Plants produce numerous secondary metabolites and rich in phytochemicals, which are potential bioresources for synthesizing Cu and CuO Nanoparticles (NPs). This green synthesis approach is environmentally friendly and more advantageous over commercial synthesis using physical and chemical methods. The green synthesized Cu and CuO NPs can be used as anticancer, antibacterial, antifungal and anti-inflammatory agents in biomedical applications. We discuss about the green synthesis of Cu and CuO NPs using various plants, factors affecting the synthesis, biomedical applications, and toxicity evaluation of the NPs. In addition, the mechanisms of the NPs entry into biological entities were also discussed.
- copper/copper oxide
- green synthesis
- nanoparticles
- biomedicine
- toxicity
1. Introduction
Green synthesis of Cu and CuO NPs is more advantageous than chemical and physical synthesis as it is a clean, nontoxic, cost-effective, and environmentally friendly approach. It bypasses the use of harsh, toxic, and expensive chemicals [1] and, instead, utilizes biological entities like bacteria [2], yeasts [3], fungi [4], algae [5], and plants [6]. Among all these natural organisms used in the green synthesis of Cu and CuO NPs, plants rich in bioactive compounds can serve as a reducing, stabilizing, and capping agent during NP synthesis; this makes them the best choice. They are nonpathogenic to humans, and the downstream processing steps are simple [7]. Unlike some bacterial and fungal strains that produce the NPs intracellularly, plant-mediated NP synthesis yields the NPs in the mixture solution, which can be easily obtained by filtering, rinsing, and drying [8][9]. The size, morphology, and stability of NPs can also be easily optimized for medicinal and pharmaceutical usage using this green method [10][11][12].
2. Plant-Based Green Synthesis of Copper and Copper Oxide Nanoparticles
The most important phytochemicals in plants are phenols and flavonoids, found in different parts of the plants, such as shoots, leaves, stems, flowers, roots, and fruits. These phenolic compounds possess hydroxyl and ketone groups, contributing to the iron chelation and subsequently demonstrating a strong antioxidant property [13]. NPs synthesized through this green method increased in stability, prevented the agglomeration and deformation of the NPs and allowed adsorption of phytochemicals on the surface of the NPs, which enhance the reaction rate of NPs [14]. One of the common techniques in synthesizing Cu and CuO NPs is mixing a known concentration of plant extract to a known concentration of precursor and heating the mixture to a fixed temperature, with continuous stirring at a given time (Figure 1).
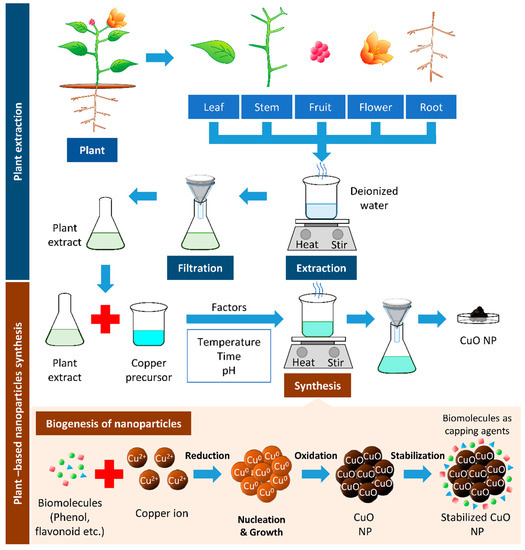
Figure 1. Plant-based synthesis of copper oxide nanoparticles. The boiling of respective parts of the plant can be used to extract biomolecules from the plant. The resulted filtrate is the plant extract, which can reduce the copper precursor to synthesize copper/copper oxide nanoparticles (Cu/CuO NPs). The biomolecules’ presence in plant extracts serves as reducing, stabilizing, and capping agents in Cu/CuO NP synthesis.
Occasionally, certain organic chemicals were added to prevent agglomeration and obtain fine NPs while maintaining the greener approach. The synthesized NPs eventually form various shapes and sizes, which carry unique properties in many applications [6]. Different characterization techniques can analyze the physical and chemical properties of NPs. In addition to size and shape, the size distribution, degree of aggregation, surface charge, surface area, and surface chemistry of the NPs can also be analyzed [15] (Figure 2).
