Gastrointestinal mucositis (GI-M) is a frequently observed side effect of chemotherapy in patients with cancer that affects the gastrointestinal microenvironment and potentially drug absorption.
- cancer
- chemotherapy
- gastrointestinal mucositis
- antibiotics
- gut microbiota
- drug pharmacokinetics
1. Introduction
|
Class of Agent |
Chemotherapeutic Agent |
Mechanism of Action |
Gastrointestinal Toxicity |
Intestinal Permeability |
|---|---|---|---|---|
|
Alkylating agents [14] |
Busulfan [21] Cyclophosphamide [22] Cisplatin [23] Melphalan [24] |
Cross-link between DNA/RNA strands |
Mucosal ulceration ↑* Cell loss ↓# Villus height |
Barrier disruption Bacterial translocation ↑ Permeability of the intestine (rats) [26] |
|
Antimetabolites |
Methotrexate [29] Gemcitabine [30] |
5-FU conversion to fluorouridine monophosphate (FUMP) Competitive inhibition of dihydrofolate reductase via displacement of dihydrofolate Incorporation of pyramidine analog into DNA |
↑Inflammation ↑Crypt apoptosis ↑Villus atrophy |
↑ Ratio of crypt cells to villous enterocytes ↑ Intestinal permeability (associated with reduced Zonula Occludens-1 expression in rats) [34] |
|
Topoisomerase I inhibitor |
Irinotecan hydrochloride [37] |
Inhibition of the DNA enzyme topoisomerase I |
↑ Villus atrophy Crypt ablation Goblet cell metaplasia ↑Inflammation [19] |
↓ Intestinal barrier function |
|
FEC |
Fluorouracil, Epirubicin, and Cyclophosphamide |
5-FU conversion to fluorouridine monophosphate (FUMP) + cross-link between DNA/RNA strands |
↑ Paracellular permeability ↓ Intestinal barrier function [40] |
↓ Glucagon-like peptide-2 circulating concentrations Mucosal ulcerations |
|
FOLFOX |
5-FU, leucovorin, and oxaliplatin |
5-FU conversion to fluorouridine monophosphate (FUMP) + inhibits the synthesis of deoxyribonucleic acid (DNA) |
↑Inflammation ↑Crypt apoptosis ↑Villus atrophy |
↑ Ratio of crypt cells to villous enterocytes ↑ Permeability of the intestine (rats) [34] ↑ Intestinal permeability (associated with reduced Zonula Occludens-1 expression in rats) |
