
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Terisha Ghazi | + 2636 word(s) | 2636 | 2021-11-08 06:50:05 |
Video Upload Options
The Developmental Origins of Health and Disease (DOHaD) concept postulates that in utero exposures influence fetal programming and health in later life. Throughout pregnancy, the placenta plays a central role in fetal programming; it regulates the in utero environment and acts as a gatekeeper for nutrient and waste exchange between the mother and the fetus. Maternal exposure to air pollution, including heavy metals, can reach the placenta, where they alter DNA methylation patterns, leading to changes in placental function and fetal reprogramming. In this entry, we explore the current knowledge on placental DNA methylation changes associated with prenatal air pollution (including heavy metals) exposure and highlight its effects on fetal development and disease susceptibility.
1. Introduction
Air pollution is an environmental problem that threatens human health and is a major cause of mortality worldwide. In 2019, air pollution caused an estimated 6.67 million deaths, accounting for 12% of all deaths globally [1]. Pregnant women and their developing fetuses are particularly vulnerable to the adverse health effects of air pollution. In pregnant women, the respiratory adaptation to pregnancy leads to an increase in tidal volume and an increase in oxygen consumption [2][3]. Air pollution particles, due to their small size, are inhaled into the lungs, and the smallest particles infiltrate into the bloodstream reaching the placenta and fetus [4][5][6]. Such in utero exposures can affect fetal development, cause adverse birth outcomes, and increase the risk of developing certain diseases in later life, as postulated by the Developmental Origins of Health and Disease (DOHaD) concept [7][8][9][10].
Substantial evidence has associated prenatal air pollution exposure with a range of adverse health outcomes including gestational diabetes [11], preeclampsia [12][13], spontaneous abortions [14][15], preterm births [16][17], low birth weights [18], macrosomia [19], and stillbirths [20]. Furthermore, air pollution exposure during pregnancy was associated with an increased risk of developing cardiovascular diseases [21], neurodevelopmental alterations [22], respiratory problems [23][24], and cancer [25][26]. However, due to the complex composition of air pollution and the intricate processes involved in fetal development, the mechanisms by which air pollution causes these adverse health effects are not yet completely understood.
During the entire pregnancy, the placenta serves as a functional interface that connects the mother to the developing fetus [27]. It secretes hormones and regulates the in utero environment for optimum fetal growth and development. Subsequently, the placenta transfers nutrients from the mother to the fetus and regulates gas and waste exchanges [27]. In this way, the placenta plays a central role in fetal programming, and we suggest that altered placental physiology and function, possibly through epigenetic modifications such as DNA methylation, can provide a mechanism linking prenatal air pollution exposure with pregnancy complications, fetal growth abnormalities, altered newborn phenotypes, and an increased risk of developing certain diseases during the lifespan. Here, we explore the current knowledge on placental DNA methylation changes associated with prenatal air pollution (including heavy metals) exposure and highlight its effects on fetal development and disease susceptibility.
2. Prenatal Air Pollution Exposure and Placental DNA Methylation
Numerous studies have shown that prenatal air pollution exposure affects global DNA methylation patterns in the human placenta [28][29][30][31][32][33]. In the ENVIRONAGE birth cohort, exposure to PM2.5 during different stages of pregnancy was associated with a lower degree of placental global DNA methylation [30]. In another study, mothers residing near major roadways, an indicator of traffic-related air pollution, showed decreased placental LINE1 but not AluYb8 methylation, common markers of global DNA methylation [31]. Furthermore, residing near a major roadway was associated with newborns that had low birth weights; however, the change in placental LINE1 methylation did not mediate this relationship [31]. In contrast, a Chinese case-control study found that exposure to PM10 in the first trimester was associated with decreased LINE1 methylation in the placenta of fetal growth-restricted newborns [29].
A nested case-control study in Iran observed a positive correlation between global DNA methylation in the placenta and exposure to PM2.5 and PM10 during the first trimester [33]. However, no significant correlation was found between exposures to particulate matter or placental global DNA methylation and birth outcomes such as gestational age, weight, length, and head and chest circumference [33]. The EDEN cohort showed a positive association with PM10 exposure the day before birth and placental Alu methylation [28]. Furthermore, the EARLI study found that nitrogen dioxide and ozone also induced changes in placental global DNA methylation levels [32].
