Video Upload Options
Nanotechnology is playing a significant role in addressing a vast range of environmental challenges by providing innovative and effective solutions. Heavy metal (HM) contamination has gained considerable attention in recent years due their rapidly increasing concentrations in agricultural soil. Due to their unique physiochemical properties, nanoparticles (NPs) can be effectively applied for stress alleviation
A1. Introduction
Rapid urbanization and industrialization, constant application of agrochemicals and fertilizers, unreasonable mining and waste management have caused heavy metal (HM) contamination to become an increasingly prominent issue [1][2][3]. There are over 10 million contaminated sites worldwide and more than 50 percent of them are contaminated with HM [4]. According to the China Ministry of Environmental Protection, the percentage of exceedance in agricultural soils is even greater than 19.4%. Among them, the average contents of Cd, Hg, As, Cu, Pb, Cr, Zn and Ni in the soil exceed the standard by 7.0%, 1.6%, 2.7%, 2.1%, 1.5%, 1.1%, 0.9% and 4.8%, respectively [5]. Due to the Chinese diet structure, the risk screening values for soil contamination of agricultural land are low. For example, the China agricultural land Cd limit is 0.3~0.6 mg/kg, while the screening value (ECOSSL) recommended by the United States Environmental Protection Agency (US-EPA) is 0.4~0.8 mg/kg [6][7]. Only in China, the HM contaminated area of farmland soil is about 2 × 107 Hm2, the annual amount of polluted grains is 1.2 × 107 t and the economic loss is $3 × 109 [8]. Recently, several studies have shown that HM pollution in farmland soil is continuously increasing [9][10].
In recent years, HM contamination has attracted public attention due to the frequent detection in food and blood. HM stress affects the plants growth and indirectly affects human health via the food chain [11]. Cereal, as a predominant food in China, are susceptible to HM pollution. Rice in particular accumulates more HM than other plants [12][13]. The notorious “Itai-Itai disease” in Japan is caused by long-term consumption of Cd-contaminated rice. Besides, excessive accumulation of HM causes systemic bone softening, nervous system damage, reproductive dysfunction and even death [14]. The Agency for Toxic Substances and Disease Registry (ATSDR) and the US-EPA have listed Cd, Pb, As and Hg among the top 20 hazardous substances. Furthermore, the current status of the rare earth elements (REEs) in the environment and potential effects on biota were described in our previous publication [15]. The deleterious effects of REEs and HM pollution in the soil environment require serious attention to overcome.
HM pollution is one of the key factors restricting crop production and threatening food security [16][17]. HM mainly affect the normal cell structure, the antioxidant system and plant growth to restrict crop production. More importantly, the global population is estimated to grow to 8.54 billion in 2030 and 9.73 billion in 2050, which means that current food production must increase by 70~100 percent to meet the needs of the world population [18][19]. Therefore, it is necessary to improve and popularize HM removal/immobilization technology in polluted farmland.
In recent years, the applications of nanoparticles (NPs) have increased dramatically in industry, medicine, agriculture and cosmetics fields [20]. NPs usually refer to materials with at least one dimension smaller than 100 nm. NPs with different particle sizes, geometries and functions can be synthesized according to the needs [21]. Compared with ordinary materials, NPs have many advantages, such as high surface activity, more surface reaction sites, good catalytic efficiency, and unique optical and magnetic properties [22][23][24]. The effects of NPs on environment have been discussed in our previous studies, such as Hao et al. demonstrated that fungal endophytes in rice were sensitive to carbon-based NMs even at 10 mg/L [25]. Rui et al. reported that biomass and quality of peanut were negatively influenced by exposure to Ag NPs at 50 mg/kg [26]. Additionally, Adeel et al. found that low doses (5 and 50 mg/kg) of NiO did not affect the survival, reproduction, and growth rate of adult earthworms, while high doses (200 and 500 mg/kg) significantly affected the physiological and biochemical endpoints [20].
NPs have been already successfully applied in agriculture and in environmental applications. Several studies have revealed that NPs could improve plant seed germination, plant photosynthesis, resistance to oxidative stress, rhizome growth and development, crop yield and quality [27][28][29][30]. On the one hand, NPs are applied as nanofertilizers [31][32][33] and nanopesticides [34][35][36][37], which have the advantages of being easily absorbed by plants and slowly released in the environment compared with traditional fertilizers [38], but the potential ecological risks still need further research [39]. On the other hand, NPs (such as CeO2, TiO2, and Mn3O4 NPs) can enhance the activity of antioxidant enzymes, which can reduce the accumulation of reactive oxygen species (ROS) in plants, alleviating plant stress and improving the quality and yield [40][41].
The development of nanoscience provides a new direction for the advancement of soil remediation [42]. Due to the unique properties, NPs not only have a better repair effect than traditional materials but also can add some new functions [43]. Except through soil, foliar spraying is another way to improve plants resistance to HM [44]. For example, selenium and silicon NPs alleviated Cd and Pb stress in rice through foliar application [45]. Foliar spraying of TiO2 NPs is more effective than soil addition in reducing Cd toxicity to maize [46][47][48][49][50].
2. Nano-Remediation in Plant Under HM Stress
2.1. Mechanisms of Nanoparticles to Alleviate the HM Stress
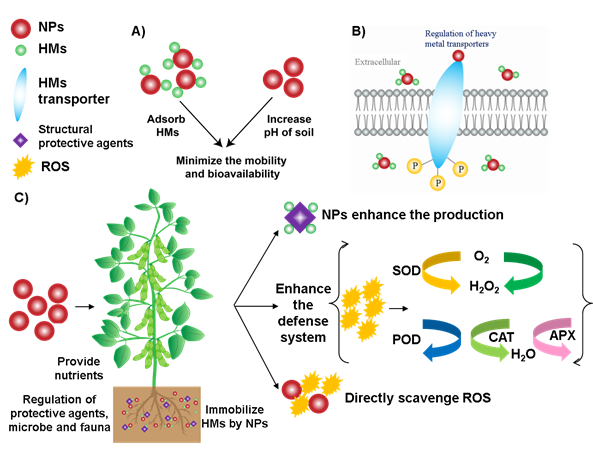
The morphology, physiology and biochemistry of the plant have been shown to be influenced by HM stress. There are several strategies applied to overcome the HM stress in plants (Figure 1). For example, 1) reducing the concentration of bioavailable HM in the soil, 2) regulating the expression of HM transport genes in plants [51], 3) enhancing the ability of plant antioxidant systems and improving the physiological functions [41]and 4) promoting the production of protective agents (such as root exudates, phytochelatin and organic acids)[52][53].
Figure 1. Mechanisms of NPs to alleviate HM stress in soil and plant tissues. (A) NPs increase the soil pH, adsorb HM, which minimize the mobility and bioavailability of HM. (B) NPs can enhance the defense ability of plants by regulating the HM transport genes. (C) NPs enhance the plant defense system, promote the production of protective agents and directly scavenge ROS, which ultimately improve the plant growth and nutritional content of fruits.
The HMs in the soil can be absorbed and transformed by the NPs, reducing the mobility and bioavailability of HM. For example, Fe3O4 NPs decreased the mobility of Cd and other HMs in the soil [54]. Wang et al. reported that mercapto Si NPs transformed Cd into a more stable constituent under field conditions after three years [55]. Besides, some NPs can improve soil properties, such as hydroxyapatite NPs can release phosphate and increase soil pH, reducing the harmful effects of HM in the soil [56].
Apoplastic barriers in the plant root have the physiological function of protecting the plant and controlling the flow of water, ions and oxygen [57]. The formation of apoplastic barriers can be influenced by NPs, which may decrease the amount of HM in the root [58]. However, the apoplastic barriers alone may not be an effective strategy to reduce the damage caused by HM stress because plant roots contain various ion and protein transporters in the root cell plasma membrane, which can transport HM simultaneously. Moreover, plant metal transport genes can be regulated by specific NPs, thereby enhancing the plant’s extracellular barrier to intercept HM.
Most NPs accumulate in the cell walls, bind with HM and make them unavailable by forming complexes. These complexes become adsorbed on the cell surface [59][60], thereby hindering the migration of HM in plants and reducing their biological activity. Besides, organic acids accumulated in the cell walls of plant roots and leaves can be chelated with HM to reduce the damage of HM stress to plants. The production of structural protective agents has been shown to be enhanced by NPs, such as applying exogenous Si NPs which promoted the synthesis of organic acids and reduced the damage of Cd to the plant.