2. Physiological Factors Contributing to Impaired Intestinal Absorption during Chemotherapy
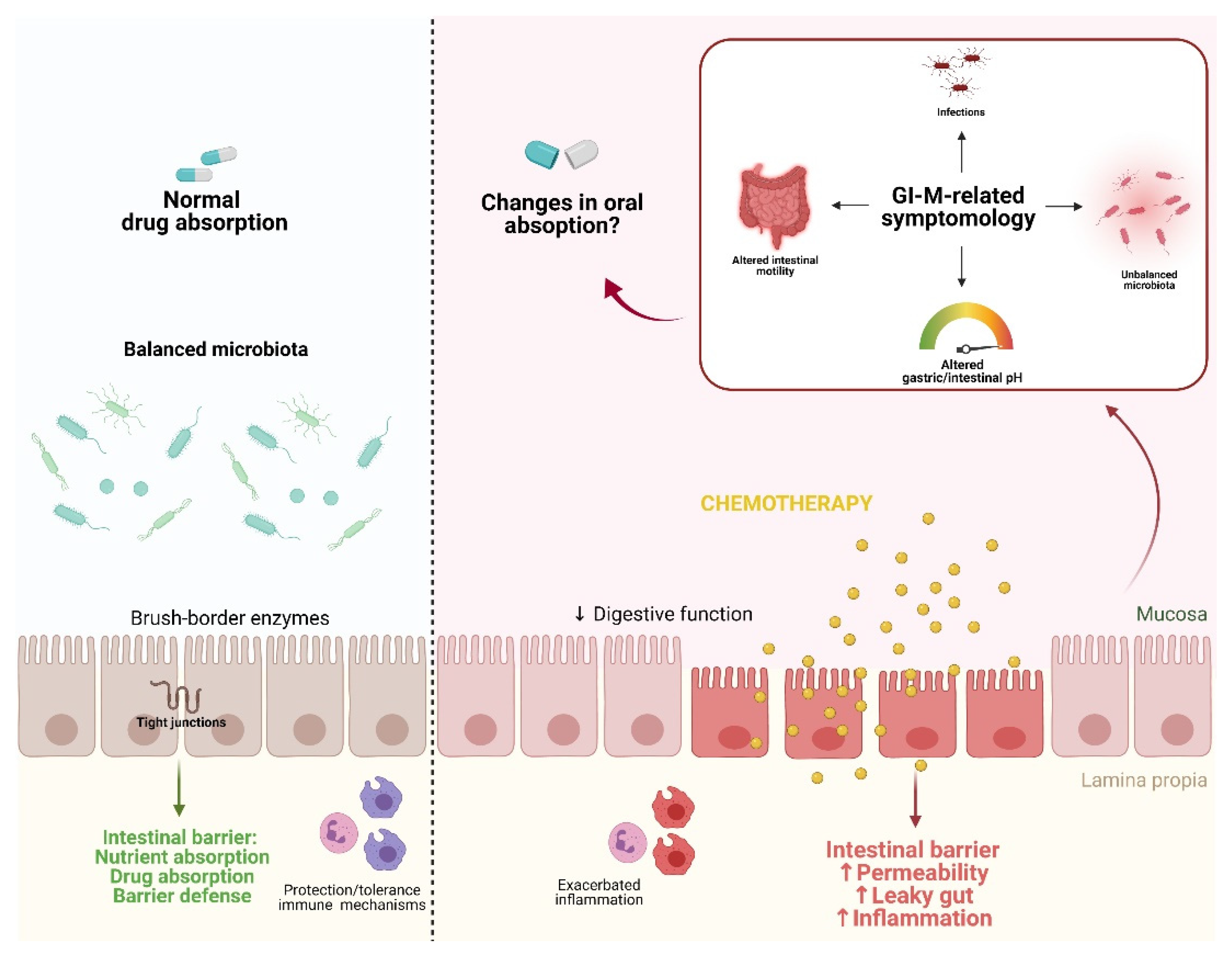
3. The Effects of Gastrointestinal Mucositis on Drug Absorption
References
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer. 2004, 4, 277–284.
- Wardill, H.R.; Van Sebille, Y.Z.A.; Ciorba, M.A.; Bowen, J.M. Prophylactic probiotics for cancer therapy-induced diarrhoea: A meta-analysis. Curr. Opin. Support. Palliat. Care 2018, 12, 187–197.
- Welch, H.G.; Schwartz, L.M.; Woloshin, S. Are increasing 5-year survival rates evidence of success against cancer? J. Am. Med. Assoc. 2000, 283, 2975–2978.
- Arnold, M.; Rutherford, M.J.; Bardot, A.; Ferlay, J.; Andersson, T.M.L.; Myklebust, T.Å.; Tervonen, H.; Thursfield, V.; Ransom, D.; Shack, L.; et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): A population-based study. Lancet Oncol. 2019, 20, 1493–1505.
- Piñana, J.L.; Montesinos, P.; Martino, R.; Vazquez, L.; Rovira, M.; López, J.; Batlle, M.; Figuera, Á.; Barba, P.; Lahuerta, J.J.; et al. Incidence, risk factors, and outcome of bacteremia following autologous hematopoietic stem cell transplantation in 720 adult patients. Ann. Hematol. 2014, 93, 299–307.
- Taur, Y.; Xavier, J.B.; Lipuma, L.; Ubeda, C.; Goldberg, J.; Gobourne, A.; Lee, Y.J.; Dubin, K.A.; Socci, N.D.; Viale, A.; et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. 2012, 55, 905–914.
- Sonis, S.T. Pathobiology of mucositis. Semin. Oncol. Nurs. 2004, 20, 11–15.
- Cario, E. Toll-like receptors in the pathogenesis of chemotherapy-induced gastrointestinal toxicity. Curr. Opin. Support. Palliat. Care 2016, 10, 157–164.
- Gafter-Gvili, A.; Fraser, A.; Paul, M.; Leibovici, L. Meta-analysis: Antibiotic prophylaxis reduces mortality in neutropenic patients. Ann. Intern. Med. 2005, 142, 979–995.
- Dahlgren, D.; Lennernäs, H. Intestinal permeability and drug absorption: Predictive experimental, computational and in vivo approaches. Pharmaceutics 2019, 11, 411.
