
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hernane Silva Barud | + 3498 word(s) | 3498 | 2021-01-07 10:04:39 | | | |
| 2 | Lily Guo | Meta information modification | 3498 | 2021-01-28 08:01:38 | | |
Video Upload Options
Bacterial cellulose (BC) is a natural polymer that has fascinating attributes, such as biocompatibility, low cost, and ease of processing, being considered a very interesting biomaterial due to its options for moldability and combination.
1. Introduction
One of the first designed and main direct applications of BC membranes in the biomedical area is as a wound dressing in the replacement of burned skin[1]. Since then, the literature has shown an increasing number of papers related to wound dressing. In terms of a temporary covering, BC dressings are mightily recommended by manufacturers for the treatment of different types of wounds, including skin tears, pressure sores, venous stasis, second-degree burns, ischemic and diabetic wounds, traumatic abrasions and lacerations, and skin graft and biopsy sites [2].
Currently, it is possible to identify the following BC-based wound dressings available on the market: Gengiflex® (for periodontal reconstruction), XCell®, Bioprocess®, and BioFill® [3][4]. Within the mentioned brands, BioFill® represents one of the first commercial products that fulfills the main requirements of an ideal wound dressing, including: water vapor permeability, good adherence to the wound, transparency, elasticity, durability, a physical barrier for bacteria, hemostatic, easy handling, and low cost with minimum exchanges. In addition, BioFill®’s efficiency in accelerating the healing and pain relief process has been successfully proven in more than 300 clinical trials in humans [5][3][4]. Additionally, it has been reported that the BC wound dressing clearly shortened wound closure and/or the healing time over standard care when applied to non-healing lower extremity ulcers in humans[5][6][7].
Likewise, Portal and co-workers [7] also observed the healing process by using BC dressing (DermafillTM, AMD/Ritmed, Tonawanda, NY, USA) for chronic wounds. They found a considerable reduction in wound closure time from 315 days to 81 days using a BC membrane, with 75% of epithelialization of the affected areas.
Despite the fact that the analgesic mechanism of action and the sensation of pain relief of these wound dressings is not yet fully understood, some authors suggested that the healing could be related to the high number of hydroxylic groups. These hydroxylic groups are able to retain a huge amount of water concomitantly with the capture of ions by means of cellulose hydrogen bonds or by the nano BC 3-D network, which mimics the skin surface, resulting in optimal conditions for healing and regeneration[4].
A variety of surface functionalization’s through biosynthetic or chemical modification was also investigated by incorporating different substrates, including polymers, inorganic nanoparticles or nanowires, and small molecules, on the surface of BC that can be easily maneuvered, forming nanocomposites with fine-tuned properties. Therefore, several BC-based nanocomposites have been developed with improved mechanical and mucoadhesive properties, such as BC/collagen[8][9][10][11] and BC/gelatin[12][13].
Kim et al. [14] prepared wet BC-gelatin nanocomposites, and NIH3T3 fibroblast cells were further seeded and compared with pure BC. After 48 h of incubation, they found that the cells spread well, presenting good adhesion in both platforms, although the biocompatibility was more superior in BC-gelatin composites than that of pure BC.
Besides collagen and gelatin protein, nanocomposites of BC modified with naturally occurring biopolymers, such as aloe vera, have been tailored as biomaterials for therapeutic applications[15][16].
Lin et al. [17] reported the skin wound healing efficacy of BC membranes modified with chitosan in experiments assessed with rat models. They found that the resulting nanocomposite reduced the time for wound healing and did not produce any toxic effect on animal cells.
BC/silk fibroin (SF) sponge-like scaffolds were obtained by Oliveira Barud et al.[18] by means of the freeze-drying technique. These nanocomposites presented a non-cytotoxic or genotoxic character as is possible to observe in SEM images, which showed a greater number of L929 cells attached to the BC/SF: 50% scaffold surface when compared with pure BC. The huge difference in terms of cell attachment may suggest that the presence of fibroin improved and created optimal conditions. So, the SF amino acid sequence may act as cell receptors guiding and allowing easy cell adhesion and growth (Figure 1), pointing that BC/SF: 50% scaffolds represent an excellent option in bioengineering and tissue regeneration in the cultivation of cells on nanobiocomposites.
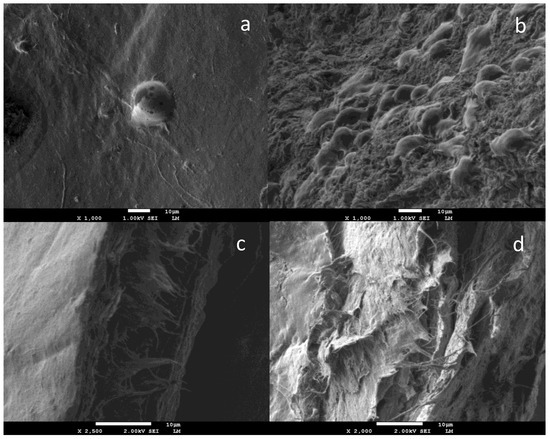
Figure 1. SEM images of L-929 cells attached to BC (a) and BC/SF (b) scaffolds surface at 48 h; cross-section SEM images of BC (c) and BC/SF (d) evidenced that the cells did not migrate into the scaffolds. Reprinted with permission from [18].
Other composites, such as BC-chitosan, BC-poly(ethylene glycol), and BC-collagen, revealed improved NIH3T3 cell activity as compared to native BC [9][19]. On the other hand, human adipose-derived stem cells proliferated on the BC-poly(2-hydroxyethyl methacrylate) scaffold to a lower extent in comparison to pure BC membranes [20]. This fact could be attributed to the presence of monomer residues present in the hybrid scaffold structure as well as the partial biocompatibility of this compound since it is considered an allergen [21].
Nanocomposites with antimicrobial activity have been prepared by incorporating silver nanoparticles [22][23]. Furthermore, BC nanocomposites modified with propolis, a naturally occurring substance, have garnered a great deal of attention as biomaterials due to its remarkable antifungal, antiviral, antioxidant, anti-inflammatory, and antibacterial properties[24].
In recent years, several controlled release systems based on nanocellulose have been conceived. Noteworthy, BC fibrils were used to deliver a myriad of drugs: Tetracycline[25], benzalkonium chloride [26], topical release of lidocaine and ibuprofen [27][28], doxorubicin[21], and release of proteins with serum albumin[29].