Apart from global DNA methylation, prenatal air pollution exposure was found to alter the promoter methylation of placental candidate genes that are involved in key biological processes [28][29][31][32][34][35][36][37][38][39][40]. In the EDEN cohort, exposure to nitrogen dioxide and PM10 altered the methylation patterns of ADORA2B, PXT1, KCTD20, CAPN10, SLC44A5, ADCK5, TGM6, TUBGCP2, and KYNU in placental tissues [28]. These genes function in placental development and were previously associated with hypoxia and preeclampsia [41][42], a pathology that has been linked with air pollution exposure during pregnancy [12][13]. In a separate study, mothers living near major roadways showed differential methylation at seven CpG sites, three of which were located in protein-coding genes (PTPRN2, TMEM125, and VPS4A) [31]. Similarly, in the EARLI cohort, differentially methylated regions were found in five protein-coding genes (F11R, ZNF442, SLC25A44, STK38,and PTPRH) in the placenta of women exposed to high levels of nitrogen dioxide and ozone during pregnancy [32]. These differentially methylated regions in the placenta did not show DNA methylation changes in cord blood, and hence, they appeared to be tissue-specific. Additionally, differentially methylated regions of three genes (RNF39, CYP2E1, and PM20D1) in cord blood showed consistent nitrogen dioxide and ozone exposure-related altered DNA methylation in the placenta [32], indicating that placental DNA methylation changes may be passed onto the developing fetus. These genes regulate immune and inflammatory responses, and their altered methylation patterns might play a role in preeclampsia.
In China, exposure to PM10 during the first and second trimesters increased HSD11B2 methylation, a gene involved in fetal growth and glucocorticoid metabolism [29]. Furthermore, the increase in HSD11B2 methylation was more prominent in the placental tissues of fetal growth-restricted newborns compared to the normal-growth newborns. Previously, HSD11B2 methylation was negatively associated with placental HSD11B2 gene expression, fetal growth indices, and adverse neurobehavioral outcomes in infants [43]. The Shanghai Mother-Child Pairs Cohort found that exposure to PM2.5 during the second and third trimesters as well as the entire pregnancy was associated with increased methylation of BID and decreased methylation of IGF2; these genes are essential for fetal growth [40]. Furthermore, for every 1% increase in BID methylation, there was a decrease in head circumference and biparietal diameter in the second trimester [40]. In Korea, the COCOA study showed that high PM2.5 exposure and low cord blood vitamin D levels during the first trimester were associated with decreased placental methylation of AHRR, DPP10, and HLA-DRB1, and early-onset persistent atopic dermatitis in children [39].
In the ENVIRONAGE birth cohort, PM2.5 and black carbon exposure increased placental DNA mutation rates as well as increased the promoter methylation of tumor suppressor (p53) and DNA repair (APEX1, OGG1, and ERCC4) genes. This study suggested that prenatal PM2.5 and black carbon exposure reduces the DNA repair capacity of the placenta and fetus, which may increase the risk for carcinogenesis in later life [36]. In the same cohort, the analysis of placental tissue from mothers exposed to PM2.5 in the first and third trimesters showed altered methylation of the genes (CLOCK, CRY1, NPAS2, and PER1–3) involved in circadian rhythm regulation [35]. Previously, dysregulation in placental methylation of these circadian pathway genes was associated with preeclampsia [44]. Another study indicated that second-trimester PM2.5 exposure decreased promoter methylation of the Lep gene, a hormone involved in intrauterine development, embryo implantation, energy regulation, and fetal growth [37]. Interestingly, the promoter methylation of Lep was higher in the placental tissue of male neonates compared to those of female neonates, suggesting that altered DNA methylation in the placenta may be sex-specific [37].
Prenatal air pollution exposure was also associated with altered placental mitochondrial DNA methylation [34][38]. In the ENVIRONAGE cohort, placental mitochondrial DNA methylation was analyzed in the D-loop control region and MT-RNR1 region. First trimester PM2.5 exposure was associated with increased mitochondrial DNA methylation at both the D-loop and MT-RNR1 regions [34]. An increase in D-loop and MT-RNR1 methylation was also observed in placental tissue following exposure to PM2.5 for the entire pregnancy [34]. These findings were confirmed in a smaller sample population of the ENVIRONAGE cohort, where PM2.5 and black carbon exposure throughout pregnancy increased D-loop and LDLR methylation [38]. In both studies, mitochondrial DNA methylation was negatively associated with mitochondrial DNA content, a measure of damaged mitochondria and mitophagy [34][38]. PM2.5 and black carbon exposure for the entire pregnancy also decreased placental promoter methylation of PINK1, a gene involved in mitochondrial quality control and mitophagy [38]. Moreover, a 0.42% increase in D-loop methylation was associated with decreased newborn birth weight [38].
3. Prenatal Heavy Metal Exposure and Placental DNA Methylation
Heavy metals such as arsenic, cadmium, lead, manganese, mercury, and nickel are common constituents of industry and traffic-related air pollution. Particulate matter, mainly PM2.5 and PM10, has a strong potential for adsorbing heavy metals, which then enter the human body through inhalation [45]. There is increasing evidence that heavy metals bound to particulate matter play a crucial role in the adverse health effects caused by particulate matter [46][47][48]. Therefore, we also included studies investigating placental DNA methylation changes and prenatal exposure to heavy metals [49][50][51][52][53][54][55][56].