- Basile, D.; Di Nardo, P.; Corvaja, C.; Garattini, S.K.; Pelizzari, G.; Lisanti, C.; Bortot, L.; Da Ros, L.; Bartoletti, M.; Borghi, M.; et al. Mucosal injury during anti-cancer treatment: From pathobiology to bedside. Cancers 2019, 11, 857.
- Elting, L.S.; Cooksley, C.; Chambers, M.; Cantor, S.B.; Manzullo, E.; Rubenstein, E.B. The burdens of cancer therapy: Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 2003, 98, 1531–1539.
- Sougiannis, A.T.; VanderVeen, B.N.; Davis, J.M.; Fan, D.; Murphy, E.A. Understanding chemotherapy-induced intestinal mucositis and strategies to improve gut resilience. Am. J. Physiol. Liver Physiol. 2021, 320, G712–G719.
- Naidu, M.U.R.; Ramana, G.V.; Rani, P.U.; Mohan, I.K.; Suman, A.; Roy, P. Chemotherapy-induced and/or radiation therapy-induced oral mucositis--complicating the treatment of cancer. Neoplasia 2004, 6, 423–431.
- Wadleigh, R.G.; Redman, R.S.; Graham, M.L.; Krasnow, S.H.; Anderson, A.; Cohen, M.H. Vitamin E in the treatment of chemotherapy-induced mucositis. Am. J. Med. 1992, 92, 481–484.
- Touchefeu, Y.; Montassier, E.; Nieman, K.; Gastinne, T.; Potel, G.; Bruley Des Varannes, S.; Le Vacon, F.; De La Cochetière, M.F. Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—Current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 2014, 40, 409–421.
- Sonis, S.; Elting, L.; Keefe, D.; Peterson, D.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.; Raber-Durlacher, J.; Donnelly, J.; Rubenstein, E. Perspectives on cancer therapy-induced mucosal injury. Cancer 2004, 100, 1995–2025.
- Carlotto, A.; Hogsett, V.L.; Maiorini, E.M.; Razulis, J.G.; Sonis, S.T. The economic burden of toxicities associated with cancer treatment: Review of the literature and analysis of nausea and vomiting, diarrhoea, oral mucositis and fatigue. Pharmacoeconomics 2013, 31, 753–766.
- Bowen, J.; Al-Dasooqi, N.; Bossi, P.; Wardill, H.; Van Sebille, Y.; Al-Azri, A.; Bateman, E.; Correa, M.E.; Raber-Durlacher, J.; Kandwal, A.; et al. The pathogenesis of mucositis: Updated perspectives and emerging targets. Support. Care Cancer 2019, 27, 4023–4033.
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New frontiers in the pathobiology and treatment of cancer regimen-related mucosal injury. Front. Pharmacol. 2017, 8, 354.
- Galaup, A.; Paci, A. Pharmacology of dimethanesulfonate alkylating agents: Busulfan and treosulfan. Expert Opin. Drug Metab. Toxicol. 2013, 9, 333–347.
- Voelcker, G. The Mechanism of Action of Cyclophosphamide and Its Consequences for the Development of a New Generation of Oxazaphosphorine Cytostatics. Sci. Pharm. 2020, 88, 42.
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378.
- Ehrsson, H.; Eksborg, S.; Österborg, A.; Mellstedt, H.; Lindfors, A. Oral melphalan pharmacokinetics—Relation to dose in patients with multiple myeloma. Med. Oncol. Tumor Pharmacother. 1989, 6, 151–154.
- Ecknauer, R.; Löhrs, U. The effect of a single dose of cyclophosphamide on the jejunum of specified pathogenfree and germfree rats. Digestion 1976, 14, 269–280.
- Wardill, H.R.; de Mooij, C.E.M.; da Silva Ferreira, A.R.; van de Peppel, I.P.; Havinga, R.; Harmsen, H.J.M.; Tissing, W.J.E.; Blijlevens, N.M.A. Translational model of melphalan-induced gut toxicity reveals drug-host-microbe interactions that drive tissue injury and fever. Cancer Chemother. Pharmacol. 2021, 88, 173–188.
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338.