Focusing on vascular applications, a German group created BASYC® (Bacterial Cellulose Synthetized), a tubular biomaterial to be used in microsurgery of arteries and veins[30][31]. Similarly, Bodin et al. [32] modified the fermentation process of Komagataeibacter xylinus using silicone guides to mold and obtain BC tubes.
Nimeskern et al.[33] designed the biofabrication of a patient-specific ear-shaped BC implant as a prototype device by applying a 3-D bioprinter technique, which consists of a high-precision motion and a microdispensing system to construct the device layer by layer. The application of this modern and innovative technique confirms BC’s great potential in bioengineering and as an ear cartilage replacement material with appropriate mechanical properties.
Bent BC films have been fashioned by using a special machine in order to shape the biocompatible wound dressing contact lens, seeking their use in the regeneration of cornea tissue or as drug delivery systems [34].
Recently, an implant for a cell trap made of BC after glioblastoma brain surgery was reported[35]. The work suggested that BC could concentrate and trap cancer cells without impact on brain parenchyma and the visibility of BC scaffolds by magnetic resonance imaging allowed increased precision of the stereotactic radiosurgery.
A plentiful number of works also unveil other interesting BC applications in tissue engineering related to neural implants/dura máter[36][37][38], urinary conduits [39][40], tympanic membrane[41][42], and vocal folds[43].
2. BC in Dentistry
Despite the great applicability in biomedicine and tissue engineering, BC-based materials are still poorly explored in dentistry.
There are few studies that evidence the applicability of BC in dental or oral fields. Some of the first applications in the dental area were related to the guided tissue regeneration technique (GTR), aiming at periodontal disease treatment. The biologic basis of GTR to promote periodontal regeneration is related to the placement of physical barriers into specific sites, which will prevent apical migration of the epithelium and gingival connective tissue cells and will allow an isolated area for the migration of mesenchymal and periodontal ligament cells (PDL) over the exposed root surface. The role of physical barriers goes far beyond aesthetic appeal: besides further selective repopulation of the affected area by the maintenance for ingrowth of a new periodontal tissue, they provide protection of the blood clot during early cicatrization stages. However, GTR membranes have limited clinical efficacy on biologic effects related to not providing differentiation and the proliferation of mesenchymal and PDL cells[44][45].
Among the lesions treated by GTR are class II furcation lesions, two-to-three wall infrabone defects, extensive defects combined or not with other techniques, bone dehiscence, and lesions associated with implants[46][47].
Novaes Jr et al.[48] treated class II furcation lesions in humans using a non-resorbable BC membrane (Gengiflex®) with the GTR technique. Clinical evaluation of attachment levels, radiographs, and a re-entry process were applied, and it was possible to observe the closure of the defect. In [49], the same group successfully treated class II furcation lesions in dogs with naturally occurring periodontitis using the same membrane.
Adequate GTR results in periodontal defects in humans as well as in GTR for bone formation were reported by Novaes[50][51] using the commercial membrane Gengiflex®. The mentioned membrane was successfully applied in association with bone-integrated implants.
An et al. [52] prepared bacterial cellulose membranes (BCMs) and performed tests in rats to assess guided bone regeneration. The electron beam (EI) irradiation technique was used to increase the biodegradation of BC. Thus, EI-BCMs membranes were evaluated by Fourier transform infrared attenuated spectroscopy (ATR-FTIR), scanning electron microscopy (SEM), thermal gravimetric analysis (TGA), and other measures of wet tensile strength as well as in vitro analysis to confirm cytocompatibility. All these chemical, mechanical, and biological analyses showed effective EI–BCMs interactions with cells, which promoted bone regeneration as a result.
On the other hand, surgical wounds of the oral mucosa are important issues in dentistry, as shown in Figure 2. Small extent defects usually heal on primary closure, but split or full-thickness grafts can be used for moderate ones. For defects involving most of the buccal mucosa, there is a need for a second surgical area[47].
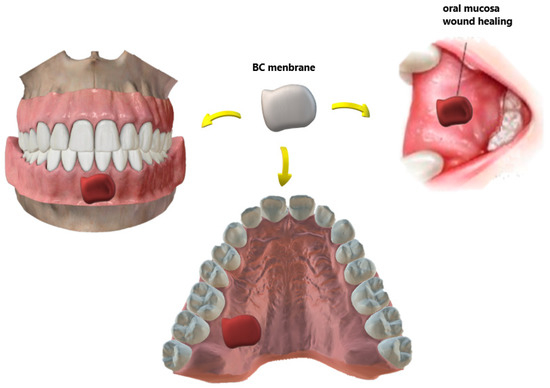
Figure 2. Different types of wounds in the oral mucosa recovered with biomaterials for BC-based dressings. In the case of an autograft donor site, the membrane minimizes the pain and morbidity of the site.
Regarding tissue reconstruction, there are several key remarkable points. One of the most relevant considerations is minimizing flap donor site morbidity, as it is known that the gold standard of a graft material is the autogenous one. Some substitutes based on biomaterials have been developed due to the following issues: morbidity of the donor site, the risk of postoperative complications, the time of the procedure surgical, the unpredictable resorption rate, and the limited amounts of soft tissue available[47].
Chiaoprakobkij et al. [53] prepared a novel three-dimensional composite based on bacterial cellulose/alginate (BCA) to be used as a temporary dressing of oral surgical flaps. In this study, HaCat cells (a keratinocyte cell line) were seeded on BC and BCA scaffolds and compared to gingival fibroblast cells. It was observed that the last-mentioned cell line had attached only onto the BCA sponge, which led researchers to conclude that the BCA sponge scaffold has good potential for use in the oral cavity to cover surgical wounds. Figure 2 illustrates some examples of the BC-based temporary wound dressing. The material features a unique design: the outer layer is thicker to prevent bacterial contamination and dehydration of the wound whereas the inner layer is porous and designed to drain exudates.
With respect to bone regeneration in dentistry as well in biomedicine, the autogenous bone graft is still considered the gold standard. Although it is the only one that presents osteoinductive, osteoconductive, and osteoprogenitor properties [54][55][56], it presents the same risks and morbidity mentioned above. Furthermore, in terms of form and function, each anatomical area offers unique challenges for bone reconstruction, and some graft donor sites (like the fibula, scapula, radio, and iliac crest) can lead to increased morbidity of the donor site[57]. As alternatives to regenerate bone tissue, allogeneic[58][59], xenogenous[60], or synthetic biomaterials have been evaluated as grafts[61][62], as shown in Figure 3.