Another strategy to alleviate HM stress is to activate the oxidation defense system of the plant. Plants usually produce ROS due to specific biochemical reactions [61]. For example, during the metabolic processes such as respiration and photosynthesis, plants continuously produce ROS in chloroplasts and other parts of cells. At low levels, ROS act as signal molecules involved in growth, development, and defense. However, under stress conditions, excessive ROS accumulation is harmful to cell membranes, proteins, and other cellular components [61]. ROS in plants is mainly scavenged by antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), glutathione peroxidase (GPX) and peroxidase (POD). In addition, non-enzymatic low-molecular weight metabolites also can scavenge ROS, such as vitamin C, vitamin E and polyphenols [62][63][64]. Metabolic pathways related to ROS clearance will be triggered under stress. For example, shikimate-phenylpropanoid biosynthesis and galactose, alanine, aspartic acid and ascorbate metabolism can alleviate the oxidative stress of the plant [65]. Therefore, application of NPs with antioxidant enzyme activity (such as CeO2 NPs, Fe3O4 NPs, Mn3O4 NPs and C60) can enhance the ability of plants to reduce ROS, ultimately minimize the effects on crop growth and yield losses [66][67][68].
2.2. Impacts of Metal-Based Nanoparticles on the HM Stress in Plants
2.2.1. Cerium Dioxide
CeO2 NPs are light rare earth elements (LREEs), recommended by the Organization for Economic Co-operation and Development (OECD) for safety evaluation compared to other ENPs due to their widespread applications and beneficial effects in agriculture systems [69]. CeO2 NPs to alleviate the stress is mainly due to the Ce3+ and Ce4+ dangling bonds on the surface that can scavenge ROS and minimize oxidative stress caused by HM [70]. For example, negatively charged CeO2 NPs effectively accumulated in chloroplasts and eliminated ROS in Arabidopsis leaf mesophyll cells compared with positively charged NPs, while the process was affected by the ratio of Ce3+/Ce4+. Several studies have indicated that the low concentration of CeO2 NPs is beneficial to the plant growth. For example, Cao et al. found that CeO2 NPs (100 mg/kg), both uncoated and polyvinylpyrrolidone (PVP) coated, can increase the photosynthesis rate of soybeans and stimulate plant growth, whereas 500 mg/kg CeO2-NPs reduced the net photosynthesis rate by 36% [71]. CeO2 NPs (100 and 200 mg/kg) also improved the physiological parameters of Brassica napus L under salt stress (100 mM NaCl) [72]. Ma et al. determined that bulk CeO2 (10 and 100 mg/L) increased biomass of Brassica rapa, while at the same concentration, CeO2 NPs exerted no significant effect [73]. This might happen due to the formation of aggregates and the selected concentration of CeO2 NPs. These studies indicate that there are still many unknowns regarding the effect of NPs surface modifications, which strongly affect the stability and mobility of NPs, on the plant health.
Foliar application of CeO2 NPs (200 mg/L) effectively stimulated the antioxidant defense system in rice grown in hydroponic conditions (CdCl2 50 μM) and inhibited the accumulation of Cd. Furthermore, CeO2 NPs (500 mg/kg) did not affect the accumulation of Cd in soybeans in Cd-contaminated soil (0.2542 and 1.0 mg/kg) while significantly increased the accumulation of Ce in plant tissues. This was partly caused by the co-exposure of Cd and CeO2 NPs that influenced root apoplastic barriers. Wang et al. reported that CeO2 NPs (100 mg/L) did not affect the accumulation of As (III) (1.0 mg/L) and As (V) (1.0 mg/L) in rice in hydroponic conditions [74]. As mentioned above, several contradictory studies are reported, and more in-depth investigations are needed to understand the alleviation mechanisms.
2.2.2. Titanium Dioxide Nanoparticles
Titanium dioxide NPs (TiO2 NPs), widely used in coatings, cosmetics and pigments, are among the most produced engineered NPs in the world due to the low toxicity, favorable optical properties, strong adhesive properties and high stability [75]. It is estimated that the global annual production of TiO2 NPs was 1,175,176 tons in 2012 [76][77]. The primary mechanisms of TiO2 NPs in alleviating HM stress include adsorption of HM and activation of the oxidation defense system. Several studies have found that application of TiO2 NPs can reduce the oxidative stress of plants. For example, Wang et al. found that adding TiO2 NPs to the nutrient solution increased antioxidant enzymes’ activity in corn tissues [78]. Singh et al. reported that the physiological parameters and photosynthetic rate of soybean increased after applying TiO2 NPs to soil, effectively limiting the toxicity of Cd to soybean plants [79]. Therefore, TiO2 NPs have great application potential in alleviating plant oxidative stress caused by HM.
Cai et al. documented that accumulation of Pb in rice co-exposed to Pb(NO3)2 (1.0 mg/L) and four types of TiO2 NPs (10 and 1000 mg/L) [80]. They found that none of the TiO2 NPs affected rice growth, but three types of NPs (anatase, pristine rutile and rutile with hydrophilic) effectively decreased the Pb accumulation in roots by >80% and in shoots by 77~97%. The same results were obtained by Ji et al., who showed that TiO2 NPs had little effect on rice seedling biomass in hydroponic cultivation [81]. However, the root length, plant height, hormone level, antioxidant enzyme activity and other physiological parameters were improved after exposure of TiO2 NPs, indicating that the addition of TiO2 NPs reduced the damage of Cd stress to rice seedlings.
Maize was grown in Cd-contaminated soil then TiO2 NPs (100 and 250 mg/L) were applied by mixing in soil and by foliar application. The results showed that foliar application of TiO2 NPs inhibited Cd absorption by maize and increased biomass, while soil application promoted the absorption of Cd by maize and substantially decreased the biomass. They also found that foliar spraying of TiO2 NPs increased SOD and GST activities and up-regulated galactose, alanine, aspartame acid and other metabolic pathways to alleviate Cd stress damage to maize. The results indicated that the application method had a significant influence on the alleviation effect.
Dai et al. studied the effect of uncoated TiO2 NPs and TiO2 NPs coated with sodium dodecylbenzene sulfonate (SDBS) on wheat seedlings under Cd stress [82]. After SDBS coating, the hydrodynamic diameter of TiO2 NPs was reduced, and the dispersion stability of TiO2 NPs was improved through spatial and electrostatic repulsion, thereby exposing more available adsorption sites and increasing the adsorption capacity of Cd2+. They also found that the effect of SDBS coated TiO2 NPs on relieving Cd toxicity were significantly better than these of bare TiO2 NPs. This suggests that NPs coated with surfactants or other materials affect the bioavailability and toxicity of HM.
Singh et al. reported that the accumulation of 133Cs in soybean effectively increased after the application of TiO2 NPs in 133Cs contaminated soil [83]. It suggests that NPs may be applied for phytoremediation, but long-term field-based studies are needed.
2.2.3. Iron
Iron (Fe) is an essential element for humans, animals and plants’ growth and development. It plays a vital role in physiological and biochemical reactions, such as cell metabolism, photosynthesis, and respiration [84]. For example, the synthesis of specific chlorophyll-protein complexes in chloroplasts requires Fe, and Fe deficiency causes yellowing leaves and reduced photosynthetic capacity [84][85]. Iron is also a cofactor for certain enzymes, closely related to enzyme activity [86]. Iron NPs (Fe NPs) are particularly powerful adsorbents due to the unique structure and electronic properties. Magnetic NPs (γ-Fe2O3 and Fe3O4) can be easily separated from the adsorbing medium by a magnetic field [84]. The alleviation mechanisms of nano Fe mainly include adsorption of HM, acting as an essential element, promotion of the formation of root surface iron film, activation of the oxidation defense system, and scavenging the ROS. Several studies have demonstrated that nano Fe has high application potential in the field of fertilizers. For example, Fe2O3 NPs have been proposed as Fe-containing fertilizers in the cultivation of peanuts, but the potential risks still need to be fully elucidated. In addition, it has been shown that legume root growthwas enhanced by 88~366% after using low dose Fe2O3 NPs pre-soaking.
