Your browser does not fully support modern features. Please upgrade for a smoother experience.
Subjects:
Cell & Tissue Engineering
A microneedle (MN) is a painless and minimally invasive drug delivery device initially developed in 1976. As microneedle technology evolves, microneedles with different shapes (cone and pyramid) and forms (solid, drug-coated, hollow, dissolvable and hydrogel-based microneedles) have been developed.
- microneedle
- classification
- manufacture
- biomedical application
- pitfalls
1. Introduction
Pain associated with conventional injection for drug administration causes poor adherence. Oral administration is convenient, but the efficiency of drug delivery is limited by the first-pass effect. A microneedle is a novel approach to drug delivery that overcomes these limitations. Microneedles can physically penetrate the stratum corneum and create micropores larger than macromolecular drugs, thus providing direct channels for drug diffusion. Alternatively, drugs can be loaded in hollow microneedles and directly injected into the circulatory system. Drugs can also be mixed with a soluble substance that penetrates the skin and reaches the circulatory system. Microneedles can be used for transdermal and non-transdermal drug delivery. In transdermal drug delivery, microneedles can reduce or eliminate the pain of injection. The length of microneedles ranges from 25 to 2000 μm [1] and can penetrate the skin barrier without damaging neural tissue (Figure 1) [2]. Furthermore, microneedles is a kind of continuous administration, so that it can reduce the frequency of administration, especially for insulin injection in patients with diabetes [3][4]. Non-transdermal administration is used for buccal mucosa [5] and surgery-exposed tissues such as eyeballs [6], vascular tissue [7] and the gastrointestinal tract [8]. For example, in 2011, Patel et al. injected particles into the suprachoroidal space through hollow microneedles and delivered a drug to the back of the eye [9].
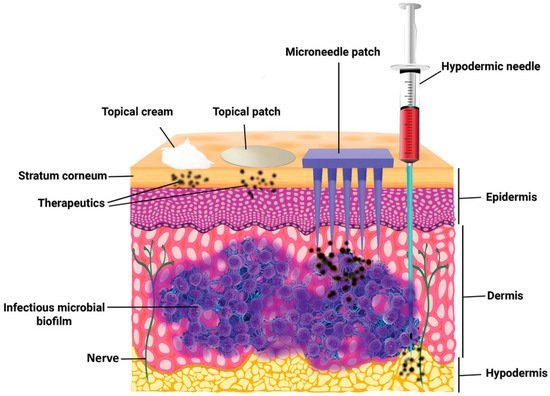
Figure 1. The comparison of skin penetration depths across different drug delivery systems. (Image was reproduced with permission from [2]).
Microneedles were developed in 1976 and used for transdermal drug delivery in 1998. They have been intensively studied over the past half-century (Figure 2) [10][11][12][13][14][15][16][17][18][19][20][21]. A “Web of Science” search with the topic of “microneedle” yielded 3407 articles published since 2000 (Figure 3), which indicates the rapid increase of microneedle studies. Moreover, most microneedles are applied in biomedical areas, especially for cancer therapy, skin disease therapy, insulin delivery for diabetes treatment, blood glucose level detection and vaccines [17][22][23][24][25][26][27][28][29][30]. Therefore, in this work, we mainly review the classification, manufacture and biomedical applications of microneedles. The pitfalls of the application of microneedles are also summarized. Given the advantages of microneedle administration, it is promising to optimize the fabrications of microneedles and avoid their disadvantages in future studies.
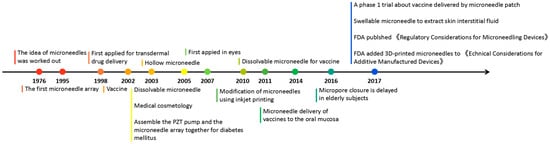
Figure 2. Timeline of microneedle development. The time point listed represented the important events associated with microneedles from the idea of microneedles in 1976.
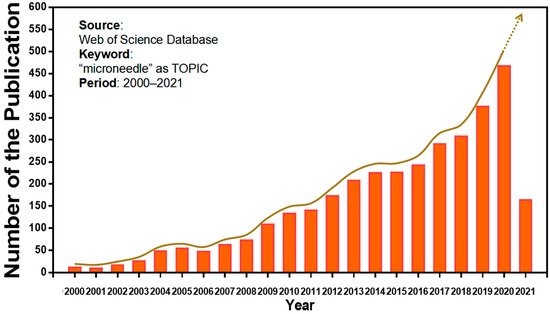
Figure 3. Keyword literature search of microneedles in Web of Science. The popularity of microneedles has been growing every year from 2000 until now.