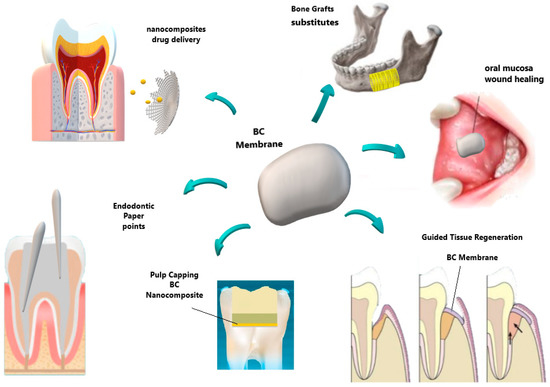
Figure 3. Schematic representation of some distinguished bacterial cellulose applications in dentistry, including the perspective of bone grafts substitutes.
At the current stage of development, the efforts of the most recent research seem to be focused on the development of synthetic bone substitutes that are able to mimic extracellular matrix functions of natural tissues and lead the host response, offering similar results to the autograft implants[62][63].
BC has been recently exploited to design a tailorable matrix to synthesize different types of calcium carbonate (CaCO3) and hydroxyapatite crystals from different starting reagents with improved biocompatibility. For instance, in order to synthesize CaCO3 over BC membranes, Stoica-Guzun et al. [64] used sodium carbonate (Na2CO3) and calcium chloride (CaCl2) as starting reactants. Other authors [65][66] produced BC-hydroxyapatite (Hap) nanocomposites by an optimal biomimetic mineralization route, inducing a negative charge on BC nanofibrils, which stimulated the nucleation of Hap via simulated body fluid (SBF) by the adsorption of polyvinylpyrrolidone (PVP). Before starting the biomimetic mineralization process, Shi et al. [67] developed an alkaline treatment in order to improve the mineralization efficiency and results. Further, Zhang et al.[68][69] promoted the growth of Hap by using a phosphorylation reaction to introduce phosphate groups to BC’s surface.
Dental implants are routine procedures in clinical practice, and often, the upper jaw region has insufficient bone height for the procedure. Thus, Boyne and James [70] created the maxillary sinus lifting technique by performing a graft, allowing implant insertion. In this way, Koike et al. [71] performed frontal bone defects in 12 rabbits that were divided in 4 groups: BC (BC grafting only), BMP-2 (treated only with BMP-2 solution), and BC + BMP-2 (BC loaded with BMP-2 graft). As a result, BC maintained the graft space and BMP-2 was released in a controlled manner into the target area, showing that BC + BMP-2 is a promising option to increase bone structure and for the placement of dental implants.
Saska et al. [72] evaluated the performance and biological properties of BC-Hap nanocomposites in defects of the rat tibiae, aiming at bone repair. The BC-Hap membranes were effective in inducing new bone formation as a slow reabsorption of the membranes occurred, suggesting that longer periods are needed for this compound to be fully reabsorbed. Similarly, Tazi et al.[73] investigated BC-Hap nanocomposite for potential bone regeneration. As a result, BC-Hap nanocomposites stimulated the growth of osteoblast cells, with a high level of alkaline phosphatase activity, and which resulted in greater sites of bone formation. The better the osteoblasts’ adhesion, the better BC-Hap biomaterials are expected to offer cell proliferation and mineralization to encourage faster bone tissue regeneration. Grande et al.[69] also developed BC-Hap scaffolds, presenting excellent results concerning bone and connective tissue regeneration.
Coelho et al. [74] synthesized an innovative BC membrane associated with HA and an antibody of a bone morphogenetic protein (anti-BMP-2) (BC-HA-anti-BMP-2). The SEM-EDS and FTIR assays confirmed the presence of BC and HA. The proposed biomaterial increased the expression of specific genes related to bone repair, proving to be non-cytotoxic, genotoxic, nor mutagenic biomaterial by using MC3T3-E1 cells.
In an attempt to manufacture new biomaterials to induce bone repair, Fan and coworkers[75] reported the introduction of goat bone apatite into BC. The designed biomaterial demonstrated in vitro cell differentiation and also stimulated cell adhesion and proliferation.
In reference to critical-sized calvarial defects in mice, Pigossi et al. [76] evaluated the performance of BC-Hap composites associated with osteogenic growth peptide (OGP) or pentapeptide OGP (10–14) as a potential biomaterial in bone regeneration. Thus, BC-Hap-OGP, BC-Hap-OGP (10–14), and BC-Hap membranes were analyzed at 3, 7, 15, 30, 60, and 90 days. Within each period of analysis, all specimens were evaluated by descriptive histology, micro-computed tomography (µCT), VEGFR-2 (vascular endothelial growth factor) quantification by ELISA, and gene expression of bone biomarkers by qPCR. The researchers found that BC-Hap-OGP (10–14) and BC-Hap membranes reveled at 60 and 90 days a high percentage of bone formation by µCT. High expression of some bone biomarkers, such as Tnfrsf11b and Alpl, Spp1, were also noticed, which lead them to conclude that the BC-Hap membrane promoted better results related to bone formation in critical-sized mice calvarial defects.
In terms of bone repair, Lee and coworkers[77] evaluated silk fibroin-BC membranes’ performance by successfully implanting them on bilateral segmental defects (2 mm in length) of rats’ zygomatic arches. Complete healing of the surgical wounds was observed at the 8-week follow-up against bone degeneration and necrosis when compared to the control side without fixation.
Concerning the field of endodontics, Figure 4 summarize some BC applications. BC has been reported as an innovative material for dental root canal treatment in animal experiments by Yoshino et al. [78]. In comparison with commercial paper point materials (ISO 45 JM paper point, Morita, Osaka, Japan), BC did not exhibit harmful effects, presenting safe compatibility and biological characteristics for dental root canal treatment. Furthermore, BC-based biomaterials under wet conditions showed an excellent absorption rate without deformation in relation to conventional ones.
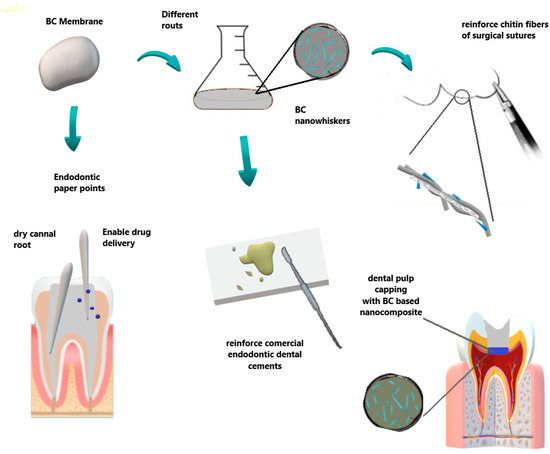
Figure 4. Some applications in endodontic and surgical areas. BC can be used as paper points to dry the root canal, also allowing desired target drug delivery. Otherwise, BC nanowhiskers can be obtained by different routes to reinforce dental cements and sutures in surgical procedures.