Nanoscale zero-valent iron (nZVI) has been shown to reduce the accumulation of HM in sunflowers, increase SOD and POD in plant leaves, and promote plant growth [87]. Guha reported that after applying nZVI (100 mg/L) to rice seedlings, gene expressions of Fe transporters (IRT1, IRT2, YSL2, YSL15) responsible for both Fe and Cd uptake were significantly down-regulated. In contrast, OsVIT1 and OsCAX4 genes were overexpressed, which lead to sequestration of Cd in vacuoles [88].
Hussain et al. found that soil and foliar application of Fe2O3 NPs (5, 10, 15, and 20 ppm) to wheat under Cd stress (available Cd 0.93 mg/kg) both reduced the leaf electrolyte leakage rate, the Cd content in grains and increased the antioxidant enzyme activity and the dry weight of the wheat [89]. They also found that the foliar spraying of Fe NPs is preferred to the soil application because the absorption of Fe in the soil may be affected by many factors, such as pH, and the combination with other minerals during the absorption process. In the case of combined application, the alleviating effects of Fe NPs (foliar) application on Cd stress in rice were further enhanced when the soil was applied with biochar [90]. However, the biochar-Fe3O4 nanocomposites facilitated Cd transport in water-saturated natural soil, indicating that the risks are still not fully understood [91]. In composite stress conditions, drought stress increased the Cd concentration in grains, and the soil application of Fe2O3 NPs to wheat under drought and Cd stress improved the photosynthesis, yield and decreased the Cd content in grains [92].
2.2.4. Zinc Oxide Nanoparticles
Zinc (Zn) is an essential trace element for plants and humans. Zinc oxide NPs (ZnO NPs) are common metal oxide NPs (MNPs), widely used in personal care products, paints and coatings. Its alleviation mechanisms include providing nutritional value, activation of oxidative stress system, and enhancement of plant’s physiological and biochemical functions. For example, seed soaking with ZnO NPs (50 mg/L) promoted glycolytic metabolism and cell wall biosynthesis, promoting the germination of corn seeds and the growth of roots and embryos [93]. The combined application of ZnO NPs (500 mg/kg) and arbuscular mycorrhiza (AM) significantly promoted soybean growth [94]. Applying ZnO NPs to wheat reduced the leaves’ electrolyte leakage (EL) rate and increased the antioxidant enzyme activity in the leaves [95]. ZnO NPs can relieve the drought stress to sorghum by increasing the absorption of nitrogen and potassium [96].
Ali et al. reported that combined application of ZnO NPs (foliar spraying) and biochar (soil application) effectively relieved the Cd stress, which was more efficient than the treatment of adding ZnO NPs or biochar separately [97][98]. It suggests that the combined application of NPs with other materials may be a promising approach in plant HM reduction.
Arsenic content in roots and shoots of rice was significantly reduced by both ZnO NPs and Zn2+ [99]. In contrast, Cd accumulation in rice shoots was only reduced by ZnO NPs, while Zn2+ increased the Cd accumulation in shoots. Khan et al. found that wheat biomass, yield and photosynthesis decreased under normal water conditions and Cd stress, while drought stress further enhanced the negative impact of Cd stress on wheat, while the addition of ZnO NPs alleviated both Cd and drought stress [100]. Hussain et al. applied different concentrations of ZnO NPs (25, 50 and 100 mg/L) to Cd-stressed wheat by foliar spray and soil application, and they found that both applications of ZnO NPs promoted wheat growth, photosynthesis and grain yield under Cd stress [101]. However, the foliar application was more efficient than soil application in increasing grain yield and reducing Cd content in grains.
Rizwan et al. soaked wheat seeds in ZnO NPs (25, 50, 75 and 100 mg/L) containing medium in the dark at 25 °C for 20 h, then selected seeds with similar germination to be sown in cadmium-contaminated soil [102]. Compared with the control group, the grain yield of wheat increased, the content of Cd in the grain decreased, the content of chlorophyll a and b, and SOD and POD activity of wheat increased significantly, and the electrolyte leakage rate decreased. The results showed that using an appropriate concentration of ZnO NPs to germinate wheat seeds can enhance wheat resistance to Cd.
2.3. Impacts of Non-Metal Based Nanoparticles Impact on the HM Stress in Plants
2.3.1. Selenium
Selenium (Se) is an essential trace element for humans, which has many important biological functions, such as immune regulation, antioxidant, antiviral and anti-cancer properties [103][104][105]. Se is mainly supplemented by eating cereal crops, but in most rice-producing countries in Asia, the content is low. A recent survey shows that about 72% of areas in China are Se-deficient areas [106]. According to WHO, approximately 15% of the world’s population is deficient in Se, and China is one of the world’s 40 Se-deficient countries [107]. Therefore, Se containing fertilizer application to increase the Se content in cereal crops is essential for human health.
As an important active component of glutathione peroxidase, Se can regulate the activity of antioxidant enzymes, remove the ROS accumulated in plants, and thus effectively enhance the stress resistance of plants. For example, it has been found that the application of Se may reduce Cd uptake and toxicity in rice [108][109][110]. Recently, Huang et al. found that the inhibition effect of Se on Cd absorption of rice may be related to the formation of root surface iron film [111]. With the addition of Fe2+, Se promoted the formation of root surface iron film of rice and greatly reduced Cd accumulation in rice.
Zhang reported that Chinese cabbage’s biomass, height, leaf chlorophyll content, SOD, and GSH-Px increased and the Cd content and MDA of leaves decreased after foliar spraying of Se NPs (1.25 and 12.5 mg/L) to Chinese cabbage under Cd stress (10 and 20 mg/kg) [112]. Both in-soil (0.5 and 2.5 mg/kg) and foliar (0.5 and 1.0 mmol/L) application of Se NPs improved the physiological indices of rice in Cd-contaminated soil (total Cd 1.62 mg/kg), among which the foliar spraying had better effects on increasing the yield, relative content of chlorophyll in rice and reducing the content of Cd in rice than soil application [113]. In Cd and Pb co-contaminated soil, foliar spraying of Se NPs on rice effectively increased rice yield, biomass, protein content and Se content and reduced phytic acid production in brown rice. It was also demonstrated that Se content and phytic acid are negatively correlated with Cd and Pb concentrations. Considering the safety factor, foliar spraying of Se NPs can significantly reduce the content of Cd in grains, making it more practical in actual production.
2.3.2. Silicon
Silicon (Si) is the second most abundant element in the Earth’s crust after oxygen. Although it is not an essential element for higher plants, it is inextricably linked to plant growth, especially for Gramineae species (such as rice) [114]. Several studies have found that the application of Si can enhance plant resistance to abiotic stress [115][116]. At present, the widely recognized alleviation of HM stress by Si is through the following four mechanisms: 1) activation of the physiological and biochemical defense systems to enhance ROS scavenging [117], 2) immobilization and complexation of HM to reduce HM biological activity [118][119], 3) acting as a nutrient source and stimulating the production of structural protective agents chelated with HM, 4) regulation of the expression of HM transport genes, such as overexpression of vacuolar H+-pyrophosphatase1 (OVP1).
Most of the early research has been carried out in the hydroponic conditions. For example, rice growing in the nutrient solution (20 μM CdCl2, foliar application of 2.5 mM nano Si) was shown to have smaller MDA and Cd content in the shoot, indicating that nano Si reduced the Cd stress [120]. In a rice cell culture experiment, the proportions of live cells in Cd solution (40 μM) were increased by 95.4%, 78.6% and 66.2% after addition of different size Si NPs (19, 48 and 202 nm: 1.0 mM), respectively. This may be explained by the down-regulation of Cd uptake and transport (OsLCT1 and OsNramp5) genes and up-regulation of Cd transport (OsHMA3) and Si uptake (OsLsi1) genes. In composite stress conditions, Si NPs treatments improved wheat growth and alleviated Cd (bioavailable Cd 1.21 mg/kg) and drought (35% of soil water holding capacity) stress [121]. Hussain et al. reported that foliar application of Si NPs (10 and 20 mg/L) effectively decreased the accumulation of Cd in grains and increased the yield. Besides, they demonstrated that combined application of Se NPs (20 mg/L) and Si NPs (10 mg/L) highly reduced Cd (62%) and Pb (52%) in rice grains.
