Lung cancer has remained one of the major causes of death worldwide. Thus, a more effective treatment approach is essential such as the inhibition of specific cancer-promoting molecules. Aurora kinases regulate the process of mitosis - a process of cell division that is necessary for normal cell proliferation. However, dysfunction of these kinases can contribute to cancer formation. The aberrant expression of Aurora kinases in lung cancer points to their crucial role in lung carcinogenesis. Therefore, understanding the fundamental functions as well as the cellular localization of Aurora kinases may contribute to evaluate their precise oncogenic function that will may lead to better specific targeting of these cancer-related molecules.
- Aurora kinases (AURK)
- AURKA
- AURKB
- AURKC
- lung cancer
- mitosis
- lung cancer therap
1. Background
Lung cancer remains the most commonly occurring cancer attributed to the leading cause of cancer-related deaths globally. Non-small cell lung cancer (NSCLC) comprises 85% to 90% of lung cancers while small cell lung cancer (SCLC) represents 10–15% [1][2][3]. Survival varies according to the clinical stage ranging from 92% for earliest stage to 0% for advanced stage, respectively [4]. While surgery is the treatment of choice for early stages NSCLC, chemo-radiotherapy has been the therapy of choice for the advanced stages for many years [5]. Nowadays, in advanced non-small cell lung cancer, molecular targeted therapy is the standard first-line treatment for patients with identified driver mutations; on the other hand, chemotherapy (platinum-based) is the standard treatment for patients without driver mutation or those with unknown mutation status [6][7][8][9]. Moreover, immunotherapy has been now established as one of the most promising therapeutic options [10]. At this time, chemo- and radiotherapies are no longer the best option for this disease [11] so that further investigation of the molecular processes surrounding the oncogenesis of this malignancy warrants the development of more effective pharmacological agents.
The normal growth and development of a cell strictly depends on its ability to divide regularly during the various stages of cell division. This process is regulated by several mitotic kinases (serine-threonine kinases) which include polo-like kinases, cyclin dependent kinase 1 (CDK1), large tumor suppressor 1 (LATS1)-related kinases, never in mitosis (NIMA) -related kinases, and Aurora kinases execute critical roles in different stages of cell division [12].
The Aurora kinases consisting of three members termed Aurora-A (AURKA), -B (AURKB), and –C (AURKC) are complementary enzymes that modulate multiple mitotic events. These mitotic kinases are expressed in a cell cycle-dependent manner, most of which are activated in the G2/M phase being involved in mitotic chromosomal segregation [13][14]. These kinases bind to protein partners to be locally functional, that are tightly regulated in a temporal and spatial manner. AURKA and AURKB are the only ones of the Aurora kinase family to be expressed at detectable levels in somatic cells during mitotic processes and their function in cellular processes in the development of tumorigenic phenotypes has been extensively studied [15].
In NCSLC, overexpression of AURKA and AURKB has been associated with poor prognosis due to reduced overall survival [16][17][18][19][20][21][22][23][24] and both kinases have been found to either potentiate or repress chemo- and/or radiotherapy in lung cancer [1][25][26][27]. Some antimitotics that control microtubule dynamics such as vinca alkaloids, taxanes, and antineoplastic drugs targeting the regulatory mechanisms of mitosis [28] have shown effectivity in reducing the pro-oncogenic properties of Aurora kinases.
2. Cellular Localization and Functions of Aurora Kinases
Aurora kinases have different subcellular distribution during the process of mitosis (Figure 1). AURKA and AURKB are constitutively present in mitotically active cells and are upregulated in highly proliferative tissues whereas the presence of AURKC is restricted to germ cells of both genders during meiosis [29]. The mitotic roles of the three Aurora kinases are thought to be dependent on their expression, temporal restriction, and localization, rather than on their kinase activity. Beside their localization, their functional differences are principally determined through binding with molecular partners and/or substrates [30].