Most studies on prenatal heavy metal exposure and placental DNA methylation changes were conducted within the RICHS cohort. In the RICHS cohort, maternal exposure to high levels of arsenic, cadmium, lead, mercury, and manganese were associated with increased placental NR3C1 methylation compared to the low exposure groups [49]. Placental NR3C1 plays a vital role in cognitive and neurodevelopment by regulating the development of the child’s hypothalamic-pituitary-adrenal (HPA) axis and cortisol levels. Therefore, altered placental NR3C1 methylation may provide insight into cognitive and neurodevelopmental abnormalities in children in later life [49]. In a separate study, mothers with high toenail cadmium concentrations were found to have low levels of placental PCDHAC1 methylation and were at an increased odds of giving birth to an infant that was small for gestational age or with a decreased head circumference [51]. Another study showed that high placental cadmium levels were associated with differential methylation at 17 CpG sites, and DNA methylation at 9 of these 17 CpG sites was associated with increased expression of genes involved in inflammatory signaling and cell growth (TNFAIP2, EXOC3L4, GAS7, SREBF1, ACOT7, and RORA) [52]. Furthermore, high placental expressions of TNFAIP2 and ACOT7 were associated with decreased birth weight. High placental expression of ACOT7 was also associated with decreased birth length and decreased head circumference [52]. Moreover, within the RICHS cohort, exposure to mercury, measured in infant toenails, was associated with differential methylation at 339 loci; 10 of these differentially methylated loci resided in the CPLX1, TTC23, and EMID2 genes and were associated with a high risk for adverse neurobehavioral profiles [55]. Exposure to high levels of manganese, measured in infant toenails, was associated with differential methylation at 5 CpG loci (EMX2OS, ATAD2B, FTO/RPGRIP1L, EN1, and LOC284276). These CpG loci resided in genes involved in neurodevelopment (EMX2OS, ATAD2B, and EN1) and fetal growth (FTO/RPGRIP1L) [54]. The function of LOC284276 is currently unknown; however, for every 10% increase in placental LOC284276 methylation, there was a decrease in birth weight [54].
In the New Hampshire Birth Cohort Study, placental arsenic levels were associated with differential methylation at 163 CpG sites. Of these, 13 CpG sites attained genome-wide significance and were located at the LYRM2 (11 CpG sites), CAMTA1 (1 CpG site), and CCDC57 (1 CpG site) genes [53]. A nested cohort in Bangladesh found that maternal exposure to arsenic, via contaminated drinking water, was associated with hypermethylation at several CpG sites, which were mainly located within open sea regions [50]. Moreover, prenatal arsenic exposure was associated with CpG methylation at the NR3C1 gene (unadjusted analysis) and the TRA2B, PLCE1, and CD36 genes (adjusted analysis) [50].
The omega cohort and pilot case-control placenta microarray study showed that cadmium levels were higher in the placental tissues from female neonates (5 ng/g) compared to male neonates (2 ng/g). High cadmium levels were associated with hypomethylation at three CpG sites (ARL9, SIAH3, and HS3ST4) and one genomic region (region 86974674 to 86975244 on chromosome 7; CROT and TP53TG1) in the placental tissue of female neonates [56]. In the placental tissue of male neonates, high cadmium levels were associated with hypomethylation at two CpG sites (MECOM) and two genomic regions (region 169379554 to 169380078 on chromosome 3 and region 1792758 to 1792758 on chromosome 8; MECOM and ARHGEF10) as well as hypermethylation at one CpG site (SALL1) [56]. These differentially methylated genes are involved in cell damage response (SIAH3, HS3ST4, and TP53TG1) in females and cell differentiation, angiogenesis, and organ development (MECOM and ARHGEF10) in males. These results suggest that cadmium-associated placental DNA methylation changes may induce fetal growth abnormalities in a sex-dependent manner [56].
4. Conclusion
As a natural barrier that directly connects the mother to the developing fetus, the placenta is continuously in contact with substances to which both the mother and fetus are exposed [27]. Perturbations in the maternal environment can be transferred to the fetus through altered placental functions, a concept known as fetal reprogramming. As a result, the placenta, which is genetically identical to the fetus, contains important information on the in utero fetal life and can be considered as a “mirror” of the future health and development of the newborn [35]. The studies included in this entry provide evidence that prenatal air pollution and heavy metal exposure is associated with altered placental DNA methylation at both the global and candidate gene promoter levels [28][29][30][31][32][33][34][35][36][37][38][39][40][49][50][51][52][53][54][55][56] (summarized in Figure 1); however, whether the changes in gene promoter methylation affected the expression of the gene was not established in the majority of studies.
Aside from the studies on placental mitochondrial DNA methylation [34][38], no genes were investigated in more than one study. This makes it difficult to determine if the alterations in candidate gene promoter methylation are specific to a particular socio-demographic population or to prenatal air pollution exposure in a certain geographical location. Different locations have different sources and chemical compositions of air pollution and individual components are not often encountered in isolation within natural settings.