Sousa et al. found that Si NPs (4 mg/kg) alleviated the toxicity of Al to maize by activating the antioxidant defense systems; at the same time, Si NPs did not influence the accumulation of Al in maize [122]. These results are contradictory with the above findings, and the reason may be related to different concentrations or types of HM used in these studies. In the field of combined application of NPs and microorganisms, Fatemi reported that Si NPs and Pb-resistant strains of the microbes more effectively alleviated the Pb toxicity to coriander than the single treatments of Si NPs or microbes [123].
2.3.3. Hydroxyapatite
Phosphorus-containing amendments have been proven effective in the in-situ remediation of HM contaminated soils because they can release phosphates to form precipitates and complexes with HM in the soil [124]. Previous studies have shown that after adding hydroxyapatite (HAP) to the soil, it can release phosphate to form complexes with HM [125][126]. Also, the Ca ions on the surface of HAP could exchange with the HM ions in the soil to reduce the harm of HMto plants [127]. HAP with different particle sizes was proved to repair HM contaminated soil, for example, normal, micro, and nano HAP increased soil pH and decreased the amount of CaCl2-extractable, exchangeable Cu and Cd.
Furthermore, effects of HAP NPs (5, 10, 20 and 30g/kg) on Brassica chinensis L under Cd stress (10 mg/kg) were studied in a pot experiment [128]. Compared with the control, the biomass, chlorophyll, vitamin C, and the activities of SOD, CAT, and POD in plant shoots were enhanced; Cd and MDA contents in the shoots were decreased. Sun et al. reported that the content of Cu and Zn in ryegrass significantly decreased by adding HAP NPs [129]. Xing investigated the soil properties and Cd, Cu, Zn and Pb content in soil and rice after addition of HAP NPs for one and three years [130]. They concluded that HAP NPs significantly decreased the Cd content in roots and grains both after one and three years. Compared with one year, the immobilization ability of HAP NPs for Pb was slightly increased after three years, while significantly decreased for Zn, Cd and Cu after three years.
The pH of the Cd-contaminated soil (10 mg/kg) increased after application of HAP NPs (0.2%, 0.5%, and 1%) and significantly increased the catalase and urease activities in the soil [131]. Besides, the plant height and biomass increased, and the Cd concentration in the edible part was reduced significantly, which shows that HAP NPs effectively alleviated the Cd stress. In Cd (37 mg/kg) and Pb (615 mg/kg) compound stress condition, the application of HAP significantly increased the soil pH, whereas the application of HAP NPs slightly reduced the soil pH, both of which significantly reduced the content of Diethylenetriaminepentaacetic acid (DTPA)-extracted Pb and Cd in soil [132]. Although HAP application reduced Cd absorption by the shoots and roots, the addition of HAP and HAP NPs both significantly increased the Cd content in tobacco leaves. It indicates that although NPs can reduce the content of HM in some parts, they may also increase it. Therefore, potential ecological risks and mechanisms still need further research.
References
- Zhai, X.; Li, Z.; Huang, B.; Luo, N.; Huang, M.; Zhang, Q.; Zeng, G. Remediation of multiple heavy metal-contaminated soil through the combination of soil washing and in situ immobilization. Sci. Total Environ. 2018, 635, 92–99, doi:10.1016/j.scitotenv.2018.04.119.
- Lu, Y.; Song, S.; Wang, R.; Liu, Z.; Meng, J.; Sweetman, A.J.; Jenkins, A.; Ferrier, R.C.; Li, H.; Luo, W., et al. Impacts of soil and water pollution on food safety and health risks in China. Environ. Int. 2015, 77, 5–15, doi:10.1016/j.envint.2014.12.010.
- Zhao, F.; Ma, Y.; Zhu, Y.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2015, 49, 750–759, doi:10.1021/es5047099.
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268, doi:10.1016/j.gexplo.2016.11.021.
- The Ministry of Environmental Protection; The Ministry of Land and Resources Report on the national soil contamination survey. http://www.mee.gov.cn/gkml/sthjbgw/qt/201404/t20140417_270670.htm. (accessed on 1 October 2020).
- Reimann, C.; de Caritat, P. New soil composition data for Europe and Australia: Demonstrating comparability, identifying continental-scale processes and learning lessons for global geochemical mapping. Sci. Total Environ. 2012, 416, 239–252, doi:https://doi.org/10.1016/j.scitotenv.2011.11.019.
- Cave, M.; Rawlins, B.; Mcgrath, S.; Scheib, A.; Breward, N.; Lister, T.; Ingham, M.; Gowing, C.; Carter. The Advanced Soil Geochemical Atlas of England and Wales. British Geological Survey: Nottingham, UK, 2012; p. 277.
- QG, Z.; YM, L. The macro strategy of soil protection in China. Bull. Chin. 2015, 4, 452–458.
- Teng, Y.; Wu, J.; Lu, S.; Wang, Y.; Jiao, X.; Song, L. Soil and soil environmental quality monitoring in China: A review. Environ. Int. 2014, 69, 177–199, doi:https://doi.org/10.1016/j.envint.2014.04.014.
- Li, M.; Xi, X.; Xiao, G.; Cheng, H.; Yang, Z.; Zhou, G.; Ye, J.; Li, Z. National multi-purpose regional geochemical survey in China. J. Geochem. Explor. 2014, 139, 21–30, doi:https://doi.org/10.1016/j.gexplo.2013.06.002.
- Arif, N.; Sharma, N.C.; Yadav, V.; Ramawat, N.; Dubey, N.K.; Tripathi, D.K.; Chauhan, D.K.; Sahi, S. Understanding Heavy Metal Stress in a Rice Crop: Toxicity, Tolerance Mechanisms, and Amelioration Strategies. J. Plant. Biol. 2019, 62, 239–253, doi:10.1007/s12374-019-0112-4.
- Xue, T.; Liao, X.; Wang, L.; Gong, X.; Zhao, F.; Ai, J.; Zhang, Y. Effects of adding selenium on different remediation measures of paddy fields with slight-moderate cadmium contamination. Environ. Geochem. Health 2020, 42, 377–388, doi:10.1007/s10653-019-00365-9.
- Allevato, E.; Stazi, S.R.; Marabottini, R.; D’Annibale, A. Mechanisms of arsenic assimilation by plants and countermeasures to attenuate its accumulation in crops other than rice. Ecotoxicol. Environ. Saf. 2019, 185, 109701, doi:10.1016/j.ecoenv.2019.109701.
- Huang, Y.; Wang, L.; Wang, W.; Li, T.; He, Z.; Yang, X. Current status of agricultural soil pollution by heavy metals in China: A meta-analysis. Sci. Total Environ. 2019, 651, 3034–3042, doi:10.1016/j.scitotenv.2018.10.185.
- Adeel, M.; Lee, J.Y.; Zain, M.; Rizwan, M.; Nawab, A.; Ahmad, M.A.; Shafiq, M.; Yi, H.; Jilani, G.; Javed, R., et al. Cryptic footprints of rare earth elements on natural resources and living organisms. Environ. Int. 2019, 127, 785–800, doi:10.1016/j.envint.2019.03.022.
- Javaid, S. Heavy metals stress, mechanism and remediation techniques in rice (Oryza sativa L.): A review. Pure Appl. Biol. 2020, 9, doi:10.19045/bspab.2020.90045.
- Irshad, H.M.K.; Noman, A.; Alhaithloul, H.; Adeel, M.; Yukui, R.; Shah, T.; Zhu, S.; Shang, J. Goethite-modified biochar ameliorates the growth of rice (Oryza sativa L.) plants by suppressing Cd and As-induced oxidative stress in Cd and As co-contaminated paddy soil. Sci. Total Environ. 2020, 137086, doi:10.1016/j.scitotenv.2020.137086.
- UnitedNations. World population prospects: the 2019 revision, key findings and advance tables. https://population.un.org/wpp/Download/Standard/Population/ (accessed on 1 October 2020).
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water management. Nature 2012, 490, 254–257, doi:10.1038/nature11420.
- Adeel, M.; Ma, C.; Ullah, S.; Rizwan, M.; Hao, Y.; Chen, C.; Jilani, G.; Shakoor, N.; Li, M.; Wang, L., et al. Exposure to nickel oxide nanoparticles insinuates physiological, ultrastructural and oxidative damage: A life cycle study on Eisenia fetida. Environ. Pollut. 2019, 254, doi:10.1016/j.envpol.2019.113032.