After the cleaning and drying steps related to endodontic treatment, the ideal sequence concerns a complete sealing of the root canal space with filling materials.
Due to the excellent properties of BC, it can enable the production of whiskers in the nanometric scale (nanowhiskers) that can be used as a reinforcement for other materials, including dental cements. Thus, Jinga et al. [79] prepared BC nanowhiskers and used commercial MTA (mineral trioxide aggregates) cement for the preparation of some composites: MTA-E (mineral trioxide aggregates-experimental), composites MTA-10% biocell, and MTA-33% biocell cements. Considering one day of hardening, XRD patterns, and the thermal analysis data, they concluded that the presence of BC nanowhiskers accelerates the hardening processes of MTA cement while a decrease in the amount of calcium hydroxide crystals was noted. Thus, it was firstly reported in the literature that BC nanowhiskers further the formation of crystalline hydrosilicates even after one day of hardening, without affecting the viability and affinity of cells.
Intending to promote a tissue regeneration response by applying the dental pulp capping technique, Manzine Costa and coworkers[80] developed an otolith/BC nanocomposite (OTL). Subsequently, exposed incisor dental pulps of dogs were divided into two groups: BC/Otolith preparation (OTL) and no protective material in the control group. The teeth were then sealed with glass ionomer and histological analyses were performed after 21 days. The proposed OTL nanocomposite induced the formation of a tissue barrier expressing osteoid-like characteristics of mineralization. The obtained results indicate that otolith/cellulose bacterial nanocomposites might work as a potential dental pulp capping biomaterial.
Recently, Voeicu et al. [81] synthesized a new family of composites starting from mineral binder powders and biocellulose membranes, showing great applications in endodontics. Silicate cement synthesized through the sol-gel technique was prepared by the addition of a BC polygranular powder previously obtained (crushed into micrometric particles via hydrothermal treatment). The resulting nanocomposite was investigated by SEM, X-ray diffraction, thermal analysis, and other mechanical tests. The most important effect of the BC content was observed in terms of shortening the setting time, where reduced clinical care time is required. In vitro tests were performed, and all materials did not exert cytotoxic effects, and promoted cell adhesion and proliferation. Moreover, they presented a pronounced mineralization process in the simulated conditions. The authors concluded that the proposed composite has high potential for applications in endodontic treatments, mainly in root canal filling, root perforations, or dentin remineralization.
Regarding the reinforcement properties, the BC presented good results, improving the mechanical properties when added to chitin fibers in surgical suture applications, and thus setting up a promising new candidate as a BC-based medical suture for dentistry[82].
Carvalho et al. [83], in 2020, developed BNC-based patches containing both HA and (Diclofenac) DCF. The objective of the material was the stimulation of healing of the aphthous ulcers in recurrent aphthous stomatitis (RAS) and the mitigation of pain. RAS is the most common form of oral mucosa ulcer, affecting from 5% to 66% of the world’s population, which is also known as aphthae or canker sores. Despite manifesting spontaneous healing in a few days, they can be extremely uncomfortable, causing stinging pain and local inflammation. From this perspective, the freestanding membrane patches were fabricated via simple diffusion of HA and DCF aqueous solutions into a wet BNC three-dimensional porous network. The resultant nanostructured patches were thermal resistant and stable up to 200 °C. In vitro assays showed that the patches were almost 100% after 24 h of incubation. In addition, the swelling ability and DCF release was conducted in simulated saliva, pointing to controlled drug-release purposes. The attained results hint at the possibility of using the proposed BNC-HA-DCF-based patches to diminish and treat aphthous stomatitis discomfort in the oral mucosa.
Finally, Table 1 shows a brief summary of the main BC advantages concerning its properties to be applied in dental and oral fields.
Table 1. Summary of the main BC advantages to be to be applied in dental and oral fields.
| and Oral Treatments | Potential Use | BC Advantages |
|---|---|---|
| Periodontal treatment | Barrier membrane in GTR technique Novaes Jr et al., [84,85] Novaes [86,87] An et al., [88] |
Allowed cell attachment and proliferation Aesthetics importance restoration of oral function Reduction in surgical steps Biocompatibility, presenting no chronic inflammatory reaction |
| Wound dressing/patches | Surgical Wounds, flaps and RAS ulcers Chiaoprakobkij et al., [89] Carvalho et al., [119] |
Biocompatible Physical barrier Allow drug delivery |
| Dental pulp tissue treatment | Root canal sealers Jinga et al., [115] Scaffold for regenerative Endodontic treatment Manzine Costa et al., [116] Voeicu et al., [117] |
Accelerates the hardening processes of cement Reinforce dental cements Mimics extracellular matrix. Induces mineralized barrier and apical closure |
| Dental surgery | Surgical suture Wu et al., [118] |
Improve reinforcement and mechanical properties |
References
- Fontana, J.D.; De Souza, A.M.; Fontana, C.K.; Torriani, I.L.; Moreschi, J.C.; Gallotti, B.J.; De Souza, S.J.; Narcisco, G.P.; Bich-ara, J.A.; Farah, L.F.X. Acetobacter cellulose pellicle as a temporary skin substitute. Appl. Biochem. Biotechnol. 1990, 24–25, 253–264, doi:10.1007/bf02920250.
- Ludwicka, K.; Cala, J.; Grobelski, B.; Sygut, D.; Jesionek-Kupnicka, D.; Kolodziejczyk, M.; Bielecki, S.; Pasieka, Z. New methods Modified bacterial cellulose tubes for regeneration of damaged peripheral nerves. Arch. Med. Sci. 2013, 3, 527–534, doi:10.5114/aoms.2013.33433.
- Farah, L.F.X. Process for the Preparation of Cellulose Film, Cellulose Film Produced Thereby, Artificial Skin Graft and its Use. 1990. Available online: https://patents.google.com/patent/US4912049A/en.
- Wouk, A.F.P.D.F.; Diniz, J.M.; Cirio, S.M.; Dos Santos, H.; Baltazar, E.L.; Acco, A. Membrana biológica (biofill)—Estudo comparativo com outros agentes promotores da cicatrização da pele em suínos: Aspectos clínicos, histopatológicos e mor-fométricos. Arch. Veter. Sci. 1998, 3, doi:10.5380/avs.v3i1.3736.