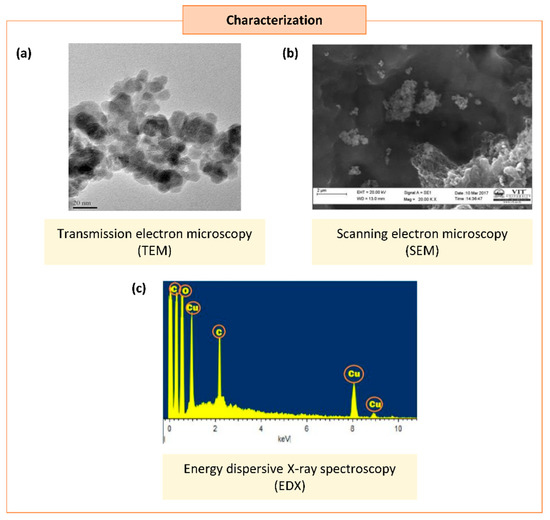
Figure 2. Characterization of synthesized copper/copper oxide nanoparticles (Cu/CuO NPs). (a) Transmission microscopy image of CuO NPs synthesized from Syzygium alternifolium (Wt.) Walp [16]. (b,c) Scanning electron microscopy image (SEM) (b) and energy dispersive X-ray spectroscopy EDS (c) of Cu NPs synthesized using Cissus vitiginea leaf extract [17].
2.1. Synthesis Strategy of CuO, Cu2O, and Cu4O3 NPs
Cu exists with variable oxidation numbers; this includes Cu(I), Cu(II), and very few Cu(III) ions. To date, the synthesis strategy reported for CuO, Cu2O, and Cu4O3 is the same in terms of parameters such as plant extract, precursor concentration, pH, and temperature. However, these parameters mainly affect the type of Cu particles formed during green synthesis [18].
2.2. Factors Affecting the Green Synthesis of Cu and CuO NPs
A few parameters play a crucial role in determining the characteristics of the Cu and CuO NPs synthesized; these include reaction temperature, pH, time, concentration of plant extracts, precursor used, and mixing speed. The reaction medium’s temperature was found to influence the nature of the NPs formed. As the reaction temperature increases, the reaction rate increases, consuming metal ions to form the nuclei of the NPs; this may lead to smaller NP sizes [19]. Generally, green synthesis is conducted below 100 °C or at ambient temperature [20]. NPs can undergo aggregation, shrink, or grow during long-term storage and it also has a shelf life that eventually affects its overall potential [21][22]. Studies have implied that the size of NPs is dependent on the reaction time [23][24][25][26]. It can be seen that increases in the time taken for green synthesis increase the size of the NPs. The biomolecules in plant extracts can act as natural capping and reducing agents during the green synthesis of NPs. These metabolite compositions vary depending on the types and parts of the plant and the extraction procedure [27]. Hence, the volume of plant extracts and the amount of biomolecules present in the plant extract used can affect the synthesis rate due to the availability of these molecules for the rapid bioreduction of metal salts and the stabilization of NPs [28]. The precursor is a salt and alkali metal solution. Numerous soluble copper salts were used in the green synthesis of Cu and CuO NPs. The pH of the reacting mixture in the biosynthesis of NPs is also one of the factors affecting the size of NPs. The optimum pH of the reaction medium ranges from 7–9, suggesting an alkaline condition is favorable for synthesizing NPs [29].
3. Application of Cu and CuO NPs
3.1. Antibacterial
Plant-mediated Cu NPs effectively repressed Gram-negative bacteria in urinary tract infection, such as Escherichia coli (E. coli), Enterococcus sp., Proteus sp., and Klebsiella sp. [17]. This implies the potential of these NPs in treating such infection. Cu and CuO NPs have also shown a suppressive effect on the growth of Gram-positive bacteria Staphylococcus aureus (S. aureus), Bacillus subtilis (B. subtilis), and Streptococcus pyrogenes (S. pyrogenes).
3.2. Antifungal
CuO NPs can induce antifungal properties via different mechanisms (Figure 3).