- Adeel, M.; Tingting, J.; Hussain, T.; He, X.; Muhammad; Ahmad, M.; Irshad, H.M.K.; Shakoor, N.; Xie, C.; Hao, Y., et al. Bioaccumulation of ytterbium oxide nanoparticles insinuate oxidative stress, inflammatory, and pathological lesions in ICR mice. Environ. Sci. Pollut. Res. 2020, 27, doi:10.1007/s11356-020-09565-8.
- Wang, Y.; Jiang, F.; Ma, C.; Rui, Y.; Tsang, D.C.W.; Xing, B. Effect of metal oxide nanoparticles on amino acids in wheat grains (Triticum aestivum) in a life cycle study. J. Environ Manag. 2019, 241, 319–327, doi:10.1016/j.jenvman.2019.04.041.
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169, doi:10.1080/17429145.2017.1310944.
- Yang, J.; Jiang, F.; Ma, C.; Rui, Y.; Rui, M.; Adeel, M.; Cao, W.; Xing, B. Alteration of Crop Yield and Quality of Wheat upon Exposure to Silver Nanoparticles in a Life Cycle Study. J. Agric. Food Chem. 2018, 66, 2589–2597, doi:10.1021/acs.jafc.7b04904.
- Hao, Y.; Ma, C.; White, J.C.; Adeel, M.; Jiang, R.; Zhao, Z.; Rao, Y.; Chen, G.; Rui, Y.; Xing, B. Carbon-based nanomaterials alter the composition of the fungal endophyte community in rice (Oryza sativa L.). Environ. Sci. Nano 2020, 7, 2047–2060, doi:10.1039/C9EN01400D.
- Rui, M.; Ma, C.; Tang, X.; Yang, J.; Jiang, F.; Pan, Y.; Xiang, Z.; Hao, Y.; Rui, Y.; Cao, W., et al. Phytotoxicity of Silver Nano-particles to Peanut (Arachis hypogaea L.): Physiological Responses and Food Safety. ACS Sustain. Chem. Eng. 2017, 5, 6557–6567, doi:10.1021/acssuschemeng.7b00736.
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540, doi:10.1038/s41565-019-0439-5.
- Li, J.; Hu, J.; Ma, C.; Wang, Y.; Wu, C.; Huang, J.; Xing, B. Uptake, translocation and physiological effects of magnetic iron oxide (γ-Fe2O3) nanoparticles in corn (Zea mays L.). Chemosphere 2016, 159, 326–334, doi:10.1016/j.chemosphere.2016.05.083.
- Palchoudhury, S.; Jungjohann, K.L.; Weerasena, L.; Arabshahi, A.; Gharge, U.; Albattah, A.; Miller, J.; Patel, K.; Holler, R.A. Enhanced legume root growth with pre-soaking in α-Fe2O3 nanoparticle fertilizer. RSC Adv. 2018, 8, 24075–24083, doi:10.1039/C8RA04680H.
- Ghafariyan, M.H.; Malakouti, M.J.; Dadpour, M.R.; Stroeve, P.; Mahmoudi, M. Effects of Magnetite Nanoparticles on Soybean Chlorophyll. Environ. Sci. Technol. 2013, 47, 10645–10652, doi:10.1021/es402249b.
- Rui, M.; Ma, C.; Hao, Y.; Guo, J.; Rui, Y.; Tang, X.; Zhao, Q.; Fan, X.; Zhang, Z.; Hou, T., et al. Iron Oxide Nanoparticles as a Potential Iron Fertilizer for Peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, doi:10.3389/fpls.2016.00815.
- Rui, M.; Ma, C.; White, J.C.; Hao, Y.; Wang, Y.; Tang, X.; Yang, J.; Jiang, F.; Ali, A.; Rui, Y., et al. Metal oxide nanoparticles alter peanut (Arachis hypogaea L.) physiological response and reduce nutritional quality: a life cycle study. Environ. Sci.-Nano 2018, 5, 2088–2102, doi:10.1039/c8en00436f.
- Li, M.; Adeel, M.; Peng, Z.; Yukui, R. Physiological impacts of zero valent iron, Fe3O4 and Fe2O3 nanoparticles in rice plants and their potential as Fe fertilizers. Environ. Pollut. 2020, doi:10.1016/j.envpol.2020.116134.
- Adeel, M.; Farooq, T.; White, J.; Hao, Y.; He, Z.; Rui, Y. Carbon-based nanomaterials suppress Tobacco Mosaic Virus (TMV) infection and induce resistance in Nicotiana benthamian. J. Hazard. Mater. 2020, doi:10.1016/j.jhazmat.2020.124167.
- Hao, Y.; Fang, P.; Ma, C.; White, J.C.; Xiang, Z.; Wang, H.; Zhang, Z.; Rui, Y.; Xing, B. Engineered nanomaterials inhibit Podosphaera pannosa infection on rose leaves by regulating phytohormones. Environ. Res. 2019, 170, 1–6, doi:10.1016/j.envres.2018.12.008.
- Hao, Y.; Yuan, W.; Ma, C.; White, J.C.; Zhang, Z.; Adeel, M.; Zhou, T.; Rui, Y.; Xing, B. Engineered nanomaterials suppress Turnip mosaic virus infection in tobacco (Nicotiana benthamiana). Environ. Sci.-Nano 2018, 5, 1685–1693, doi:10.1039/c8en00014j.
- Zhao, X.; Jia, Y.; Li, J.; Dong, R.; Zhang, J.; Ma, C.; Wang, H.; Rui, Y.; Jiang, X. Indole Derivative-Capped Gold Nanoparticles as an Effective Bactericide in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 29398–29406, doi:10.1021/acsami.8b11980.
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 2019, 14, 517–522, doi:10.1038/s41565-019-0461-7.
- Lombi, E.; Donner, E.; Dusinska, M.; Wickson, F. A One Health approach to managing the applications and implications of nanotechnologies in agriculture. Nat. Nanotechnol. 2019, 14, 523–531, doi:10.1038/s41565-019-0460-8.
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778, doi:10.1016/j.scitotenv.2020.137778.
- Wang, Z.; Yue, L.; Dhankher, O.P.; Xing, B. Nano-enabled improvements of growth and nutritional quality in food plants driven by rhizosphere processes. Environ. Int. 2020, 142, 105831, doi:10.1016/j.envint.2020.105831.
- Liu, Y.; Wu, T.; White, J.C.; Lin, D. A new strategy using nanoscale zero-valent iron to simultaneously promote remediation and safe crop production in contaminated soil. Nat. Nanotechnol. 2020, doi:10.1038/s41565-020-00803-1.
- Moharem, M.; Elkhatib, E.; Mesalem, M. Remediation of chromium and mercury polluted calcareous soils using nanoparticles: Sorption-desorption kinetics, speciation and fractionation. Environ. Res. 2019, 170, 366–373, doi:10.1016/j.envres.2018.12.054.
- Toumey, C. Notes on environmental nanoscience. Nat. Nanotechnol. 2020, 15, 250–251, doi:10.1038/s41565-020-0677-6.
- Hussain, B.; Lin, Q.; Hamid, Y.; Sanaullah, M.; Di, L.; Hashmi, M.L.U.R.; Khan, M.B.; He, Z.; Yang, X. Foliage application of selenium and silicon nanoparticles alleviates Cd and Pb toxicity in rice (Oryza sativa L.). The Sci. Total Environ. 2020, 712, 136497, doi:10.1016/j.scitotenv.2020.136497.
- Lian, J.; Zhao, L.; Wu, J.; Xiong, H.; Bao, Y.; Zeb, A.; Tang, J.; Liu, W. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.). Chemosphere 2020, 239, 124794, doi:10.1016/j.chemosphere.2019.124794.
- Olawumi, T.O.; Chan, D.W.M. A scientometric review of global research on sustainability and sustainable development. J. Clean. Prod. 2018, 183, 231–250, doi:https://doi.org/10.1016/j.jclepro.2018.02.162.
- Chen, C.; Song, M. Visualizing a field of research: A methodology of systematic scientometric reviews. PLoS ONE 2019, 14, e223994.
- Chen, C.; Ibekwe-SanJuan, F.; Hou, J. The structure and dynamics of cocitation clusters: A multiple-perspective cocitation analysis. J. Am. Soc. Inf. Sci. Technol. 2010, 61, 1386–1409, doi:10.1002/asi.21309.
- Chen, C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377, doi:10.1002/asi.20317.