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules 2007, 8, 1–12, doi:10.1021/bm060620d.
- Portal, O.; Clark, W.A.; Levinson, D.J. Microbial cellulose wound dressing in the treatment of nonhealing lower extremity ulcers. Wounds 2009, 21, 1–3.
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brown, R.M. Microbial cellulose—The natural power to heal wounds. Biomaterials 2006, 27, 145–151, doi:10.1016/j.biomaterials.2005.07.035.
- Zhijiang, C.; Guang, Y. Bacterial cellulose/collagen composite: Characterization and first evaluation of cytocompatibility. J. Appl. Polym. Sci. 2011, 120, 2938–2944, doi:10.1002/app.33318.
- Kaya, M.G.A.; Vuluga, Z.; Panaitescu, D.M.; Vuluga, D.M.; Căşărică, A.; Ghiurea, M. Morphology and thermal stability of bacterial cellulose/collagen composites. Open Chem. 2014, 12, 968–975, doi:10.2478/s11532-014-0545-z.
- Moraes, P.R.F.D.S.; Saska, S.; Barud, H.; De Lima, L.R.; Martins, V.D.C.A.; Plepis, A.M.D.G.; Ribeiro, S.J.L.; Gaspar, A.M.M. Bacterial Cellulose/Collagen Hydrogel for Wound Healing. Mater. Res. 2016, 19, 106–116, doi:10.1590/1980-5373-mr-2015-0249.
- Nakayama, A.; Kakugo, A.; Gong, J.P.; Osada, Y.; Takai, M.; Erata, T.; Kawano, S. High Mechanical Strength Dou-ble-Network Hydrogel with Bacterial Cellulose. Adv. Funct. Mater. 2004, 14, 1124–1128, doi:10.1002/adfm.200305197.
- Saska, S.; Teixeira, L.N.; De Oliveira, P.T.; Gaspar, A.M.M.; Ribeiro, S.J.L.; Messaddeq, Y.; Marchetto, R. Bacterial cellu-lose-collagen nanocomposite for bone tissue engineering. J. Mater. Chem. 2012, 22, 22102–22112, doi:10.1039/c2jm33762b.
- Wang, J.; Wan, Y.; Luo, H.; Gao, C.; Huang, Y. Immobilization of gelatin on bacterial cellulose nanofibers surface via cross-linking technique. Mater. Sci. Eng. C 2012, 32, 536–541, doi:10.1016/j.msec.2011.12.006.
- Kim, J.-H.; Cai, Z.; Chen, Y. Biocompatible Bacterial Cellulose Composites for Biomedical Application. J. Nanotechnol. Eng. Med. 2009, 1, 011006, doi:10.1115/1.4000062.
- Saibuatong, O.-A.; Phisalaphong, M. Novo aloe vera–bacterial cellulose composite film from biosynthesis. Carbohydr. Polym. 2010, 79, 455–460, doi:10.1016/j.carbpol.2009.08.039.
- Piaia, L.; Paes, C.Q.; Porto, L.M. Viability of human dermal fibroblasts cultured on bacterial cellulose and Aloe vera com-posites. BMC Proc. 2014, 8, P61, doi:10.1186/1753-6561-8-S4-P61.
- Lin, W.-C.; Lien, C.-C.; Yeh, H.-J.; Yu, C.-M.; Hsu, S.-H. Bacterial cellulose and bacterial cellulose–chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611, doi:10.1016/j.carbpol.2013.01.076.
- Barud, H.S.; Cavicchioli, M.; Amaral, T.S.D.; Junior, O.B.D.O.; Santos, D.M.; Petersen, A.L.D.O.A.; Celes, F.S.; Borges, V.D.M.; De Oliveira, C.I.; De Oliveira, P.F.; et al. Preparation and characterization of a bacterial cellulose/silk fibroin sponge scaf-fold for tissue regeneration. Carbohydr. Polym. 2015, 128, 41–51, doi:10.1016/j.carbpol.2015.04.007.
- Cai, Z.; Kim, J. Bacterial cellulose/poly(ethylene glycol) composite: Characterization and first evaluation of biocompatibil-ity. Cellulose 2010, 17, 83–91, doi:10.1007/s10570-009-9362-5.
- Figueiredo, A.G.P.R.; Figueiredo, A.R.P.; Alonso-Varona, A.; Fernandes, S.C.M.; Palomares, T.; Rubio-Azpeitia, E.; Bar-ros-Timmons, A.; Silvestre, A.J.D.; Neto, C.; Freire, C.S. Biocompatible Bacterial Cellulose-Poly(2-hydroxyethyl methacry-late) Nanocomposite Films. BioMed. Res. Int. 2013, 2013, 1–14, doi:10.1155/2013/698141.
- Horue, M.; Cacicedo, M.L.; Castro, G.R. New insights into bacterial cellulose materials: Production and modification strate-gies. Int. J. Adv. Med Biotechnol. IJAMB 2018, 1, 44–59, doi:10.25061/2595-3931/ijamb/2018.v1i2.20.
- Maria, L.C.D.S.; Santos, A.L.; Oliveira, P.C.; Barud, H.S.; Messaddeq, Y.; Ribeiro, S.J. Synthesis and characterization of silver nanoparticles impregnated into bacterial cellulose. Mater. Lett. 2009, 63, 797–799, doi:10.1016/j.matlet.2009.01.007.
- Barud, H.S.; Barrios, C.; Regiani, T.; Marques, R.F.; Verelst, M.; Dexpert-Ghys, J.; Messaddeq, Y.; Ribeiro, S.J. Self-supported silver nanoparticles containing bacterial cellulose membranes. Mater. Sci. Eng. C 2008, 28, 515–518, doi:10.1016/j.msec.2007.05.001.
- Barud, H.D.S.; Júnior, A.M.D.A.; Saska, S.; Mestieri, L.B.; Campos, J.A.D.B.; De Freitas, R.M.; Ferreira, N.U.; Nascimento, A.P.; Miguel, F.G.; Vaz, M.M.D.O.L.L.; et al. Antimicrobial Brazilian Propolis (EPP-AF) Containing Biocellulose Membranes as Promising Biomaterial for Skin Wound Healing. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–10, doi:10.1155/2013/703024.
- Stoica-Guzun, A.; Stroescu, M.; Tache, F.; Zaharescu, T.; Grosu, E. Effect of electron beam irradiation on bacterial cellulose membranes used as transdermal drug delivery systems. Nucl. Instr. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2007, 265, 434–438, doi:10.1016/j.nimb.2007.09.036.