2. Classification of Microneedles
Here, in this review, according to microneedle-based devices, MNs can be divided into five categories: solid, drug-coated, dissolvable, hollow and hydrogel-based microneedles (Figure 4) [31][32].
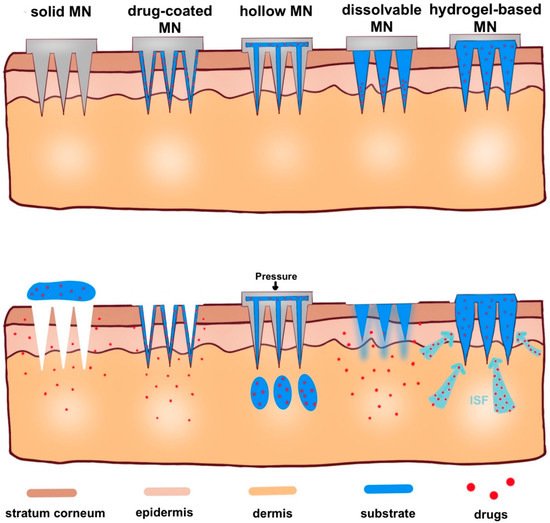
Figure 4. Schematic representation of five types of microneedle administration methods. Solid MNs are inserted into the skin and removed, leaving a channel through which the drug enters. Drug-coated MNs are the same as solid MN, except the drug is on the surface of the microneedles. For hollow MNs, after adding pressure, drug is released from the hollow microneedle. For dissolvable MN, when the microneedle substrate is dissolving, the drugs on the tip of the needle are released. When hydrogel-based MNs swell up from absorbing (ISF), the drug is released into the body.
2.1. Solid Microneedles
Solid microneedles are generally made of metal, silicon or polymer for pretreatment of fine incisions and improvement of drug permeability. Sabri et al. found that solid microneedles promoted the ex vivo transdermal absorption of imiquimod (one of the most effective drugs for the treatment of basal cell carcinoma), and the intradermal depot persisted for up to 24 h [33]. However, the application of solid microneedles requires two steps, and they may not be convenient for patients.
2.2. Drug-Coated Microneedles
Drug-coated microneedles can be used for delivering active molecules such as small molecules [34], proteins [35] and vaccines [36]. At the same time, drug-coated microneedles takes advantage of remaining active for a long time while the dose of the coated drug may be low [37]. Moreover, the drug amount loaded in the coated MNs system is limited. For instance, a maximum of 1 mg of drug can be coated on MNs, while much larger amounts of drugs (up to 33 mg) can be delivered by dissolvable MNs [38].
2.3. Dissolvable/Biodegradable Microneedles
Dissolvable/biodegradable microneedles dissolve completely upon insertion into the skin and have high biocompatibility—because safe polymers can be used as raw materials—and have high loading capacity [39]. When soluble microneedles were used on mouse ears, the length of microneedles within the skin rapidly decreased to 25% of the initial depth within the first 3 min and then slowly but constantly dissolved over the next 10 min (Figure 5A) [40]. This feature can be used for sustained drug delivery, but it also suggests that patients may need to wait for the microneedles to dissolve in the skin. Thus, Wang et al. designed a kind of microneedle which had bubble-structure microstructures in the body of the needle. The bubble structure promotes the concentration of the drug to the needle tip and enables the drug delivery efficiency to reach over 8% in 20 s in mice (Figure 5B) [41]. However, attention should be paid to whether the mechanical characteristics can be maintained in a humid environment [42].