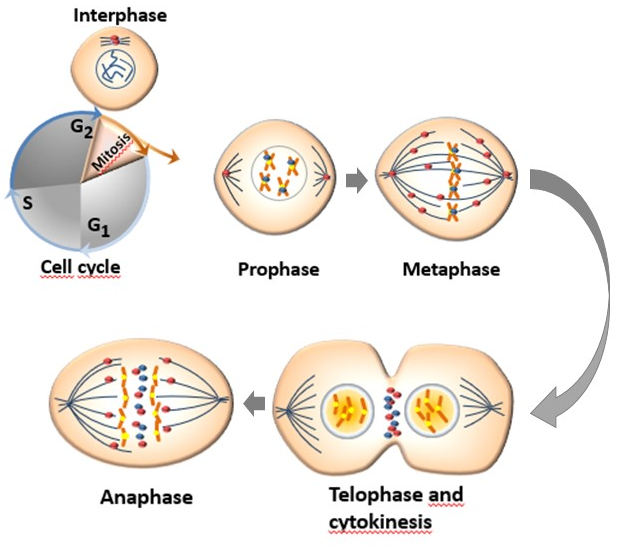
The human AURKA is encoded by the AURKA gene located on chromosome 20q13.2 and is the most studied of Aurora kinase family. The kinase activity of AURKA and the levels of proteins linked to this kinase increase from G2 phase through the mitosis phase, reaching an activity peak during pro-metaphase. Its activation in the late G2 phase is required to trigger the initial activation of cyclin B1-CDK 1 at the centrosome and provide entry into mitosis [34]. Thr288 phosphorylation determines the activation of the kinase activity of AURKA while its deactivation is determined by the dephosphorylation of Thr288 by protein phosphatase 1 (PP1). The activity of AURKA is determined by a protein associated with microtubules targeting protein for xenopus kinesin-like protein (TPX2), which triggers its autophosphorylation and protects it from the inhibitory property of PP1 [12][35]. The organization of the anaphase spindle at the end of mitosis is determined by the presence of AURKA, whose abundance is downregulated by the proteolysis process mediated by the APC/C-Cdh1-dependent proteasome [13].
Aurora-B is a protein encoded by the AURKB gene found on chromosome 17p13.1 [36]. AURKB guarantees a correct kinetochore–microtubule attachment thanks to the collaboration of other substrates such as survivin, borealin, and the internal centromere protein (INCEP). In fact, AURKB phosphorylates INCEP directly, increasing its kinase activity in vitro [37] In mitotic cells, AURKB is localized to the kinetochore of prometaphase chromosome, contributing to their proper alignment at metaphase, and to the spindle midzone of anaphase cells and execute important actions for the completion of cytokinesis reviewed in [38]. Aurora-B can cause defects in chromosome separation and cytokinesis, implying its pivotal role in cell division [31]. At the end of mitosis, both AURKA and AURKB are subjected to ubiquitination and proteasome degradation after dephosphorylation by PP2A or PP1 [39].
The Aurora-C is encoded by the AURKC gene localized at chromosome 19q13.43 (Gene ID: 6795). AURKB and AURKC are thought to have similar distribution pattern sequences, substrates such as INCENP [40], surviving, and borealin, and functions during cell division [33]. The only detectable difference is the localization of AURKC in interphase germ cells that involves the centrosomes rather than the nuclei [41].
This entry is adapted from the peer-reviewed paper 10.3390/cancers12113371
References
- Bertran-Alamillo, J.; Cattan, V.; Schoumacher, M.; Codony-Servat, J.; Giménez-Capitán, A.; Cantero, F.; Burbridge, M.; Rodríguez, S.; Teixidó, C.; Roman, R.; et al. AURKB as a target in non-small cell lung cancer with acquired resistance to anti-EGFR therapy. Nat. Commun. 2019, 10, 1–14.
- Mascaux, C.; Tomasini, P.; Greillier, L.; Barlesi, F. Personalised medicine for nonsmall cell lung cancer. Eur. Respir. Rev. 2017, 26.
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300.
- Neal, R.D.; Sun, F.; Emery, J.D.; Callister, M.E. Lung cancer. BMJ 2019, 365.
- Ohe, Y. Chemoradiotherapy for lung cancer: Current status and perspectives. Int. J. Clin. Oncol. 2004, 9, 435–443.
- Zhang, C.; Leighl, N.B.; Wu, Y.-L.; Zhong, W.-Z. Emerging therapies for non-small cell lung cancer. J. Hematol. Oncol. 2019, 12, 45.
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311.
- Ramalingam, S.; Belani, C. Systemic Chemotherapy for Advanced Non-Small Cell Lung Cancer: Recent Advances and Future Directions. Oncologist 2008, 13, 5–13.
- Gridelli, C.; de Marinis, F.; Cappuzzo, F.; Maio, M.D.; Hirsch, F.R.; Mok, T.; Morgillo, F.; Rosell, R.; Spigel, D.R.; Yang, J.C.-H.; et al. Treatment of Advanced Non–Small-Cell Lung Cancer With Epidermal Growth Factor Receptor (EGFR) Mutation or ALK Gene Rearrangement: Results of an International Expert Panel Meeting of the Italian Association of Thoracic Oncology. Clin. Lung Cancer 2014, 15, 173–181.
