofibrils hydrogels enriched with melatonin loaded forsterite nanoparticles for cartilage tissue engineering: Fabrication, characterization and cell culture studies. Mater. Sci. Eng. C 2020, 115, 111114.
- Akalin, O.B.; Bayraktar, H. Alteration of cell motility dynamics through collagen fiber density in photopolymerized polyethylene glycol hydrogels. Int. J. Biol. Macromol. 2020, 157, 414–423.
- Arumugam, S.; Ramamoorthy, P.; Chakkarapani, L.D. Synthesis and characterizations of biocompatible polymers and carbon nanotubes-based hybrids for biomedical applications. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 786–797.
- Hernandez-Gordillo, V.; Kassis, T.; Lampejo, A.; Choi, G.; Gamboa, M.E.; Gnecco, J.S.; Brown, A.; Breault, D.T.; Carrier, R.; Griffith, L.G. Fully synthetic matrices for in vitro culture of primary human intestinal enteroids and endometrial organoids. Biomaterials 2020, 254, 120125.
- Yao, Y.; Zaw, A.M.; Anderson, D.E.J.; Hinds, M.T.; Yim, E.K.F. Fucoidan functionalization on poly(vinyl alcohol) hydrogels for improved endothelialization and hemocompatibility. Biomaterials 2020, 249, 120011.
- Van Hoorick, J.; Delaey, J.; Vercammen, H.; Van Erps, J.; Thienpont, H.; Dubruel, P.; Zakaria, N.; Koppen, C.; Van Vlierberghe, S.; Van den Bogerd, B. Designer Descemet Membranes Containing PDLLA and Functionalized Gelatins as Corneal Endothelial Scaffold. Adv. Healthc. Mater. 2020, 2000760.
- Merkel, T.J.; Jones, S.W.; Herlihy, K.P.; Kersey, F.R.; Shields, A.R.; Napier, M.; Luft, J.C.; Wu, H.; Zamboni, W.C.; Wang, A.Z.; et al. Using mechanobiological mimicry of red blood cells to extend circulation times of hydrogel microparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 586.
- Vakkipurath Kodakkadan, Y.N.; Idzakovicova, K.; Sepitka, J.; ten Napel, D.; Safai, E.; Cigler, P.; Štěpánek, F.; Rehor, I. Arbitrarily-shaped microgels composed of chemically unmodified biopolymers. Biomater. Sci. 2020, 8, 3044–3051.
- Galdioli Pellá, M.C.; Simão, A.R.; Lima-Tenório, M.K.; Tenório-Neto, E.; Scariot, D.B.; Nakamura, C.V.; Rubira, A.F. Chitosan hybrid microgels for oral drug delivery. Carbohydr. Polym. 2020, 239, 116236.
- Jooybar, E.; Abdekhodaie, M.J.; Karperien, M.; Mousavi, A.; Alvi, M.; Dijkstra, P.J. Developing hyaluronic acid microgels for sustained delivery of platelet lysate for tissue engineering applications. Int. J. Biol. Macromol. 2020, 144, 837–846.
- Rose, J.C.; Gehlen, D.B.; Haraszti, T.; Köhler, J.; Licht, C.J.; De Laporte, L. Biofunctionalized aligned microgels provide 3D cell guidance to mimic complex tissue matrices. Biomaterials 2018, 163, 128–141.
- Compaan, A.M.; Song, K.; Chai, W.; Huang, Y. Cross-Linkable Microgel Composite Matrix Bath for Embedded Bioprinting of Perfusable Tissue Constructs and Sculpting of Solid Objects. ACS Appl. Mater. Interfaces 2020, 12, 7855–7868.
- Ji, Y. Microgels prepared from corn starch with an improved capacity for uptake and release of lysozyme. J. Food Eng. 2020, 285, 110088.
- Feldman, D. Polymers and Polymer Nanocomposites for Cancer Therapy. Appl. Sci. 2019, 9, 3899.