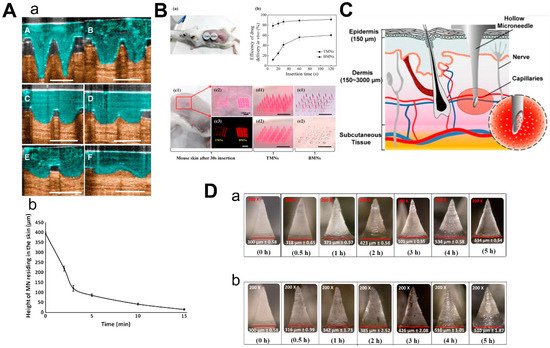
Figure 5. Characteristics of 3 types of microneedles. (A) Diagram (a) and dissolution curve (b) of dissolvable microneedles in mice. (B) Pharmacokinetics of TMN and BMN in mice with different inserting times (a,b) and the comparison of images before and after TMN and BMN are inserted into the skin (from 10 s to 120 s). TMN: traditional microneedle. BMN: bubble microneedle. (C) Schematic diagram of hollow microneedles for blood extraction. (D) Swelling images for hydrogel-based MNs loaded with α-arbutin in porcine skin (ex vivo) and in mice skin (in vivo) at different time points from 30 min to 5 h. (Images were reproduced with the permission from [40][41][43][44]).
2.4. Hollow Microneedles
Hollow microneedles can be used to inject solutions or suspensions to provide specific channels for therapies targeting specific tissues. Li et al. reported a method to optimize the manufacturing process of hollow microneedles to produce long and sharp microneedles that could reach the vessels for blood analysis (Figure 5C) [43]. Furthermore, hollow microneedles have a good command of the amount of drug and control of the time of the drug’s release, while fabrication was difficult and had risks linked to needle breakage and lumen blockage [45].
2.5. Hydrogel-Based Microneedles
The mechanism of hydrogel-based MN drug delivery is the same as dissolvable MN: insert into the skin, release the drugs and there is no need to discard the needle [31]. Due to the hydrophilic nature of the hydrogel, the hydrogel-based microneedles will absorb the interstitial fluid and swell when they are inserted into the skin [46]. Materials with biocompatibility and good swelling properties should be selected to make hydrogel-based microneedles [47]. Aung et al. investigated the swelling of the poly(vinyl-alcohol) (PVA) combined polyacrylic acid-co-maleic acid (PAMA) hydrogel-based microneedles at different times after they were inserted into the skin of mice and pigs, respectively (Figure 5D). Although the microneedles were slightly deformed, they still kept mechanical strength. Compared to dissolvable MN, hydrogel-based microneedles could load a larger amount of drugs [44].
References
- Donnelly, R.F.; Singh, T.R.R.; Woolfson, A.D. Microneedle-based drug delivery systems: Microfabrication, drug delivery, and safety. Drug Deliv. 2010, 17, 187–207.
- Jamaledin, R.; Yiu, C.K.Y.; Zare, E.N.; Niu, L.-N.; Vecchione, R.; Chen, G.; Gu, Z.; Tay, F.R.; Makvandi, P. Advances in Antimicrobial Microneedle Patches for Combating Infections. Adv. Mater. 2020, 32, 2002129.
- Zhu, M.; Liu, Y.; Jiang, F.; Cao, J.; Kundu, S.C.; Lu, S. Combined Silk Fibroin Microneedles for Insulin Delivery. ACS Biomater. Sci. Eng. 2020, 6, 3422–3429.
- Zhang, Y.; Jiang, G.; Yu, W.; Liu, D.; Xu, B. Microneedles fabricated from alginate and maltose for transdermal delivery of insulin on diabetic rats. Mater. Sci. Eng. C 2018, 85, 18–26.
- Caffarel-Salvador, E.; Kim, S.; Soares, V.; Tian, R.Y.; Stern, S.R.; Minahan, D.; Yona, R.; Lu, X.; Zakaria, F.R.; Collins, J.; et al. A microneedle platform for buccal macromolecule delivery. Sci. Adv. 2021, 7, eabe2620.
- Lee, K.; Goudie, M.J.; Tebon, P.; Sun, W.; Luo, Z.; Lee, J.; Zhang, S.; Fetah, K.; Kim, H.-J.; Xue, Y.; et al. Non-transdermal microneedles for advanced drug delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 41–59.
- Lee, K.J.; Park, S.H.; Lee, J.Y.; Joo, H.C.; Jang, E.H.; Youn, Y.-N.; Ryu, W. Perivascular biodegradable microneedle cuff for reduction of neointima formation after vascular injury. J. Control. Release 2014, 192, 174–181.
- Traverso, G.; Schoellhammer, C.M.; Schroeder, A.; Maa, R.; Lauwers, G.Y.; Polat, B.E.; Anderson, D.G.; Blankschtein, D.; Langer, R. Microneedles for Drug Delivery via the Gastrointestinal Tract. J. Pharm. Sci. 2015, 104, 362–367.