- Remon, J.; Passiglia, F.; Ahn, M.-J.; Barlesi, F.; Forde, P.M.; Garon, E.B.; Gettinger, S.; Goldberg, S.B.; Herbst, R.S.; Horn, L.; et al. Immune Checkpoint Inhibitors in Thoracic Malignancies: Review of the Existing Evidence by an IASLC Expert Panel and Recommendations. J. Thorac. Oncol. 2020, 15, 914–947.
- Yuan, M.; Huang, L.-L.; Chen, J.-H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal. Transduct. Target. Ther. 2019, 4, 1–14.
- Dar, A.A.; Goff, L.W.; Majid, S.; Berlin, J.; El-Rifai, W. Aurora Kinase Inhibitors-Rising Stars in Cancer Therapeutics? Mol. Cancer Ther. 2010, 9, 268–278.
- D’Assoro, A.B.; Haddad, T.; Galanis, E. Aurora-A Kinase as a Promising Therapeutic Target in Cancer. Front. Oncol. 2016, 5.
- Lo Iacono, M.; Monica, V.; Saviozzi, S.; Ceppi, P.; Bracco, E.; Papotti, M.; Scagliotti, G.V. Aurora Kinase A expression is associated with lung cancer histological-subtypes and with tumor de-differentiation. J. Transl. Med. 2011, 9, 100.
- Katayama, H.; Sen, S. Aurora kinase inhibitors as anticancer molecules. Biochim. Biophys. Acta BBA Gene Regul. Mech. 2010, 1799, 829–839.
- Liu, N.; Wang, Y.A.; Sun, Y.; Ecsedy, J.; Sun, J.; Li, X.; Wang, P. Inhibition of Aurora A enhances radiosensitivity in selected lung cancer cell lines. Respir. Res. 2019, 20, 230.
- Zhang, M.-Y.; Liu, X.-X.; Li, H.; Li, R.; Liu, X.; Qu, Y.-Q. Elevated mRNA Levels of AURKA, CDC20 and TPX2 are associated with poor prognosis of smoking related lung adenocarcinoma using bioinformatics analysis. Int. J. Med. Sci. 2018, 15, 1676–1685.
- Al-Khafaji, A.S.K.; Marcus, M.W.; Davies, M.P.A.; Risk, J.M.; Shaw, R.J.; Field, J.K.; Liloglou, T. AURKA mRNA expression is an independent predictor of poor prognosis in patients with non-small cell lung cancer. Oncol. Lett. 2017, 13, 4463–4468.
- Schneider, M.A.; Christopoulos, P.; Muley, T.; Warth, A.; Klingmueller, U.; Thomas, M.; Herth, F.J.F.; Dienemann, H.; Mueller, N.S.; Theis, F.; et al. AURKA, DLGAP5, TPX2, KIF11 and CKAP5: Five specific mitosis-associated genes correlate with poor prognosis for non-small cell lung cancer patients. Int. J. Oncol. 2017, 50, 365–372.
- Li, X.; Liu, N.; Ren, P.; Cao, Q.; Wang, P.; Zhao, L.; Lu, B.; Wang, P. Prognostic value of combined expression of Aurora A, p53 and p21 WAF1 in patients after curative resection of non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2015, 37, 512–516.
- Xu, J.; Yue, C.; Zhou, W.; Qian, Y.; Zhang, Y.; Wang, S.; Liu, A.; Liu, Q. Aurora-A contributes to cisplatin resistance and lymphatic metastasis in non-small cell lung cancer and predicts poor prognosis. J. Transl. Med. 2014, 12, 200.
- Takeshita, M.; Koga, T.; Takayama, K.; Ijichi, K.; Yano, T.; Maehara, Y.; Nakanishi, Y.; Sueishi, K. Aurora-B overexpression is correlated with aneuploidy and poor prognosis in non-small cell lung cancer. Lung Cancer Amst. Neth. 2013, 80, 85–90.
- Hayama, S.; Daigo, Y.; Yamabuki, T.; Hirata, D.; Kato, T.; Miyamoto, M.; Ito, T.; Tsuchiya, E.; Kondo, S.; Nakamura, Y. Phosphorylation and activation of cell division cycle associated 8 by aurora kinase B plays a significant role in human lung carcinogenesis. Cancer Res. 2007, 67, 4113–4122.
- Vischioni, B.; Oudejans, J.J.; Vos, W.; Rodriguez, J.A.; Giaccone, G. Frequent overexpression of aurora B kinase, a novel drug target, in non-small cell lung carcinoma patients. Mol. Cancer Ther. 2006, 5, 2905–2913.