Two studies also showed that PM2.5 and cadmium-induced DNA methylation of genes were different in the placentae from male and female neonates [37][56], suggesting that the adverse health effects of these air pollutants may be sex-specific. Furthermore, prenatal exposure to air pollution induced inconsistent placental global DNA methylation changes, which may result from several other factors such as maternal diet or nutritional status and folate supplementation; folate is an essential micronutrient and methyl donor required during pregnancy to prevent neural tube defects in the fetus. Interestingly, trimester-specific analyses indicated that most of the placental DNA methylation changes observed at birth followed air pollution exposures during the early stages of pregnancy [28][29][30][33][34][35][39], which is also when fetal epigenetic reprogramming occurs. Although this suggests that air pollution and heavy metal exposure during the early stages of pregnancy may, through altered placental DNA methylation, be responsible for fetal growth abnormalities, there was insufficient evidence linking prenatal air pollution and heavy metal-induced placental DNA methylation changes with specific diseases. Follow-up studies are required to determine if placental DNA methylation changes persist to the fetus and into adulthood and its possible implications on fetal development and disease susceptibility throughout the lifespan. Together, the findings depicted in this entry highlight the need for more effective and stricter environmental and public health policies to reduce air pollution and protect human health.
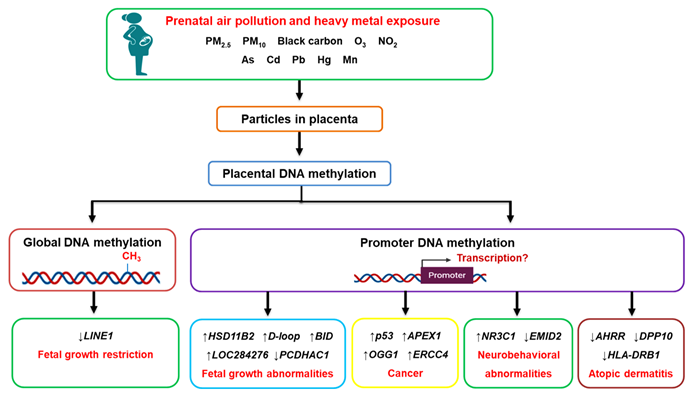
Figure 1. The effects of prenatal air pollution exposure on placental DNA methylation patterns and its implications on fetal development and future disease susceptibility. Maternal exposure to air pollution, including heavy metals, can reach the placenta, where they alter DNA methylation patterns at both the global and gene promoter level. The aberrant methylation of genes affects fetal growth (HSD11B2, BID, D-loop, PCDHAC1, LOC284276) and increases the risk of developing cancer (p53, APEX1, OGG1, ERCC4), neurobehavioral abnormalities (NR3C1, EMID2), and atopic dermatitis (AHRR, DPP10, HLA-DRB1) in later life.
References
- State of global air 2020: A special report on global exposure to air pollution and its health impacts . Health Effects Institute. Retrieved 2021-11-14
- Justine Chang; David Streitman; Physiologic Adaptations to Pregnancy. Neurologic Clinics 2012, 30, 781-789, 10.1016/j.ncl.2012.05.001.
- Matthew J. Hegewald; Robert O. Crapo; Respiratory Physiology in Pregnancy. Clinics in Chest Medicine 2011, 32, 1-13, 10.1016/j.ccm.2010.11.001.
- Hannelore Bové; Eva Bongaerts; Eli Slenders; Esmée M. Bijnens; Nelly D. Saenen; Wilfried Gyselaers; Peter Van Eyken; Michelle Plusquin; Maarten Roeffaers; Marcel Ameloot; et al.Tim S. Nawrot Ambient black carbon particles reach the fetal side of human placenta. Nature Communications 2019, 10, 1-7, 10.1038/s41467-019-11654-3.
- Norrice M. Liu; Lisa Miyashita; Barbara A. Maher; Graham McPhail; Carolyn J.P. Jones; Benjamin Barratt; Shakila Thangaratinam; Vassil Karloukovski; Imad A. Ahmed; Zabeada Aslam; et al.Jonathan Grigg Evidence for the presence of air pollution nanoparticles in placental tissue cells. Science of The Total Environment 2020, 751, 142235, 10.1016/j.scitotenv.2020.142235.
- Eva Reichrtová; František Dorociak; Lubica Palkovičová; Sites of lead and nickel accumulation in the placental tissue. Human & Experimental Toxicology 1998, 17, 176-181, 10.1177/096032719801700309.
- D. J. P. Barker; In utero programming of chronic disease. Clinical Science 1998, 95, 115-128, 10.1042/cs0950115.
- D.J.P. Barker; The Developmental Origins of Adult Disease. Journal of the American College of Nutrition 2004, 23, 588S-595S, 10.1080/07315724.2004.10719428.