- Chen, C. Science Mapping: A Systematic Review of the Literature. J. Data Inf. Sci. 2017, 2, 1–40, doi:10.1515/jdis-2017-0006.
- Cao, F.; Dai, H.; Hao, P.F.; Wu, F. Silicon regulates the expression of vacuolar H(+)-pyrophosphatase 1 and decreases cadmium accumulation in rice (Oryza sativa L.). Chemosphere 2020, 240, 124907, doi:10.1016/j.chemosphere.2019.124907.
- Mengmeng, S.; Jie, X.; Lingling, Z.; Dejian, S.; Xitong, Z. Research Progress on Effects of Silicon on Plant Growth under Cad-mium Stress. Guangzhou Chem. Ind. 2019, 47, 41–43.
- Sebastian, A.; Nangia, A.; Prasad, M.N.V. Cadmium and sodium adsorption properties of magnetite nanoparticles synthesized from Hevea brasiliensis Muell. Arg. bark: Relevance in amelioration of metal stress in rice. J. Hazard. Mater. 2019, 371, 261–272, doi:10.1016/j.jhazmat.2019.03.021.
- Wang, Y.; Liu, Y.; Zhan, W.; Zheng, K.; Lian, M.; Zhang, C.; Ruan, X.; Li, T. Long-term stabilization of Cd in agricultural soil using mercapto-functionalized nano-silica (MPTS/nano-silica): A three-year field study. Ecotoxicol. Environ. Saf. 2020, 197, 110600, doi:10.1016/j.ecoenv.2020.110600.
- Cui, H.; Shi, Y.; Zhou, J.; Chu, H.; Cang, L.; Zhou, D. Effect of different grain sizes of hydroxyapatite on soil heavy metal bioavailability and microbial community composition. Agric. Ecosyst. Environ. 2018, 267, 165–173, doi:10.1016/j.agee.2018.08.017.
- Chao-Dong, Y.; Xia, Z.; Guo-Feng, L.; Jun-Wei, Z.; Man-Zhu, B.; Zhi-Xiang, Z. Progress on the Structure and Physiological Functions of Apoplastic Barriers in Root. Bull. Bot. Res. 2013, 33, 114–119.
- Rossi, L.; Zhang, W.; Schwab, A.P.; Ma, X. Uptake, Uptake, Accumulation, and in Planta Distribution of Coexisting Cerium Oxide Nanoparticles and Cadmium inGlycine max (L.) Merr Environ. Sci. Technol. 2017, 51, 12815–12824, doi:10.1021/acs.est.7b03363.
- Wang, K.; Wang, Y.; Wan, Y.; Mi, Z.; Wang, Q.; Wang, Q.; Li, H. The fate of arsenic in rice plants (Oryza sativa L.): Influence of different forms of selenium. Chemosphere 2021, 264, 128417, doi:10.1016/j.chemosphere.2020.128417.
- Cui, J.; Liu, T.; Li, F.; Yi, J.; Liu, C.; Yu, H. Silica nanoparticles alleviate cadmium toxicity in rice cells: Mechanisms and size effects. Environ. Pollut. 2017, 228, 363–369, doi:10.1016/j.envpol.2017.05.014.
- Wu, H.; Tito, N.; Giraldo, J.P. Anionic Cerium Oxide Nanoparticles Protect Plant Photosynthesis from Abiotic Stress by Scavenging Reactive Oxygen Species. ACS Nano 2017, 11, 11283–11297, doi:10.1021/acsnano.7b05723.
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-Biotechnology in Agriculture: Use of Nanomaterials to Promote Plant Growth and Stress Tolerance. J. Agric. Food Chem. 2020, 68, 1935–1947, doi:10.1021/acs.jafc.9b06615.
- Ullah, S.; Adeel, M.; Zain, M.; Rizwan, M.; Irshad, M.K.; Jilani, G.; Hameed, A.; Khan, A.; Arshad, M.; Raza, A., et al. Physi-ological and biochemical response of wheat (Triticum aestivum) to TiO2 nanoparticles in phosphorous amended soil: A full life cycle study. J. Environ. Manag. 2020, 263, doi:10.1016/j.jenvman.2020.110365.
- Zhang, P.; Ma, Y.; Liu, S.; Wang, G.; Zhang, J.; He, X.; Zhang, J.; Rui, Y.; Zhang, Z. Phytotoxicity, uptake and transformation of nano-CeO2 in sand cultured romaine lettuce. Environ. Pollut. 2017, 220, 1400–1408, doi:10.1016/j.envpol.2016.10.094.
- Zhang, H.; Du, W.; Peralta-videa, J.; Gardea-Torresdey, J.; White, J.; Keller, A.; Guo, H.; Ji, R.; Zhao, L. Metabolomics Reveals How Cucumber (Cucumis sativus) Reprograms Metabolites To Cope with Silver Ions and Silver Nanoparticle-Induced Oxi-dative Stress. Environ. Sci. Technol. 2018, 52, doi:10.1021/acs.est.8b02440.
- Konate, A.; He, X.; Zhang, Z.; Ma, Y.; Zhang, P.; Alugongo, G.M.; Rui, Y. Magnetic (Fe3O4) Nanoparticles Reduce Heavy Metals Uptake and Mitigate Their Toxicity in Wheat Seedling. Sustainability 2017, 9, 790, doi:10.3390/su9050790.
- Guo, K.; Hu, A.; Wang, K.; Wang, L.; Fu, D.; Hao, Y.; Wang, Y.; Ali, A.; Adeel, M.; Rui, Y., et al. Effects of spraying nano-materials on the absorption of metal(loid)s in cucumber. IET Nanobiotechnol. 2019, 13, 712–719, doi:10.1049/iet-nbt.2019.0060.
- Yao, J.; Cheng, Y.; Zhou, M.; Zhao, S.; Lin, S.; Wang, X.; Wu, J.; Li, S.; Wei, H. ROS scavenging Mn3O4 nanozymes for in vivo anti-inflammation. Chem. Sci. 2018, 9, doi:10.1039/C7SC05476A.
- Wang, Y.; Wang, L.; Ma, C.; Wang, K.; Hao, Y.; Chen, Q.; Mo, Y.; Rui, Y. Effects of cerium oxide on rice seedlings as affected by co-exposure of cadmium and salt. Environ. Pollut. 2019, 252, 1087–1096, doi:10.1016/j.envpol.2019.06.007.
- Dutta, P.; Pal, S.; Seehra, M.S.; Shi, Y.; Eyring, E.M.; Ernst, R.D. Concentration of Ce3+ and Oxygen Vacancies in Cerium Oxide Nanoparticles. Chem. Mater. 2006, 18, 5144–5146, doi:10.1021/cm061580n.
- Cao, Z.; Stowers, C.; Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. Physiological effects of cerium oxide nanoparticles on the photosynthesis and water use efficiency of soybean (Glycine max (L.) Merr.). Environ Sci.-Nano 2017, 4, 1086–1094, doi:10.1039/c7en00015d.
- Rossi, L.; Zhang, W.; Lombardini, L.; Ma, X. The impact of cerium oxide nanoparticles on the salt stress responses of Brassica napus L. Environ. Pollut. 2016, 219, 28–36, doi:10.1016/j.envpol.2016.09.060.
- Ma, Y.; Zhang, P.; Zhang, Z.; He, X.; Li, Y.; Zhang, J.; Zheng, L.; Chu, S.; Yang, K.; Zhao, Y., et al. Origin of the different phytotoxicity and biotransformation of cerium and lanthanum oxide nanoparticles in cucumber. Nanotoxicology 2014, 9, 262–270, doi:10.3109/17435390.2014.921344.
- Wang, X.; Sun, W.; Zhang, S.; Sharifan, H.; Ma, X. Elucidating the Effects of Cerium Oxide Nanoparticles and Zinc Oxide Nanoparticles on Arsenic Uptake and Speciation in Rice (Oryza sativa) in a Hydroponic System. Environ. Sci. Technol. 2018, 52, 10040–10047, doi:10.1021/acs.est.8b01664.
- Mueller, N.C.; Nowack, B. Exposure Modeling of Engineered Nanoparticles in the Environment. Environ. Sci. Technol. 2008, 42, 4447–4453, doi:10.1021/es7029637.