- Patel, S.R.; Lin, A.S.P.; Edelhauser, H.F.; Prausnitz, M.R. Suprachoroidal Drug Delivery to the Back of the Eye Using Hollow Microneedles. Pharm. Res. 2011, 28, 166–176.
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated Microneedles: A Novel Approach to Transdermal Drug Delivery. J. Pharm. Sci. 1998, 87, 922–925.
- Mikszta, J.A.; Alarcon, J.B.; Brittingham, J.M.; Sutter, D.E.; Pettis, R.J.; Harvey, N.G. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat. Med. 2002, 8, 415–419.
- Hashmi, S.; Ling, P.; Hashmi, G.; Reed, M.; Gaugler, R.; Trimmer, W. Genetic transformation of nematodes using arrays of micromechanical piercing structures. Biotechniques 1995, 19, 766–770.
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.-H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755.
- Miyano, T.; Tobinaga, Y.; Kanno, T.; Matsuzaki, Y.; Takeda, H.; Wakui, M.; Hanada, K. Sugar micro needles as transdermic drug delivery system. Biomed. Microdevices 2005, 7, 185–188.
- Fernandes, D. Minimally Invasive Percutaneous Collagen Induction. Oral Maxillofac. Surg. Clin. N. Am. 2005, 17, 51–63.
- Ma, B.; Liu, S.; Gan, Z.; Liu, G.; Cai, X.; Zhang, H.; Yang, Z. A PZT insulin pump integrated with a silicon microneedle array for transdermal drug delivery. Microfluid. Nanofluid. 2006, 2, 417–423.
- Sullivan, S.P.; Koutsonanos, D.G.; del Pilar Martin, M.; Lee, J.W.; Zarnitsyn, V.; Choi, S.-O.; Murthy, N.; Compans, R.W.; Skountzou, I.; Prausnitz, M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010, 16, 915–920.
- Boehm, R.D.; Miller, P.R.; Hayes, S.L.; Monteiro-Riviere, N.A.; Narayan, R.J. Modification of microneedles using inkjet printing. AIP Adv. 2011, 1, 22139.
- Kelchen, M.N.; Siefers, K.J.; Converse, C.C.; Farley, M.J.; Holdren, G.O.; Brogden, N.K. Micropore closure kinetics are delayed following microneedle insertion in elderly subjects. J. Control. Release 2016, 225, 294–300.
- Rouphael, N.G.; Paine, M.; Mosley, R.; Henry, S.; McAllister, D.V.; Kalluri, H.; Pewin, W.; Frew, P.M.; Yu, T.; Thornburg, N.J.; et al. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): A randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 2017, 390, 649–658.
- Chang, H.; Zheng, M.; Yu, X.; Than, A.; Seeni, R.Z.; Kang, R.; Tian, J.; Khanh, D.P.; Liu, L.; Chen, P.; et al. A Swellable Microneedle Patch to Rapidly Extract Skin Interstitial Fluid for Timely Metabolic Analysis. Adv. Mater. 2017, 29, 1702243.
- Yu, J.; Wang, J.; Zhang, Y.; Chen, G.; Mao, W.; Ye, Y.; Kahkoska, A.R.; Buse, J.B.; Langer, R.; Gu, Z. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat. Biomed. Eng. 2020, 4, 499–506.
- Ye, Y.; Yu, J.; Wang, C.; Nguyen, N.-Y.; Walker, G.M.; Buse, J.B.; Gu, Z. Microneedles Integrated with Pancreatic Cells and Synthetic Glucose-Signal Amplifiers for Smart Insulin Delivery. Adv. Mater. 2016, 28, 3115–3121.
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568.
- O’Mahony, C. Structural characterization and in-vivo reliability evaluation of silicon microneedles. Biomed. Microdevices 2014, 16, 333–343.
- Zhu, M.W.; Li, H.W.; Chen, X.L.; Tang, Y.F.; Lu, M.H.; Chen, Y.F. Silica needle template fabrication of metal hollow microneedle arrays. J. Micromech. Microeng. 2009, 19, 115010.
- Yang, S.-J.; Jeong, J.-O.; Lim, Y.-M.; Park, J.-S. Synthesis and characterization of PVP microneedle patch using metal bioelectrodes for novel drug delivery system. Mater. Des. 2021, 201, 109485.