- Wei, B.; Yang, G.; Hong, F. Preparation and evaluation of a kind of bacterial cellulose dry films with antibacterial proper-ties. Carbohydr. Polym. 2011, 84, 533–538, doi:10.1016/j.carbpol.2010.12.017.
- Trovatti, E.; Silva, N.H.C.S.; Duarte, I.F.; Rosado, C.F.; Almeida, I.F.; Costa, P.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Biocellulose Membranes as Supports for Dermal Release of Lidocaine. Biomacromolecules 2011, 12, 4162–4168, doi:10.1021/bm201303r.
- Trovatti, E.; Freire, C.S.R.; Pinto, P.C.; Almeida, I.F.; Costa, P.; Silvestre, A.J.D.; Neto, C.P.; Rosado, C. Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: In vitro diffusion stud-ies. Int. J. Pharm. 2012, 435, 83–87, doi:10.1016/j.ijpharm.2012.01.002.
- Müller, A.; Ni, Z.; Hessler, N.; Wesarg, F.; Müller, F.A.; Kralisch, D.; Fischer, D. The Biopolymer Bacterial Nanocellulose as Drug Delivery System: Investigation of Drug Loading and Release using the Model Protein Albumin. J. Pharm. Sci. 2013, 102, 579–592, doi:10.1002/jps.23385.
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsur-gery. Prog. Polym. Sci. 2001, 26, 1561–1603, doi:10.1016/s0079-6700(01)00021-1.
- Wippermann, J.; Schumann, D.; Klemm, D.; Kosmehl, H.; Salehi-Gelani, S.; Wahlers, T. Preliminary Results of Small Arterial Substitute Performed with a New Cylindrical Biomaterial Composed of Bacterial Cellulose. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 592–596, doi:10.1016/j.ejvs.2009.01.007.
- Bodin, A.; Bäckdahl, H.; Fink, H.; Gustafsson, L.; Risberg, B.; Gatenholm, P. Influence of cultivation conditions on mechani-cal and morphological properties of bacterial cellulose tubes. Biotechnol. Bioeng. 2007, 97, 425–434, doi:10.1002/bit.21314.
- Nimeskern, L.; Ávila, H.M.; Sundberg, J.; Gatenholm, P.; Müller, R.; Stok, K.S. Mechanical evaluation of bacterial nanocel-lulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013, 22, 12–21, doi:10.1016/j.jmbbm.2013.03.005.
- Cavicchioli, M.; Corso, C.T.; Coelho, F.; Mendes, L.; Saska, S.; Soares, C.P.; Souza, F.O.; Franchi, L.P.; Capote, T.S.O.; Scarel-Caminaga, R.M.; et al. Characterization and cytotoxic, genotoxic and mutagenic evaluations of bacterial cellulose membranes incorporated with ciprofloxacin: A potential material for use as therapeutic contact lens. World J. Pharm. Pharm. Sci. 2015, 4, 1626–1647.
- Autier, L.; Clavreul, A.; Cacicedo, M.L.; Franconi, F.; Sindji, L.; Rousseau, A.; Perrot, R.; Montero-Menei, C.N.; Castro, G.R.; Menei, P. A new glioblastoma cell trap for implantation after surgical resection. Acta Biomater. 2019, 84, 268–279, doi:10.1016/j.actbio.2018.11.027.
- Pértile, R.A.N.; Moreira, S.M.G.; Andrade, F.K.; Domingues, L.; Gama, M. Bacterial cellulose modified using recombinant proteins to improve neuronal and mesenchymal cell adhesion. Biotechnol. Prog. 2012, 28, 526–532, doi:10.1002/btpr.1501.
- Xu, C.; Ma, X.; Chen, S.-W.; Tao, M.; Yuan, L.; Jing, Y. Bacterial Cellulose Membranes Used as Artificial Substitutes for Dural Defection in Rabbits. Int. J. Mol. Sci. 2014, 15, 10855–10867, doi:10.3390/ijms150610855.
- Mello, L.R.; Feltrin, L.T.; Neto, P.T.F.; Ferraz, F.A.P. Duraplasty with biosynthetic cellulose: An experimental study. J. Neu-rosurg. 1997, 86, 143–150, doi:10.3171/jns.1997.86.1.0143.
- Bodin, A.; Bharadwaj, S.; Wu, S.; Gatenholm, P.; Atala, A.; Zhang, Y. Tissue-engineered conduit using urine-derived stem cells seeded bacterial cellulose polymer in urinary reconstruction and diversion. Biomater. 2010, 31, 8889–8901, doi:10.1016/j.biomaterials.2010.07.108.
- Huang, J.-W.; Lv, X.-G.; Li, Z.; Song, L.-J.; Feng, C.; Xie, M.-K.; Li, C.; Li, H.-B.; Wang, J.-H.; Zhu, W.-D.; et al. Urethral recon-struction with a 3D porous bacterial cellulose scaffold seeded with lingual keratinocytes in a rabbit model. Biomed. Mater. 2015, 10, 055005, doi:10.1088/1748-6041/10/5/055005.
- Kim, J.; Kim, S.W.; Park, S.; Lim, K.-T.; Seonwoo, H.; Kim, Y.; Hong, B.H.; Choung, Y.-H.; Chung, J.H. Bacterial Cellulose Nanofibrillar Patch as a Wound Healing Platform of Tympanic Membrane Perforation. Adv. Heal. Mater. 2013, 2, 1525–1531, doi:10.1002/adhm.201200368.
- Silveira, F.C.A.; Pinto, F.C.M.; Neto, S.D.S.C.; Leal, M.D.C.; Cesário, J.; Aguiar, J.L.D.A. Treatment of tympanic membrane perforation using bacterial cellulose: A randomized controlled trial. Braz. J. Otorhinolaryngol. 2016, 82, 203–208, doi:10.1016/j.bjorl.2015.03.015.
- De Souza, F.C.; Olival-Costa, H.; Da Silva, L.; Pontes, P.A.; Lancellotti, C.L.P. Bacterial Cellulose as Laryngeal Medialization Material: An Experimental Study. J. Voice 2011, 25, 765–769, doi:10.1016/j.jvoice.2010.07.005.
- Needleman, I.G.; Worthington, H.V.; Giedrys-Leeper, E.; Tucker, R.J. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst. Rev. 2006, CD001724, doi:10.1002/14651858.CD001724.pub2.