- Peter D. Gluckman; Mark Hanson; Cyrus Cooper; Kent Thornburg; Effect of In Utero and Early-Life Conditions on Adult Health and Disease. New England Journal of Medicine 2008, 359, 61-73, 10.1056/nejmra0708473.
- James M. Swanson; Sonja Entringer; Claudia Buss; Pathik D. Wadhwa; Developmental Origins of Health and Disease: Environmental Exposures. Seminars in Reproductive Medicine 2009, 27, 391-402, 10.1055/s-0029-1237427.
- Huanhuan Zhang; Qiong Wang; Simin He; Kaipu Wu; Meng Ren; Haotian Dong; Jiangli Di; Zengli Yu; Cunrui Huang; Ambient air pollution and gestational diabetes mellitus: A review of evidence from biological mechanisms to population epidemiology. Science of The Total Environment 2020, 719, 137349, 10.1016/j.scitotenv.2020.137349.
- Gavin Pereira; Fatima Haggar; Antonia W Shand; Carol Bower; Angus Cook; Natasha Nassar; Association between pre-eclampsia and locally derived traffic-related air pollution: a retrospective cohort study. Journal of Epidemiology and Community Health 2012, 67, 147-152, 10.1136/jech-2011-200805.
- Qiong Wang; Huanhuan Zhang; Qianhong Liang; Luke Knibbs; Meng Ren; Changchang Li; Junzhe Bao; Suhan Wang; Yiling He; Lei Zhu; et al.Xuemei WangQingguo ZhaoCunrui Huang Effects of prenatal exposure to air pollution on preeclampsia in Shenzhen, China. Environmental Pollution 2018, 237, 18-27, 10.1016/j.envpol.2018.02.010.
- Audrey J Gaskins; Jaime E Hart; Jorge E Chavarro; Stacey A Missmer; Janet W Rich-Edwards; Francine Laden; Shruthi Mahalingaiah; Air pollution exposure and risk of spontaneous abortion in the Nurses’ Health Study II. Human Reproduction 2019, 34, 1809-1817, 10.1093/humrep/dez111.
- Marianthi-Anna Kioumourtzoglou; Raanan Raz; Ander Wilson; Ronen Fluss; Ronit Nirel; David M. Broday; Yuval; Michele R. Hacker; Thomas F. McElrath; Itamar Grotto; et al.Petros KoutrakisMarc G. Weisskopf Traffic-related Air Pollution and Pregnancy Loss. Epidemiology 2019, 30, 4-10, 10.1097/ede.0000000000000918.
- Xinhua Ji; Xia Meng; Cong Liu; Renjie Chen; Yihui Ge; Lena Kan; Qingyan Fu; Weihua Li; Lap Ah Tse; Haidong Kan; et al. Nitrogen dioxide air pollution and preterm birth in Shanghai, China. Environmental Research 2018, 169, 79-85, 10.1016/j.envres.2018.11.007.
- Xin Liu; Yufeng Ye; Yi Chen; Xiaona Li; Baixiang Feng; Ganxiang Cao; Jianpeng Xiao; Weilin Zeng; Xing Li; Jiufeng Sun; et al.Dan NingYi YangZhenjiang YaoYuming GuoQiong WangYonghui ZhangWenjun MaQingfeng DuBo ZhangTao Liu Effects of prenatal exposure to air particulate matter on the risk of preterm birth and roles of maternal and cord blood LINE-1 methylation: A birth cohort study in Guangzhou, China. Environment International 2019, 133, 105177, 10.1016/j.envint.2019.105177.
- Xi Gong; Yan Lin; F. Benjamin Zhan; Industrial air pollution and low birth weight: a case-control study in Texas, USA. Environmental Science and Pollution Research 2018, 25, 30375-30389, 10.1007/s11356-018-2941-y.
- Shi Chen; Shirui Wang; Tiantian Li; Huijuan Zhu; Siyu Liang; Ke Xu; Yuelun Zhang; Xianxian Yuan; Yingying Yang; Hui Pan; et al.XiaoMing Shi Effect of PM2.5 on macrosomia in China: A nationwide prospective cohort study. Pediatric Obesity 2019, 15, e12584, 10.1111/ijpo.12584.
- Hongyan Zang; Han Cheng; Wenya Song; Mei Yang; Ping Han; Chunxiao Chen; Rui Ding; Ambient air pollution and the risk of stillbirth: a population-based prospective birth cohort study in the coastal area of China. Environmental Science and Pollution Research 2019, 26, 6717-6724, 10.1007/s11356-019-04157-7.
- Carrie V. Breton; Wendy J. Mack; Jin Yao; Kiros Berhane; Milena Amadeus; Fred Lurmann; Frank Gilliland; Rob McConnell; Howard N. Hodis; Nino Kunzli; et al.Ed Avol Prenatal Air Pollution Exposure and Early Cardiovascular Phenotypes in Young Adults. PLOS ONE 2016, 11, e0150825, 10.1371/journal.pone.0150825.