- Balmert, S.C.; Carey, C.D.; Falo, G.D.; Sethi, S.K.; Erdos, G.; Korkmaz, E.; Falo, L.D., Jr. Dissolving undercut microneedle arrays for multicomponent cutaneous vaccination. J. Control. Release 2020, 317, 336–346.
- Bystrova, S.N.; Luttge, R. Micromolding for ceramic microneedle arrays. Microelectron. Eng. 2011, 88, 1681–1684.
- Lim, J.; Tahk, D.; Yu, J.; Min, D.-H.; Jeon, N.L. Design rules for a tunable merged-tip microneedle. Microsyst. Nanoeng. 2018, 4, 29.
- Ahmed Saeed Al-Japairai, K.; Mahmood, S.; Hamed Almurisi, S.; Reddy Venugopal, J.; Rebhi Hilles, A.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673.
- McAlister, E.; Kirkby, M.; Domínguez-Robles, J.; Paredes, A.J.; Anjani, Q.K.; Moffatt, K.; Vora, L.K.; Hutton, A.R.J.; McKenna, P.E.; Larrañeta, E.; et al. The role of microneedle arrays in drug delivery and patient monitoring to prevent diabetes induced fibrosis. Adv. Drug Deliv. Rev. 2021, 175, 113825.
- Sabri, A.; Ogilvie, J.; McKenna, J.; Segal, J.; Scurr, D.; Marlow, M. Intradermal Delivery of an Immunomodulator for Basal Cell Carcinoma; Expanding the Mechanistic Insight into Solid Microneedle-Enhanced Delivery of Hydrophobic Molecules. Mol. Pharm. 2020, 17, 2925–2937.
- Baek, S.-H.; Shin, J.-H.; Kim, Y.-C. Drug-coated microneedles for rapid and painless local anesthesia. Biomed. Microdevices 2017, 19, 2.
- Caudill, C.L.; Perry, J.L.; Tian, S.; Luft, J.C.; DeSimone, J.M. Spatially controlled coating of continuous liquid interface production microneedles for transdermal protein delivery. J. Control. Release 2018, 284, 122–132.
- Shakya, A.K.; Lee, C.H.; Gill, H.S. Cutaneous vaccination with coated microneedles prevents development of airway allergy. J. Control. Release 2017, 265, 75–82.
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237.
- Pere, C.P.P.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432.
- Zhu, D.D.; Zhang, X.P.; Zhang, B.L.; Hao, Y.Y.; Guo, X.D. Safety Assessment of Microneedle Technology for Transdermal Drug Delivery: A Review. Adv. Ther. 2020, 3, 2000033.
- Pattani, A.; McKay, P.F.; Garland, M.J.; Curran, R.M.; Migalska, K.; Cassidy, C.M.; Malcolm, R.K.; Shattock, R.J.; McCarthy, H.O.; Donnelly, R.F. Microneedle mediated intradermal delivery of adjuvanted recombinant HIV-1 CN54gp140 effectively primes mucosal boost inoculations. J. Control. Release 2012, 162, 529–537.
- Wang, Q.L.; Zhu, D.D.; Liu, X.B.; Chen, B.Z.; Guo, X.D. Microneedles with Controlled Bubble Sizes and Drug Distributions for Efficient Transdermal Drug Delivery. Sci. Rep. 2016, 6, 38755.
- Wang, Q.L.; Ren, J.W.; Chen, B.Z.; Jin, X.; Zhang, C.Y.; Guo, X.D. Effect of humidity on mechanical properties of dissolving microneedles for transdermal drug delivery. J. Ind. Eng. Chem. 2018, 59, 251–258.
- Li, C.G.; Lee, C.Y.; Lee, K.; Jung, H. An optimized hollow microneedle for minimally invasive blood extraction. Biomed. Microdevices 2013, 15, 17–25.
- Jina, A.; Tierney, M.J.; Tamada, J.A.; McGill, S.; Desai, S.; Chua, B.; Chang, A.; Christiansen, M. Design, Development, and Evaluation of a Novel Microneedle Array-based Continuous Glucose Monitor. J. Diabetes Sci. Technol. 2014, 8, 483–487.
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258.
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-Forming Microneedles: Current Advancements and Future Trends. Macromol. Biosci. 2021, 21, 2000307.
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Pamornpathomkul, B.; Opanasopit, P. Fabrication, characterization and comparison of α-arbutin loaded dissolving and hydrogel forming microneedles. Int. J. Pharm. 2020, 586, 119508.