- Servin, A.D.; Morales, M.I.; Castillo-Michel, H.; Hernandez-Viezcas, J.A.; Munoz, B.; Zhao, L.; Nunez, J.E.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Synchrotron Verification of TiO2 Accumulation in Cucumber Fruit: A Possible Pathway of TiO2 Na-noparticle Transfer from Soil into the Food Chain. Environ. Sci. Technol. 2013, 47, 11592–11598, doi:10.1021/es403368j.
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanoparticle Res. 2012, 14, doi:10.1007/s11051-012-1109-9.
- Wang, Z.Y.; Yu, X.L.; Gao, D.M.; Feng, W.Q.; Xing, B.S.; Li, F.M. Effect of Nano-rutile TiO2 and Multiwalled Carbon Nanotubes on the Growth of Maize (Zea mays L.) Seedlings and the Relevant Antioxidant Response. Environ. Sci. 2010, 31, 480–487, doi:10.13227/j.hjkx.2010.02.026.
- Singh, J.; Lee, B. Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): A possible mechanism for the removal of Cd from the contaminated soil. J. Environ. Manag. 2016, 170, 88–96, doi:10.1016/j.jenvman.2016.01.015.
- Cai, F.; Wu, X.; Zhang, H.; Shen, X.; Zhang, M.; Chen, W.; Gao, Q.; White, J.C.; Tao, S.; Wang, X. Impact of TiO2 nanoparticles on lead uptake and bioaccumulation in rice (Oryza sativa L.). Nanoimpact 2017, 5, 101–108, doi:10.1016/j.impact.2017.01.006.
- Ji, Y.; Zhou, Y.; Ma, C.; Feng, Y.; Hao, Y.; Rui, Y.; Wu, W.; Gui, X.; Le, V.N.; Han, Y., et al. Jointed toxicity of TiO2 NPs and Cd to rice seedlings: NPs alleviated Cd toxicity and Cd promoted NPs uptake. Plant Physiol. Biochem. 2017, 110, 82.
- Dai, C.; Shen, H.; Duan, Y.; Liu, S.; Zhou, F.; Wu, D.; Zhong, G.; Javadi, A.; Tu, Y. TiO2 and SiO2 Nanoparticles Combined with Surfactants Mitigate the Toxicity of Cd2+ to Wheat Seedlings. Water Air Soil Pollut. 2019, 230, doi:10.1007/s11270-019-4297-4.
- Singh, J.; Lee, B. Effects of Nano-TiO2 particles on bioaccumulation of Cs-133 from the contaminated soil by Soybean (Glycine max). Process. Saf. Environ. 2018, 116, 301–311, doi:10.1016/j.psep.2018.02.016.
- Lin, J.; He, F.; Su, B.; Sun, M.; Owens, G.; Chen, Z. The stabilizing mechanism of cadmium in contaminated soil using green synthesized iron oxide nanoparticles under long-term incubation. J. Hazard. Mater. 2019, 379, 120832, doi:10.1016/j.jhazmat.2019.120832.
- Konate, A.; Wang, Y.; He, X.; Adeel, M.; Zhang, P.; Ma, Y.; Ding, Y.; Zhang, J.; Yang, J.; Kizito, S., et al. Comparative effects of nano and bulk-Fe3O4 on the growth of cucumber (Cucumis sativus). Ecotoxicol. Environ. Saf. 2018, 165, 547–554, doi:10.1016/j.ecoenv.2018.09.053.
- Briat, J.; Curie, C.; de Ric Gaymard, F. Iron utilization and metabolism in plants Jean-Franc¸ois Briat, Catherine Curie and Fre’ de’ ric Gaymard. Curr. Opin. Plant Biol. 2007, 10, 276–282.
- Michálková, Z.; Fernández, D.M.; Komárek, M. Interactions of two novel stabilizing amendments with sunflower plants grown in a contaminated soil. Chemosphere 2017, doi:10.1016/j.chemosphere.2017.08.009.
- Guha, T.; Barman, S.; Mukherjee, A.; Kundu, R. Nano-scale zero valent iron modulates Fe/Cd transporters and immobilizes soil Cd for production of Cd free rice. Chemosphere 2020, 260, 127533, doi:10.1016/j.chemosphere.2020.127533.
- Hussain, A.; Ali, S.; Rizwan, M.; Rehman, M.Z.U.; Qayyum, M.F.; Wang, H.; Rinklebe, J. Responses of wheat (Triticum aes-tivum) plants grown in a Cd contaminated soil to the application of iron oxide nanoparticles. Ecotoxicol. Environ. Saf. 2019, 173, 156–164, doi:10.1016/j.ecoenv.2019.01.118.
- Rizwan, M.; Noureen, S.; Ali, S.; Anwar, S.; Rehman, M.Z.U.; Qayyum, M.F.; Hussain, A. Influence of biochar amendment and foliar application of iron oxide nanoparticles on growth, photosynthesis, and cadmium accumulation in rice biomass. J. Soils Sediments 2019, 19, 3749–3759, doi:10.1007/s11368-019-02327-1.
- Chen, M.; Tao, X.; Wang, D.; Xu, Z.; Xu, X.; Hu, X.; Xu, N.; Cao, X. Facilitated transport of cadmium by biochar-Fe3O4 nano-composites in water-saturated natural soils. Sci. Total Environ. 2019, 684, 265–275, doi:10.1016/j.scitotenv.2019.05.326.
- Adrees, M.; Khan, Z.S.; Ali, S.; Hafeez, M.; Khalid, S.; Ur Rehman, M.Z.; Hussain, A.; Hussain, K.; Shahid Chatha, S.A.; Rizwan, M. Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere 2020, 238, 124681, doi:10.1016/j.chemosphere.2019.124681.
- Luying, S.; Fengbin, S.; Xiangnan, L.; Xiancan, Z.; Shengqun, L.; Yang, W.; Xiaoning, Q. Effects of ZnO nanoparticles on seed germination and root carbon metabolism in maize (Zea mays L.). Soils Crop. 2020, 9, 40–49.
- Lihua, W.; Fayuan, W.; Xinxin, J.; Shuai, L.; Xueqin, L. Effect of ZnO nanoparticles and inoculation with arbuscular mycorrhizal fungus on growth and nutrient uptake of soybean. Acta Ecol. Sin. 2015, 35, 5254–5261.
- Bashir, A.; Rizwan, M.; Ali, S.; Adrees, M.; Rehman, M.Z.; UrQayyum, M.F. Effect of composted organic amendments and zinc oxide nanoparticles on growth and cadmium accumulation by wheat; a life cycle study. Environ. Sci. Pollut. Res. 2020, 27, 23926–23936, doi:10.1007/s11356-020-08739-8.
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Zinc oxide nanoparticles alleviate drought-induced alterations in sorghum performance, nutrient acquisition, and grain fortification. Sci. Total Environ. 2019, 688, 926–934, doi:10.1016/j.scitotenv.2019.06.392.
- Ali, S.; Rizwan, M.; Noureen, S.; Anwar, S.; Ali, B.; Naveed, M.; Abd Allah, E.F.; Alqarawi, A.A.; Ahmad, P. Combined use of biochar and zinc oxide nanoparticle foliar spray improved the plant growth and decreased the cadmium accumulation in rice (Oryza sativa L.) plant. Environ. Sci. Pollut. Res. 2019, 26, 11288–11299, doi:10.1007/s11356-019-04554-y.
- Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Adrees, M.; Arshad, M.; Qayyum, M.F.; Hussain, A.; Chatha, S.A.S.; Imran, M. Allevi-ation of cadmium accumulation in maize (Zea mays L.) by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environ. Pollut. 2019, 358-367, doi:10.1016/j.envpol.2019.02.031.
- Ma, X.; Sharifan, H.; Dou, F.; Sun, W. Simultaneous reduction of arsenic (As) and cadmium (Cd) accumulation in rice by zinc oxide nanoparticles. Chem. Eng. J. 2020, 384, doi:10.1016/j.cej.2019.123802.
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Javed, M.R.; Adrees, M. The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ. Sci. Pollut. Res. Int. 2019, 26, 19859–19870, doi:10.1007/s11356-019-05333-5.
- Hussain, A.; Ali, S.; Rizwan, M.; Rehman, M.Z.U.; Javed, M.R.; Imran, M.; Chatha, S.A.S.; Nazir, R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ. Pollut. 2018, doi:10.1016/j.envpol.2018.08.036.