- Mònica Guxens; Małgorzata J. Lubczyńska; Ryan L. Muetzel; Albert Dalmau-Bueno; Vincent W.V. Jaddoe; Gerard Hoek; Aad van der Lugt; Frank C. Verhulst; Tonya White; Bert Brunekreef; et al.Henning TiemeierHanan El Marroun Air Pollution Exposure During Fetal Life, Brain Morphology, and Cognitive Function in School-Age Children. Biological Psychiatry 2018, 84, 295-303, 10.1016/j.biopsych.2018.01.016.
- Zhang Hehua; Chang Qing; Gao Shanyan; Wu Qijun; Zhao Yuhong; The impact of prenatal exposure to air pollution on childhood wheezing and asthma: A systematic review. Environmental Research 2017, 159, 519-530, 10.1016/j.envres.2017.08.038.
- Hind Sbihi; Lillian Tamburic; Mieke Koehoorn; Michael Brauer; Perinatal air pollution exposure and development of asthma from birth to age 10 years. European Respiratory Journal 2016, 47, 1062-1071, 10.1183/13993003.00746-2015.
- Jo Kay C. Ghosh; Julia Heck; Myles Cockburn; Jason Su; Michael Jerrett; Beate Ritz; Prenatal Exposure to Traffic-related Air Pollution and Risk of Early Childhood Cancers. American Journal of Epidemiology 2013, 178, 1233-1239, 10.1093/aje/kwt129.
- Éric Lavigne; Marc-André Bélair; Minh T. Do; David M. Stieb; Perry Hystad; Aaron van Donkelaar; Randall V. Martin; Daniel L. Crouse; Eric Crighton; Hong Chen; et al.Jeffrey R. BrookRichard T. BurnettScott WeichenthalPaul J. VilleneuveTeresa ToSabit CakmakMarkey JohnsonAbdool S. YasseenKenneth C. JohnsonMarianna OfnerLin XieMark Walker Maternal exposure to ambient air pollution and risk of early childhood cancers: A population-based study in Ontario, Canada. Environment International 2017, 100, 139-147, 10.1016/j.envint.2017.01.004.
- Graham J. Burton; Abigail L. Fowden; The placenta: a multifaceted, transient organ. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 2015, 370, 20140066-20140066, 10.1098/rstb.2014.0066.
- Emilie Abraham; Sophie Rousseaux; Lydiane Agier; Lise Giorgis-Allemand; Jörg Tost; Julien Galineau; Agnès Hulin; Valérie Siroux; Daniel Vaiman; Marie Aline Charles; et al.Barbara HeudeAnne ForhanJoel SchwartzFlorent ChuffartEkaterina Bourova-FlinSaadi KhochbinRémy SlamaJohanna Lepeule Pregnancy exposure to atmospheric pollution and meteorological conditions and placental DNA methylation. Environment International 2018, 118, 334-347, 10.1016/j.envint.2018.05.007.
- Jing Cai; Yan Zhao; Pengcheng Liu; Bin Xia; Qingyang Zhu; Xiu Wang; Qi Song; Haidong Kan; Yunhui Zhang; Exposure to particulate air pollution during early pregnancy is associated with placental DNA methylation. Science of The Total Environment 2017, 607-608, 1103-1108, 10.1016/j.scitotenv.2017.07.029.
- Bram G Janssen; Lode Godderis; Nicky Pieters; Katrien Poels; Michał Kiciński; Ann Cuypers; Frans Fierens; Joris Penders; Michelle Plusquin; Wilfried Gyselaers; et al.Tim S Nawrot Placental DNA hypomethylation in association with particulate air pollution in early life. Particle and Fibre Toxicology 2013, 10, 22-22, 10.1186/1743-8977-10-22.
- Samantha L. Kingsley; Melissa N. Eliot; Eric A. Whitsel; Yen-Tsung Huang; Karl T. Kelsey; Carmen Marsit; Gregory A. Wellenius; Maternal residential proximity to major roadways, birth weight, and placental DNA methylation. Environment International 2016, 92-93, 43-49, 10.1016/j.envint.2016.03.020.
- Christine Ladd-Acosta; Jason I. Feinberg; Shannon C. Brown; Frederick W. Lurmann; Lisa A. Croen; Irva Hertz-Picciotto; Craig J. Newschaffer; Andrew P. Feinberg; M. Daniele Fallin; Heather E. Volk; et al. Epigenetic marks of prenatal air pollution exposure found in multiple tissues relevant for child health. Environment International 2019, 126, 363-376, 10.1016/j.envint.2019.02.028.
- Zhila Maghbooli; Arash Hossein-Nezhad; Elham Adabi; Effat Asadollah-Pour; Mahsa Sadeghi; Sara Mohammad-Nabi; Leila Zakeri Rad; Ali-Asghar Malek Hosseini; Mehrnaz Radmehr; Fatemeh Faghihi; et al.Atoosa AghaeiAbolfazl OmidifarYasaman AghababeiHadis Behzadi Air pollution during pregnancy and placental adaptation in the levels of global DNA methylation. PLoS ONE 2018, 13, e0199772, 10.1371/journal.pone.0199772.