- Shah, K.N.; Bhatt, R.; Rotow, J.; Rohrberg, J.; Olivas, V.; Wang, V.E.; Hemmati, G.; Martins, M.M.; Maynard, A.; Kuhn, J.; et al. Aurora kinase A drives the evolution of resistance to third-generation EGFR inhibitors in lung cancer. Nat. Med. 2019, 25, 111–118.
- Wu, C.C.; Yu, C.-T.R.; Chang, G.-C.; Lai, J.-M.; Hsu, S.-L. Aurora-A promotes gefitinib resistance via a NF-κB signaling pathway in p53 knockdown lung cancer cells. Biochem. Biophys. Res. Commun. 2011, 405, 168–172.
- Orth, M.; Unger, K.; Schoetz, U.; Belka, C.; Lauber, K. Taxane-mediated radiosensitization derives from chromosomal missegregation on tripolar mitotic spindles orchestrated by AURKA and TPX2. Oncogene 2018, 37, 52–62.
- Lapenna, S.; Giordano, A. Cell cycle kinases as therapeutic targets for cancer. Nat. Rev. Drug Discov. 2009, 8, 547–566.
- Tang, A.; Gao, K.; Chu, L.; Zhang, R.; Yang, J.; Zheng, J. Aurora kinases: Novel therapy targets in cancers. Oncotarget 2017, 8, 23937–23954.
- Li, S.; Deng, Z.; Fu, J.; Xu, C.; Xin, G.; Wu, Z.; Luo, J.; Wang, G.; Zhang, S.; Zhang, B.; et al. Spatial Compartmentalization Specializes the Function of Aurora A and Aurora B. J. Biol. Chem. 2015, 290, 17546–17558.
- Ke, Y.W.; Dou, Z.; Zhang, J.; Yao, X.B. Function and regulation of Aurora/Ipl1p kinase family in cell division. Cell Res. 2003, 13, 69–81.
- Giet, R.; Prigent, C. Aurora/Ipl1p-related kinases, a new oncogenic family of mitotic serine-threonine kinases. J. Cell Sci. 1999, 112 Pt 21, 3591–3601.
- Quartuccio, S.M.; Schindler, K. Functions of Aurora kinase C in meiosis and cancer. Front. Cell Dev. Biol. 2015, 3.
- Hirota, T.; Kunitoku, N.; Sasayama, T.; Marumoto, T.; Zhang, D.; Nitta, M.; Hatakeyama, K.; Saya, H. Aurora-A and an Interacting Activator, the LIM Protein Ajuba, Are Required for Mitotic Commitment in Human Cells. Cell 2003, 114, 585–598.
- Eyers, P.A.; Erikson, E.; Chen, L.G.; Maller, J.L. A novel mechanism for activation of the protein kinase Aurora A. Curr. Biol. CB 2003, 13, 691–697.
- Baldini, E.; Tuccilli, C.; Sorrenti, S.; Mascagni, D.; Arcieri, S.; Filippini, A.; Ulisse, S. Aurora Kinases: New Molecular Targets for the Therapy of Aggressive Thyroid Cancers. Anti Cancer Drugs Nat. Synth. Cell 2016.
- Bishop, J.D.; Schumacher, J.M. Phosphorylation of the Carboxyl Terminus of Inner Centromere Protein (INCENP) by the Aurora B Kinase Stimulates Aurora B Kinase Activity. J. Biol. Chem. 2002, 277, 27577–27580.
- Carmena, M.; Earnshaw, W.C. The cellular geography of Aurora kinases. Nat. Rev. Mol. Cell Biol. 2003, 4, 842–854.
- Willems, E.; Dedobbeleer, M.; Digregorio, M.; Lombard, A.; Lumapat, P.N.; Rogister, B. The functional diversity of Aurora kinases: A comprehensive review. Cell Div. 2018, 13.
- Abdul Azeez, K.R.; Chatterjee, S.; Yu, C.; Golub, T.R.; Sobott, F.; Elkins, J.M. Structural mechanism of synergistic activation of Aurora kinase B/C by phosphorylated INCENP. Nat. Commun. 2019, 10, 1–11.
- Fernández-Miranda, G.; Trakala, M.; Martín, J.; Escobar, B.; González, A.; Ghyselinck, N.B.; Ortega, S.; Cañamero, M.; Castro, I.P.d.; Malumbres, M. Genetic disruption of aurora B uncovers an essential role for aurora C during early mammalian development. Development 2011, 138, 2661–2672.
 Encyclopedia
Encyclopedia