- Verma, P.K.; Srivastava, R.; Gupta, K.K.; Chaturvedi, T.P. Treatment strategy for guided tissue regeneration in various class II furcation defect: Case series. Dent. Res. J. 2013, 10, 689–694.
- Gottlow, J.; Nyman, S.; Karring, T.; Lindhe, J. New attachment formation as the result of controlled tissue regeneration. J. Clin. Periodontol. 1984, 11, 494–503, doi:10.1111/j.1600-051x.1984.tb00901.x.
- Villar, C.C.; Cochran, D.L. Regeneration of Periodontal Tissues: Guided Tissue Regeneration. Dent. Clin. North Am. 2010, 54, 73–92, doi:10.1016/j.cden.2009.08.011.
- Novaes, A.B., Jr.; Moraes, N.H.; Novaes, A.B. The use of BioFill as biological membrane in the treatment of furcation lesion with and without the utilization of porous hydroxyapatite. Rev. Bras. Odontol. 1990, 47, 29–32.
- Novaes, A.B., Jr.; Novaes, A.B.; Grisi, M.F.; Soares, U.N.; Gabarra, F.R. Gengiflex, an Alkali-Cellulose Membrane for GTR. Braz. Dent. J. 1994, 4, 65–73.
- Novaes, A.B. IMZ implants placed into extraction sockets in association with membrane therapy (Gengiflex) and porous hydroxyapatite: A case report. Int. J. Oral Maxillofac. Implant. 1992, 7, 536–540.
- Novaes, A.B. Bone formation over a TiAl6 V4 (IMZ) implant placed into an extraction socket in association with membrane therapy (Gengiflex). Clin. Oral Implant. Res. 1993, 4, 106–110, doi:10.1034/j.1600-0501.1993.040207.x.
- An, S.-J.; Lee, S.-H.; Huh, J.-B.; Jeong, S.-I.; Park, J.-S.; Gwon, H.-J.; Kang, E.-S.; Jeong, C.-M.; Lim, Y.-M. Preparation and Characterization of Resorbable Bacterial Cellulose Membranes Treated by Electron Beam Irradiation for Guided Bone Re-generation. Int. J. Mol. Sci. 2017, 18, 2236, doi:10.3390/ijms18112236.
- Chiaoprakobkij, N.; Sanchavanakit, N.; Subbalekha, K.; Pavasant, P.; Phisalaphong, M. Characterization and biocompatibil-ity of bacterial cellulose/alginate composite sponges with human keratinocytes and gingival fibroblasts. Carbohydr. Polym. 2011, 85, 548–553, doi:10.1016/j.carbpol.2011.03.011.
- Block, M.S.; Kent, J.N. Sinus augmentation for dental implants: The use of autogenous bone. J. Oral Maxillofac. Surg. 1997, 55, 1281–1286, doi:10.1016/s0278-2391(97)90185-3.
- Gerressen, M.; Hermanns-Sachweh, B.; Riediger, D.; Hilgers, R.-D.; Spiekermann, H.; Ghassemi, A. Purely cancellous vs. corticocancellous bone in sinus floor augmentation with autogenous iliac crest: A prospective clinical trial. Clin. Oral Im-plant. Res. 2009, 20, 109–115, doi:10.1111/j.1600-0501.2008.01619.x.
- Rogers, G.F.; Greene, A.K. Autogenous Bone Graft. J. Craniofacial Surg. 2012, 23, 323–327, doi:10.1097/scs.0b013e318241dcba.
- Ayub, L.G.; Ramos, U.D.; Reino, D.M.; Grisi, M.F.; Taba, M.; Souza, S.L.S.; Palioto, D.B.; Novaes, A.B.; Taba, M. A Modified Surgical Technique for Root Coverage with an Allograft: A 12-Month Randomized Clinical Trial. J. Periodontol. 2014, 85, 1529–1536, doi:10.1902/jop.2014.140111.
- Barone, A.; Varanini, P.; Orlando, B.; Tonelli, P.; Covani, U. Deep-Frozen Allogeneic Onlay Bone Grafts for Reconstruction of Atrophic Maxillary Alveolar Ridges: A Preliminary Study. J. Oral Maxillofac. Surg. 2009, 67, 1300–1306, doi:10.1016/j.joms.2008.12.043.
- Khojasteh, A.; Motamedian, S.R.; Khojaste, M. Success rate of implants placed in autogenous bone blocks versus allogenic bone blocks: A systematic literature review. Ann. Maxillofac. Surg. 2016, 6, 78–90, doi:10.4103/2231-0746.186143.
- Hatano, N.; Shimizu, Y.; Ooya, K. A clinical long-term radiographic evaluation of graft height changes after maxillary sinus floor augmentation with a 2:1 autogenous bone/xenograft mixture and simultaneous placement of dental implants. Clin. Oral Implant. Res. 2004, 15, 339–345, doi:10.1111/j.1600-0501.2004.00996.x.
- Xu, C.; Su, P.; Chen, X.; Meng, Y.; Yu, W.; Xiang, A.P.; Wang, Y. Biocompatibility and osteogenesis of biomimetic Bio-glass-Collagen-Phosphatidylserine composite scaffolds for bone tissue engineering. Biomater. 2011, 32, 1051–1058, doi:10.1016/j.biomaterials.2010.09.068.
- de Souza, S.L.S.; Martins, S.H.L.; Bezerra, F.J.B.; Shibli, J.A.; Mantovani, R.V.; da Costa, L.F.A.; Júnior, J.A.B.G. Alveolar ridge preservation and regeneration with biomaterials. In 50 Years Osseointegration Reflections Perspect; Rossetti, P.H.O., Bonachela, W.C., Eds.; São Paulo, Brazilian, 2015; pp. 145–179.
- Barrere, F.; Mahmood, T.; De Groot, K.; Van Blitterswijk, C.; Van Blitterswijk, C.A. Advanced biomaterials for skeletal tis-sue regeneration: Instructive and smart functions. Mater. Sci. Eng. R Rep. 2008, 59, 38–71, doi:10.1016/j.mser.2007.12.001.
- Stoica-Guzun, A.; Stroescu, M.; Jinga, S.; Jipa, I.; Dobre, T.; Dobre, L. Ultrasound influence upon calcium carbonate precipita-tion on bacterial cellulose membranes. Ultrason. Sonochem. 2012, 19, 909–915, doi:10.1016/j.ultsonch.2011.12.002.
- Yin, N.; Chen, S.; Ouyang, Y.; Tang, L.; Yang, J.-X.; Wang, H. Biomimetic mineralization synthesis of hydroxyapatite bacte-rial cellulose nanocomposites. Prog. Nat. Sci. 2011, 21, 472–477, doi:10.1016/s1002-0071(12)60085-9.