- Chang, C.T.; Ho, T.Y.; Lin, H.; Liang, J.A.; Huang, H.C.; Li, C.C.; Lo, H.Y.; Wu, S.L.; Huang, Y.F.; Hsiang, C.Y. 5-fluorouracil induced intestinal mucositis via nuclear factor-??B activation by transcriptomic analysis and in vivo bioluminescence imaging. PLoS ONE 2012, 7, e31808.
- Cronstein, B.N.; Aune, T.M. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat. Rev. Rheumatol. 2020, 16, 145–154.
- De Sousa Cavalcante, L.; Monteiro, G. Gemcitabine: Metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur. J. Pharmacol. 2014, 741, 8–16.
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M.K. Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered? Cancer Chemother. Pharmacol. 2009, 63, 239–251.
- Chang, C.-W.; Lee, H.-C.; Li, L.-H.; Chiang Chiau, J.-S.; Wang, T.-E.; Chuang, W.-H.; Chen, M.-J.; Wang, H.-Y.; Shih, S.-C.; Liu, C.-Y.; et al. Fecal Microbiota Transplantation Prevents Intestinal Injury, Upregulation of Toll-Like Receptors, and 5-Fluorouracil/Oxaliplatin-Induced Toxicity in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 386.
- Vanhoecke, B.; Bateman, E.; Mayo, B.; Vanlancker, E.; Stringer, A.; Thorpe, D.; Keefe, D. Dark Agouti rat model of chemotherapy-induced mucositis: Establishment and current state of the art. Exp. Biol. Med. 2015, 240, 725–741.
- Daniele, B.; Secondulfo, M.; De Vivo, R.; Pignata, S.; De Magistris, L.; Delrio, P.; Palaia, R.; Barletta, E.; Tambaro, R.; Carratù, R. Effect of Chemotherapy with 5-Fluorouracil on Intestinal Permeability and Absorption in Patients with Advanced Colorectal Cancer. J. Clin. Gastroenterol. 2001, 32, 228–230.
- Fijlstra, M.; Ferdous, M.; Koning, A.M.; Rings, E.H.H.M.; Harmsen, H.J.M.; Tissing, W.J.E. Substantial decreases in the number and diversity of microbiota during chemotherapy-induced gastrointestinal mucositis in a rat model. Support. Care Cancer 2015, 23, 1513–1522.
- Hamada, K.; Shitara, Y.; Sekine, S.; Horie, T. Zonula Occludens-1 alterations and enhanced intestinal permeability in methotrexate-treated rats. Cancer Chemother. Pharmacol. 2010, 66, 1031–1038.
- Fujita, K.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248.
- Wardill, H.R.; Bowen, J.M.; Van Sebille, Y.Z.A.; Secombe, K.R.; Coller, J.K.; Ball, I.A.; Logan, R.M.; Gibson, R.J. TLR4-dependent claudin-1 internalization and secretagogue-mediated chloride secretion regulate irinotecan-induced diarrhea. Mol. Cancer Ther. 2016, 15, 2767–2779.
- Stringer, A.M.; Gibson, R.J.; Bowen, J.M.; Logan, R.M.; Ashton, K.; Yeoh, A.S.J.; Al-Dasooqi, N.; Keefe, D.M.K. Irinotecan-induced mucositis manifesting as diarrhoea corresponds with an amended intestinal flora and mucin profile. Int. J. Exp. Pathol. 2009, 90, 489–499.
- Russo, F.; Linsalata, M.; Clemente, C.; D’Attoma, B.; Orlando, A.; Campanella, G.; Giotta, F.; Riezzo, G. The effects of fluorouracil, epirubicin, and cyclophosphamide (FEC60) on the intestinal barrier function and gut peptides in breast cancer patients: An observational study. BMC Cancer 2013, 13, 56.
- Secombe, K.R.; Coller, J.K.; Gibson, R.J.; Wardill, H.R.; Bowen, J.M. The bidirectional interaction of the gut microbiome and the innate immune system: Implications for chemotherapy-induced gastrointestinal toxicity. Int. J. Cancer 2019, 144, 2365–2376.
- Al-Dasooqi, N.; Sonis, S.T.; Bowen, J.M.; Bateman, E.; Blijlevens, N.; Gibson, R.J.; Logan, R.M.; Nair, R.G.; Stringer, A.M.; Yazbeck, R.; et al. Emerging evidence on the pathobiology of mucositis. Support. Care Cancer 2013, 21, 2075–2083.
- Villa, A.; Sonis, S.T. Mucositis: Pathobiology and management. Curr. Opin. Oncol. 2015, 27, 159–164.