- Bram G Janssen; Hyang-Min Byun; Wilfried Gyselaers; Wouter Lefebvre; Andrea Baccarelli; Tim S Nawrot; Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: An ENVIRONAGE birth cohort study. Epigenetics 2015, 10, 536-544, 10.1080/15592294.2015.1048412.
- Tim S. Nawrot; Nelly D. Saenen; Julie Schenk; Bram G. Janssen; Valeria Motta; Letizia Tarantini; Bianca Cox; Wouter Lefebvre; Charlotte Vanpoucke; Cristina Maggioni; et al.Valentina Bollati Placental circadian pathway methylation and in utero exposure to fine particle air pollution. Environment International 2018, 114, 231-241, 10.1016/j.envint.2018.02.034.
- Kristof Neven; Nelly D Saenen; Letitzia Tarantini; Bram G Janssen; Wouter Lefebvre; Charlotte Vanpoucke; Valentina Bollati; Tim S Nawrot; Placental promoter methylation of DNA repair genes and prenatal exposure to particulate air pollution: an ENVIR ON AGE cohort study. The Lancet Planetary Health 2018, 2, e174-e183, 10.1016/s2542-5196(18)30049-4.
- Nelly D. Saenen; Karen Vrijens; Bram G. Janssen; Harry A. Roels; Kristof Y. Neven; Wim Vanden Berghe; Wilfried Gyselaers; Charlotte Vanpoucke; Wouter Lefebvre; Patrick De Boever; et al.Tim S. Nawrot Lower Placental Leptin Promoter Methylation in Association with Fine Particulate Matter Air Pollution during Pregnancy and Placental Nitrosative Stress at Birth in the ENVIR ON AGE Cohort. Environmental Health Perspectives 2017, 125, 262-268, 10.1289/ehp38.
- Stijn Vos; Tim S. Nawrot; Dries S. Martens; Hyang-Min Byun; Bram G. Janssen; Mitochondrial DNA methylation in placental tissue: a proof of concept study by means of prenatal environmental stressors. Epigenetics 2020, 16, 121-131, 10.1080/15592294.2020.1790923.
- Song-I Yang; Seung-Hwa Lee; So-Yeon Lee; Hwan-Cheol Kim; Hyo-Bin Kim; Jeong-Hyun Kim; Hyeyeun Lim; Min Jee Park; Hyun-Ju Cho; Jisun Yoon; et al.Sungsu JungHyeon-Jong YangKangmo AhnKyung Won KimYoun Ho ShinDong In SuhHye-Sung WonMi-Young LeeSoo Hyun KimSuk-Joo ChoiJa-Young KwonJong Kwan JunSoo-Jong Hong Prenatal PM2.5 exposure and vitamin D–associated early persistent atopic dermatitis via placental methylation. Annals of Allergy, Asthma & Immunology 2020, 125, 665-673.e1, 10.1016/j.anai.2020.09.008.
- Yingya Zhao; Pengpeng Wang; Yuhan Zhou; Bin Xia; Qingyang Zhu; Wenzhen Ge; Jialin Li; Huijing Shi; Xirong Xiao; Yunhui Zhang; et al. Prenatal fine particulate matter exposure, placental DNA methylation changes, and fetal growth. Environment International 2020, 147, 106313, 10.1016/j.envint.2020.106313.
- Jesenia Acurio; Felipe Troncoso; Patricio Bertoglia; Carlos Salomón; Claudio Aguayo; Luis Sobrevia; Carlos Escudero; Potential Role of A2BAdenosine Receptors on Proliferation/Migration of Fetal Endothelium Derived from Preeclamptic Pregnancies. BioMed Research International 2014, 2014, 1-11, 10.1155/2014/274507.
- Hong-Juan Ding; Rui-Zhe Jia; Xiang Zhang; Ping Hu; Xiao-Mei Liu; Xiang-Dong Hua; Xin Wang; Screening for differential methylation status in human placenta in preeclampsia using a CpG island plus promoter microarray. International Journal of Molecular Medicine 2012, 30, 133-141, 10.3892/ijmm.2012.983.
- Carmen J. Marsit; Matthew A. Maccani; James F. Padbury; Barry M. Lester; Placental 11-Beta Hydroxysteroid Dehydrogenase Methylation Is Associated with Newborn Growth and a Measure of Neurobehavioral Outcome. PLoS ONE 2012, 7, e33794, 10.1371/journal.pone.0033794.
- Caroline Van Den Berg; Ines Chaves; E. M. Herzog; S. P. Willemsen; G. T. J. Van Der Horst; R. P. M. Steegers-Theunissen; Early- and late-onset preeclampsia and the DNA methylation of circadian clock and clock-controlled genes in placental and newborn tissues. Chronobiology International 2017, 34, 921-932, 10.1080/07420528.2017.1326125.