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Rehman, M.Z.U.; Waris, A.A. Zinc and iron oxide nanopar-ticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. 2018 Chemosphere 2018, 214, 269–277..
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in Human Health and Disease. Antioxid. Redox Signal. 2011, 14, 1337–1383, doi:10.1089/ars.2010.3275.
- Lv, Y.; Yu, T.; Yang, Z.; Zhao, W.; Zhang, M.; Wang, Q. Constraint on selenium bioavailability caused by its geochemical behavior in typical Kaschin-Beck disease areas in Aba, Sichuan Province of China. Sci. Total Environ. 2014, 493, 737–749, doi:10.1016/j.scitotenv.2014.06.050.
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268, doi:10.1016/S0140-6736(11)61452-9.
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309, doi:10.1016/j.envint.2017.12.035.
- Tan, L.C.; Nancharaiah, Y.V.; van Hullebusch, E.D.; Lens, P.N.L. Selenium: environmental significance, pollution, and bio-logical treatment technologies. Biotechnol. Adv. 2016, 34, 886–907, doi:10.1016/j.biotechadv.2016.05.005.
- Chang, H.; Zhou, X.; Wang, W.; Zhou, Y.; Dai, W.; Zhang, C.; Yu, S. Effects of Selenium Application in Soil on Formation of Iron Plaque outside Roots and Cadmium Uptake by Rice Plants. In Advanced Materials Research; Bu, J., Kim, Y.H., Eds.; Trans Tech Publications Ltd.: Zurich, Switzerland, 2013; Volume 750–752, pp. 1573–1576.
- Hu, Y.; Norton, G.J.; Duan, G.; Huang, Y.; Liu, Y. Effect of selenium fertilization on the accumulation of cadmium and lead in rice plants. Plant Soil 2014, 384, 131–140, doi:10.1007/s11104-014-2189-3.
- Lin, L.; Zhou, W.; Dai, H.; Cao, F.; Zhang, G.; Wu, F. Selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. J. Hazard. Mater. 2012, 235–236, 343–351, doi:10.1016/j.jhazmat.2012.08.012.
- Huang, G.; Ding, C.; Li, Y.; Zhang, T.; Wang, X. Selenium enhances iron plaque formation by elevating the radial oxygen loss of roots to reduce cadmium accumulation in rice (Oryza sativa L.). J. Hazard. Mater. 2020, 398, 122860, doi:10.1016/j.jhazmat.2020.122860.
- Shengrong, Z. Mechanism of Migration and Transformation of Nano Selenium and Mitigates Cadmium Stress in Plants. Master’s Thesis, Shandong University, Jinan, China, 2019.
- Mengting, L. Effect of Selenium application on Cadmium accumulation and in Rice (Oryza sativa). Master’s Thesis, Guangxi University, Nanning, China, 2018.
- Gaur, S.; Kumar, J.; Kumar, D.; Chauhan, D.K.; Prasad, S.M.; Srivastava, P.K. Fascinating impact of silicon and silicon transporters in plants: A review. Ecotoxicol. Environ. Saf. 2020, 202, 110885, doi:10.1016/j.ecoenv.2020.110885.
- Mateos-Naranjo, E.; Gallé, A.; Florez-Sarasa, I.; Perdomo, J.A.; Galmés, J.; Ribas-Carbó, M.; Flexas, J. Assessment of the role of silicon in the Cu-tolerance of the C4 grass Spartina densiflora. J. Plant Physiol. 2015, 178, 74–83, doi:10.1016/j.jplph.2015.03.001.
- Bhat, J.A.; Shivaraj, S.M.; Singh, P.; Navadagi, D.B.; Tripathi, D.K.; Dash, P.K.; Solanke, A.U.; Sonah, H.; Deshmukh, R. Role of Silicon in Mitigation of Heavy Metal Stresses in Crop Plants. Plants 2019, 8, 71, doi:10.3390/plants8030071.
- Emamverdian, A.; Ding, Y.; Xie, Y.; Sangari, S. Silicon Mechanisms to Ameliorate Heavy Metal Stress in Plants. BioMed Res. Int. 2018, 2018, 1–10, doi:10.1155/2018/8492898.
- Liang, Y.; Sun, W.; Zhu, Y.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428, doi:10.1016/j.envpol.2006.06.008.
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic.-Amst. 2015, 196, 66–81, doi:10.1016/j.scienta.2015.09.010.
- Wang, S.; Wang, F.; Gao, S. Foliar application with nano-silicon alleviates Cd toxicity in rice seedlings. Environ. Sci. Pollut. Res. 2015, 22, 2837–2845, doi:10.1007/s11356-014-3525-0.
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Adrees, M.; Qayyum, M.F.; Khalid, S.; Ur Rehman, M.Z.; Sarwar, M.A. Effects of silicon nanoparticles on growth and physiology of wheat in cadmium contaminated soil under different soil moisture levels. Environ. Sci. Pollut. Res. 2020, 27, 4958–4968, doi:10.1007/s11356-019-06673-y.
- de Sousa, A.; Saleh, A.M.; Habeeb, T.H.; Hassan, Y.M.; Zrieq, R.; Wadaan, M.A.M.; Hozzein, W.N.; Selim, S.; Matos, M.; AbdElgawad, H. Silicon dioxide nanoparticles ameliorate the phytotoxic hazards of aluminum in maize grown on acidic soil. Sci. Total Environ. 2019, 693, doi:10.1016/j.scitotenv.2019.133636.
- Fatemi, H.; Pour, B.E.; Rizwan, M. Isolation and characterization of lead (Pb) resistant microbes and their combined use with silicon nanoparticles improved the growth, photosynthesis and antioxidant capacity of coriander (Coriandrum sativum L.) under Pb stress. Environ. Pollut. 2020, 266, doi:10.1016/j.envpol.2020.114982.
- Oliva Moncunill, J.; Pablo Ribas, J.D.; Cortina Pallás, J.L.; Cama, J.; Ayora Ibáñez, C. Removal of cadmium, copper, nickel, cobalt and mercury from water by Apatite IITM: Column experiments. J. Hazard. Mater. 2011, 194, 312–323.
- Mignardi, S.; Corami, A.; Ferrini, V. Evaluation of the effectiveness of phosphate treatment for the remediation of mine waste soils contaminated with Cd, Cu, Pb, and Zn. Chemosphere 2012, 86, 354–360, doi:10.1016/j.chemosphere.2011.09.050.
- Xu, L.; Cui, H.; Zheng, X.; Zhu, Z.; Liang, J.; Zhou, J. Immobilization of copper and cadmium by hydroxyapatite combined with phytoextraction and changes in microbial community structure in a smelter-impacted soil. RSC Adv. 2016, 6, 103955–103964, doi:10.1039/c6ra23487a.
- Mingxin, W.; Caicai, W.; Jinyong, Z.; Yang, X.; Shize, W. Joint remediation of heavy metal contaminated soil by EDTA and nano-hydroxyapatite. Chin. J. Environ. Eng. 2019, 13, 396–405.
- Li, Z.; Huang, J. Effects of Nanoparticle Hydroxyapatite on Growth and Antioxidant System in Pakchoi (Brassica chinensis L.) from Cadmium-Contaminated Soil. J. Nanomater. 2014, doi:10.1155/2014/470962.
- Sun, R.; Chen, J.; Fan, T.; Zhou, D.; Wang, Y. Effect of nanoparticle hydroxyapatite on the immobilization of Cu and Zn in polluted soil. Environ. Sci. Pollut. Res. 2018, 25, 73–80, doi:10.1007/s11356-016-8063-5.
- Jin-feng, X.; Long, C.; Li-qiang, G.; Dong-mei, Z. Long-term stability of immobilizing remediation of a heavy metal contaminated soil with nano-hydroxyapatite. J. Agro-Environ. Sci 2016, 35, 1271–1277.
- Qingqing, Z.; Shuokang, W.; Chenchen, Z.; Qing, W.; Ying, Z.; Lin, N.; Shu Xuan, L.; Wei, L. Adsorption and Desorptlon of Cd on nHAP and Remediation test on Cd Contaminated Soil. Environ. Eng. 2017, 35, 179–184.
- Fayuan, W.; Ling, W.; Xugang, W.; Zhaoyong, S. Role of immobilization amendments in phytoremediation of Pb-Cd-contaminated soil using tobacco plants. Chin. J. Environ. Eng. 2014, 8, 789–794.