- Nugent, S.G.; Kumar, D.; Rampton, D.S.; Evans, D.F. Intestinal luminal pH in inflammatory bowel disease: Possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 2001, 48, 571–577.
- Zhang, X.; Han, Y.; Huang, W.; Jin, M.; Gao, Z. The influence of the gut microbiota on the bioavailability of oral drugs. Acta Pharm. Sin. B 2020, 11, 1789–1812.
- Parsons, R.L. Drug Absorption in Gastrointestinal Disease With Particular Reference to Malabsorption Syndromes. Clin. Pharmacokinet. 1977, 2, 45–60.
- Effinger, A.; O’Driscoll, C.M.; McAllister, M.; Fotaki, N. Impact of gastrointestinal disease states on oral drug absorption—Implications for formulation design—A PEARRL review. J. Pharm. Pharmacol. 2019, 71, 674–698.
- Castoldi, A.; Favero de Aguiar, C.; Moraes-Vieira, P.; Olsen Saraiva Câmara, N. They Must Hold Tight: Junction Proteins, Microbiota And Immunity In Intestinal Mucosa. Curr. Protein Pept. Sci. 2015, 16, 655–671.
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 2–3.
- Zhang, J.; Zhang, J.; Wang, R. Gut microbiota modulates drug pharmacokinetics. Drug Metab. Rev. 2018, 50, 357–368.
- Kwon, Y. Mechanism-based management for mucositis: Option for treating side effects without compromising the efficacy of cancer therapy. Onco. Targets. Ther. 2016, 9, 2007–2016.
- Karbelkar, S.A.; Majumdar, A.S. Altered systemic bioavailability and organ distribution of azathioprine in methotrexate-induced intestinal mucositis in rats. Indian J. Pharmacol. 2016, 48, 241.
- Fijlstra, M.; Rings, E.H.H.M.; Verkade, H.J.; van Dijk, T.H.; Kamps, W.A.; Tissing, W.J.E. Lactose maldigestion during methotrexate-induced gastrointestinal mucositis in a rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G283–G291.
- Fijlstra, M.; Tissing, W.J.E.; Stellaard, F.; Verkade, H.J.; Rings, E.H.H.M. Reduced absorption of long-chain fatty acids during methotrexate-induced gastrointestinal mucositis in the rat. Clin. Nutr. 2013, 32, 452–459.
- Kovanda, L.L.; Marty, F.M.; Maertens, J.; Desai, A.V.; Lademacher, C.; Engelhardt, M.; Lu, Q.; Hope, W.W. Impact of mucositis on absorption and systemic drug exposure of isavuconazole. Antimicrob. Agents Chemother. 2017, 61, e00101-17.
- Gattis, W.A.; Petros, W.P.; Pickard, W.W.; Drew, R.H.; May, D.B.; Hathorn, J.W. A prospective, open-label study of single-dose ciprofloxacin absorption after chemotherapy in patients with malignancy. Pharmacotherapy 1997, 17, 836–840.
- Vanstraelen, K.; Prattes, J.; Maertens, J.; Lagrou, K.; Schoemans, H.; Peersman, N.; Vermeersch, P.; Theunissen, K.; Mols, R.; Augustijns, P.; et al. Posaconazole plasma exposure correlated to intestinal mucositis in allogeneic stem cell transplant patients. Eur. J. Clin. Pharmacol. 2016, 72, 953–963.
- Alqahtani, S.; Mohamed, L.A.; Kaddoumi, A. Experimental models for predicting drug absorption and metabolism. Expert Opin. Drug Metab. Toxicol. 2013, 9, 1241–1254.
- Xiang, Y.; Wen, H.; Yu, Y.; Li, M.; Fu, X.; Huang, S. Gut-on-chip: Recreating human intestine in vitro. J. Tissue Eng. 2020, 11, 2041731420965318.
- Pilmis, B.; Le Monnier, A.; Zahar, J.R. Gut microbiota, antibiotic therapy and antimicrobial resistance: A narrative review. Microorganisms 2020, 8, 269.
- Lee, S.H.; Choi, N.; Sung, J.H. Pharmacokinetic and pharmacodynamic insights from microfluidic intestine-on-a-chip models. Expert Opin. Drug Metab. Toxicol. 2019, 15, 1005–1019.
 Encyclopedia
Encyclopedia