- Huiming Li; Xin Qian; Qin’Geng Wang; Heavy Metals in Atmospheric Particulate Matter: A Comprehensive Understanding Is Needed for Monitoring and Risk Mitigation. Environmental Science & Technology 2013, 47, 13210-13211, 10.1021/es404751a.
- Valentina Bollati; Barbara Marinelli; Pietro Apostoli; Matteo Bonzini; Francesco Nordio; Mirjam Hoxha; Valeria Pegoraro; Valeria Motta; Letizia Tarantini; Laura Cantone; et al.Joel SchwartzPier Alberto BertazziAndrea Baccarelli Exposure to Metal-Rich Particulate Matter Modifies the Expression of Candidate MicroRNAs in Peripheral Blood Leukocytes. Environmental Health Perspectives 2010, 118, 763-768, 10.1289/ehp.0901300.
- Kyung Hwa Jung; David Torrone; Stephanie Lovinsky-Desir; Matthew Perzanowski; Joshua Bautista; Jacqueline R. Jezioro; Lori Hoepner; Jamie Ross; Frederica P. Perera; Steven N. Chillrud; et al.Rachel L. Miller Short-term exposure to PM2.5 and vanadium and changes in asthma gene DNA methylation and lung function decrements among urban children. Respiratory Research 2017, 18, 1-11, 10.1186/s12931-017-0550-9.
- Yingying Zhang; Xiaotong Ji; Tingting Ku; Guangke Li; Nan Sang; Heavy metals bound to fine particulate matter from northern China induce season-dependent health risks: A study based on myocardial toxicity. Environmental Pollution 2016, 216, 380-390, 10.1016/j.envpol.2016.05.072.
- Allison A. Appleton; Brian P. Jackson; Margaret Karagas; Carmen Marsit; Prenatal exposure to neurotoxic metals is associated with increased placental glucocorticoid receptor DNA methylation. Epigenetics 2017, 12, 607-615, 10.1080/15592294.2017.1320637.
- Andres Cardenas; Eugene Andres Houseman; Andrea A Baccarelli; Quazi Quamruzzaman; Mahmuder Rahman; Golam Mostofa; Robert Wright; David C Christiani; Molly L Kile; Inuteroarsenic exposure and epigenome-wide associations in placenta, umbilical artery, and human umbilical vein endothelial cells. Epigenetics 2015, 10, 1054-1063, 10.1080/15592294.2015.1105424.
- Todd M. Everson; David A. Armstrong; Brian P. Jackson; Benjamin B. Green; Margaret R. Karagas; Carmen J. Marsit; Maternal cadmium, placental PCDHAC1 , and fetal development. Reproductive Toxicology 2016, 65, 263-271, 10.1016/j.reprotox.2016.08.011.
- Todd Everson; Tracy Punshon; Brian P. Jackson; Ke Hao; Luca Lambertini; Jia Chen; Margaret R. Karagas; Carmen J. Marsit; Cadmium-Associated Differential Methylation throughout the Placental Genome: Epigenome-Wide Association Study of Two U.S. Birth Cohorts. Environmental Health Perspectives 2018, 126, 017010, 10.1289/ehp2192.
- Benjamin B. Green; Margaret R. Karagas; Tracy Punshon; Brian P. Jackson; David J. Robbins; Eugene Andres Houseman; Carmen J. Marsit; Epigenome-Wide Assessment of DNA Methylation in the Placenta and Arsenic Exposure in the New Hampshire Birth Cohort Study (USA). Environmental Health Perspectives 2016, 124, 1253-1260, 10.1289/ehp.1510437.
- Jennifer Z.J. Maccani; Devin C. Koestler; E. Andrés Houseman; David A. Armstrong; Carmen J. Marsit; Karl T. Kelsey; DNA methylation changes in the placenta are associated with fetal manganese exposure. Reproductive Toxicology 2015, 57, 43-49, 10.1016/j.reprotox.2015.05.002.
- Jennifer Z.J. Maccani; Devin C. Koestler; Barry Lester; Eugene Andres Houseman; David A. Armstrong; Karl T. Kelsey; Carmen J. Marsit; Placental DNA Methylation Related to Both Infant Toenail Mercury and Adverse Neurobehavioral Outcomes. Environmental Health Perspectives 2015, 123, 723-729, 10.1289/ehp.1408561.
- April F. Mohanty; Fred M. Farin; Theo K. Bammler; James W. MacDonald; Zahra Afsharinejad; Thomas M. Burbacher; David S. Siscovick; Michelle A. Williams; Daniel A. Enquobahrie; Infant sex-specific placental cadmium and DNA methylation associations. Environmental Research 2015, 138, 74-81, 10.1016/j.envres.2015.02.004.