- Pedrosa, S.S.; Pereira, P.; Correia, A.; Moreira, S.; Rocha, H.; Gama, F.M. Biocompatibility of a Self-Assembled Crosslinkable Hyaluronic Acid Nanogel. Macromol. Biosci. 2016, 16, 1610–1620.
- Qi, X.; Su, T.; Zhang, M.; Tong, X.; Pan, W.; Zeng, Q.; Shen, J. Sustainable, flexible and biocompatible hydrogels derived from microbial polysaccharides with tailorable structures for tissue engineering. Carbohydr. Polym. 2020, 237, 116160.
- Liu, L.; Zhang, Y.; Yu, S.; Yang, Z.; He, C.; Chen, X. Dual Stimuli-Responsive Nanoparticle-Incorporated Hydrogels as an Oral Insulin Carrier for Intestine-Targeted Delivery and Enhanced Paracellular Permeation. ACS Biomater. Sci. Eng. 2018, 4, 2889–2902.
- Zhou, M.; Hu, Q.; Wang, T.; Xue, J.; Luo, Y. Alginate hydrogel beads as a carrier of low density lipoprotein/pectin nanogels for potential oral delivery applications. Int. J. Biol. Macromol. 2018, 120, 859–864.
- Gálvez-Montón, C.; Prat-Vidal, C.; Roura, S.; Soler-Botija, C.; Bayes-Genis, A. Cardiac Tissue Engineering and the Bioartificial Heart. Rev. Española Cardiol. (Engl. Ed.) 2013, 66, 391–399.
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95.
- Pok, S.; Myers, J.D.; Madihally, S.V.; Jacot, J.G. A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater. 2013, 9, 5630–5642.
- Efraim, Y.; Sarig, H.; Cohen Anavy, N.; Sarig, U.; de Berardinis, E.; Chaw, S.-Y.; Krishnamoorthi, M.; Kalifa, J.; Bogireddi, H.; Duc, T.V.; et al. Biohybrid cardiac ECM-based hydrogels improve long term cardiac function post myocardial infarction. Acta Biomater. 2017, 50, 220–233.
- Verma, M.L.; Dhanya, B.S.; Sukriti; Rani, V.; Thakur, M.; Jeslin, J.; Kushwaha, R. Carbohydrate and protein based biopolymeric nanoparticles: Current status and biotechnological applications. Int. J. Biol. Macromol. 2020, 154, 390–412.
- Novak, U.; Bajić, M.; Kõrge, K.; Oberlintner, A.; Murn, J.; Lokar, K.; Triler, K.V.; Likozar, B. From waste/residual marine biomass to active biopolymer-based packaging film materials for food industry applications—A review. Phys. Sci. Rev. 2019, 5, 20190099.
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector—Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837.
- Tamimi, M.; Rajabi, S.; Pezeshki-Modaress, M. Cardiac ECM/chitosan/alginate ternary scaffolds for cardiac tissue engineering application. Int. J. Biol. Macromol. 2020, 164, 389–402.
- Dunn, D.A.; Hodge, A.J.; Lipke, E.A. Biomimetic materials design for cardiac tissue regeneration. WIREs Nanomed. Nanobiotechnol. 2014, 6, 15–39.
- Zhang, J.; Zhou, A.; Deng, A.; Yang, Y.; Gao, L.; Zhong, Z.; Yang, S. Pore architecture and cell viability on freeze dried 3D recombinant human collagen-peptide (RHC)–chitosan scaffolds. Mater. Sci. Eng. C 2015, 49, 174–182.
- Campos, F.; Bonhome-Espinosa, A.B.; Chato-Astrain, J.; Sánchez-Porras, D.; García-García, Ó.D.; Carmona, R.; López-López, M.T.; Alaminos, M.; Carriel, V.; Rodriguez, I.A. Evaluation of Fibrin-Agarose Tissue-Like Hydrogels Biocompatibility for Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2020, 8, 596.
- Bonhome-Espinosa, A.B.; Campos, F.; Durand-Herrera, D.; Sánchez-López, J.D.; Schaub, S.; Durán, J.D.G.; Lopez-Lopez, M.T.; Carriel, V. In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2020, 104, 103619.