- Wan, Y.Z.; Gao, C.; Luo, H.L.; He, F.; Liang, H.; Li, X.L.; Wang, Y.L. Early growth of nano-sized calcium phosphate on phos-phorylated bacterial cellulose nanofibers. J. Nanosci. Nanotechnol. 2009, 9, 6494–6500, doi:10.1166/jnn.2009.1311.
- Shi, S.; Chen, S.; Zhang, X.; Shen, W.; Li, X.; Hu, W.; Wang, H. Biomimetic mineralization synthesis of calcium-deficient carbonate-containing hydroxyapatite in a three-dimensional network of bacterial cellulose. J. Chem. Technol. Biotechnol. 2008, 84, 285–290, doi:10.1002/jctb.2037.
- Zhang, S.; Xiong, G.; He, F.; Huang, Y.; Wang, Y.; Wan, Y. Characterisation of Hydroxyapatite/Bacterial Cellulose Nano-composites. Polym. Polym. Compos. 2009, 17, 353–358, doi:10.1177/096739110901700602.
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Bañó, M.C. Nanocomposites of bacterial cellulose/hydroxyapatite for biomedical applications. Acta Biomater. 2009, 5, 1605–1615, doi:10.1016/j.actbio.2009.01.022.
- Boyne, P.J.; James, R.A. Grafting of the maxillary sinus floor with autogenous marrow and bone. J. Oral Surg. (American Dent. Assoc. 1965) 1980, 38, 613–616.
- Koike, T.; Sha, J.; Bai, Y.; Matsuda, Y.; Hideshima, K.; Yamada, T.; Kanno, T. Efficacy of Bacterial Cellulose as a Carrier of BMP-2 for Bone Regeneration in a Rabbit Frontal Sinus Model. Materials 2019, 12, 2489, doi:10.3390/ma12152489.
- Saska, S.; Barud, H.S.; Gaspar, A.M.M.; Marchetto, R.; Ribeiro, S.J.L.; Messaddeq, Y. Bacterial Cellulose-Hydroxyapatite Nanocomposites for Bone Regeneration. Int. J. Biomater. 2011, 2011, 1–8, doi:10.1155/2011/175362.
- Tazi, N.; Zhang, Z.; Messaddeq, Y.; Almeida-Lopes, L.; Zanardi, L.M.; Levinson, D.J.; Rouabhia, M. Hydroxyapatite bioacti-vated bacterial cellulose promotes osteoblast growth and the formation of bone nodules. AMB Express 2012, 2, 61, doi:10.1186/2191-0855-2-61.
- Coelho, F.; Cavicchioli, M.; Specian, S.S.; Scarel-Caminaga, R.M.; Penteado, L.D.A.; De Medeiros, A.I.; Ribeiro, S.J.D.L.; Ca-pote, T.S.D.O. Bacterial cellulose membrane functionalized with hydroxiapatite and anti-bone morphogenetic protein 2: A promising material for bone regeneration. PLoS ONE 2019, 14, e0221286, doi:10.1371/journal.pone.0221286.
- Fan, X.; Zhang, T.; Zhao, Z.; Ren, H.; Zhang, Q.; Yan, Y.; Lv, G. Preparation and characterization of bacterial cellulose micro-fiber/goat bone apatite composites for bone repair. J. Appl. Polym. Sci. 2013, 129, 595–603, doi:10.1002/app.38702.
- Pigossi, S.C.; De Oliveira, G.J.P.L.; Finoti, L.S.; Nepomuceno, R.; Spolidorio, L.C.; Rossa, C.; Ribeiro, S.J.; Saska, S.; Scarel-Caminaga, R.M. Bacterial cellulose-hydroxyapatite composites with osteogenic growth peptide (OGP) or pentapep-tide OGP on bone regeneration in critical-size calvarial defect model. J. Biomed. Mater. Res. Part A 2015, 103, 3397–3406, doi:10.1002/jbm.a.35472.
- Lee, J.M.; Kim, J.H.; Lee, O.J.; Park, C.H. The Fixation Effect of a Silk Fibroin–Bacterial Cellulose Composite Plate in Seg-mental Defects of the Zygomatic ArchAn Experimental StudySilk Fibroin–Bacterial Cellulose Composite Plate. JAMA Oto-laryngol. Neck Surg. 2013, 139, 629–635, doi:10.1001/jamaoto.2013.3044.
- Yoshino, A.; Tabuchi, M.; Uo, M.; Tatsumi, H.; Hideshima, K.; Kondo, S.; Sekine, J. Applicability of bacterial cellulose as an alternative to paper points in endodontic treatment. Acta Biomater. 2013, 9, 6116–6122, doi:10.1016/j.actbio.2012.12.022.
- Jinga, S.I.; Voicu, G.; Stoica-Guzun, A.; Stroescu, M.; Grumezescu, A.M.; Bleotu, C. Biocellulose nanowhiskers cement com-posites for endodontic use. Dig. J. Nanomater. Biostruct. 2014, 9, 543–550.
- Costa, L.M.M.; De Olyveira, G.M.; Basmaji, P.; Valido, D.P.; Gois, P.B.P.; Júnior, R.L.A.C.; Filho, L.X. Novel Otoliths/Bacterial Cellulose Nanocomposites as a Potential Natural Product for Direct Dental Pulp Capping. J. Biomater. Tissue Eng. 2012, 2, 48–53, doi:10.1166/jbt.2012.1031.
- Voicu, G.; Jinga, S.-I.; Drosu, B.-G.; Busuioc, C. Improvement of silicate cement properties with bacterial cellulose powder addition for applications in dentistry. Carbohydr. Polym. 2017, 174, 160–170, doi:10.1016/j.carbpol.2017.06.062.
- Wu, H.; Williams, G.R.; Wu, J.; Wu, J.; Niu, S.; Li, H.; Wang, H.; Zhu, L.-M. Regenerated chitin fibers reinforced with bacteri-al cellulose nanocrystals as suture biomaterials. Carbohydr. Polym. 2018, 180, 304–313, doi:10.1016/j.carbpol.2017.10.022.
- Carvalho, J.P.F.; Silva, A.C.Q.; Bastos, V.; Oliveira, H.; Pinto, R.J.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S. Nanocellu-lose-Based Patches Loaded with Hyaluronic Acid and Diclofenac towards Aphthous Stomatitis Treatment. Nanomaterials 2020, 10, 628, doi:10.3390/nano10040628.