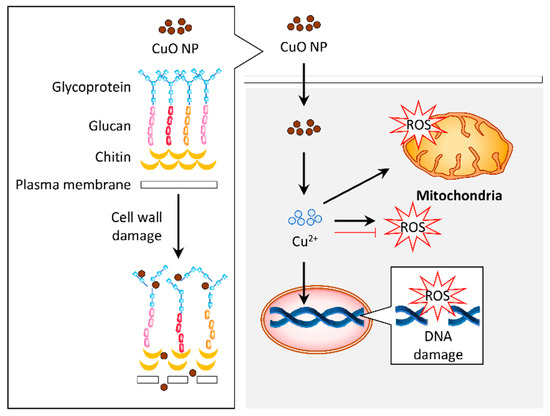
Figure 3. Graphical representation of the proposed mechanism for antifungal activity in response to copper oxide nanoparticles (CuO NPs). The CuO NPs distorted the cell wall of fungus such as Aspergillus flavus. Internalization of these particles induced reactive oxygen species (ROS) generation, which resulted in DNA and mitochondrial damage that contribute to the antifungal activity. Alternatively, the antifungal activity of CuO NPs may be attributed to their antioxidant property.
3.3. Anticancer
Numerous studies have reported the anticancer effect of plant-mediated Cu/CuO NPs, particularly breast [30][31][32][33][34][35][36][37], cervical [31][32][38], colon [30], skin (epithelioma) [32], gastric [39], blood (leukemia) [30], liver [40], lung [32][41][42][43] and ovarian [34] cancers (Table 1).
Table 1. Anticancer effects of the plant-derived Cu and CuO NPs biosynthesized.
| Types of Cells/ Cell Line |
Cu/CuO NPs | Toxicity (IC50) (μg/mL) | Biological Function (Targeting) | Reference | |
|---|---|---|---|---|---|
| Size (nm) | Shapes | ||||
| Breast cancer | |||||
| AMJ-13 | 20–50 | Spherical | 1.47 | Antioxidant, loss of membrane potential, and DNA fragmentation | [34] |
| MCF-7 | 5–24 | Cuboidal, spherical, and oval-shaped | >100 | ROS generation, loss of mitochondrial membrane potential, apoptosis, and cell cycle arrest | [33] |
| 10–40 | Spherical | 50.3 | Growth inhibition | [30] | |
| 12 | Spherical | 19.77–27.44 (depends on source of plant extract) |
Antioxidant and apoptosis | [32] | |
| 18.9–45.2 | Spherical | 53.89 | Growth inhibition | [44] | |
| 20 | Spherical | 85.58 | Growth inhibition | [45] | |
| 20 | Spherical | 24.5 | ROS generation and antiangiogenic | [36] | |
| 30–40 | Spherical, cubical | 35 | Growth inhibition | [46] | |
| 36 ± 8 | Spherical | 21.56 | ROS and NO generation, apoptosis, DNA fragmentation, induces proinflammatory (TNF-α) cytokines, and inhibits anti-inflammatory cytokine (IL-10) | [31] | |
| >200 | Spherical | 21.5 | ROS generation and antiangiogenic | [36] | |
| MDA-MB-231 | 10–50 | Spherical | 30 | ROS generation | [35] |
| 20 | Spherical | 11 | ROS generation and antiangiogenic | [36] | |
| >200 | Spherical | 7.5 | ROS generation and antiangiogenic | [36] | |
| Cervical cancer | |||||
| HeLa | 12 | Spherical | 26.73–20.32 (depends on the source of plant extract) |
Antioxidant and apoptosis | [32] |
| ~26.6 | Spherical | ~0.5 mg/mL | ROS generation, loss of mitochondrial membrane potential, and apoptosis | [38] | |
| 36 ± 8 | Spherical | 24.74 | ROS and NO generation, apoptosis, DNA fragmentation, induces proinflammatory (TNF-α) cytokines, and inhibits anti-inflammatory cytokine (IL-10) | [31] | |
| 60–100 | Spherical | 119.1 μg/mL | Growth inhibition | [47] | |
| Colon cancer | |||||
| HT-29 | 10–40 | Spherical | 33.0 | Growth inhibition | [30] |
| Epithelioma | |||||
| Hep-2 | 12 | Spherical | 21.66–29.58 (depends on source of plant extract) | Antioxidant and apoptosis | [32] |
| Gastric cancer | |||||
| AGS | 5–22 | Spherical | 25–50 | Apoptosis | [39] |
| Leukemia | |||||
| MOLT-4 | 10–40 | Spherical | >80 | Growth inhibition | [30] |
| Liver cancer | |||||
| HepG2 | 23–57 | Spherical, hexagonal, cubical | >500 | Antioxidant | [40] |
| Lung cancer | |||||
| A549 | 12 | Spherical | 18.11–37.19 (depends on the source of plant extract) | Antioxidant and apoptosis | [32] |
| 20 | Spherical | 81.57 | Growth inhibition | [45] | |
| 33.47 | Spherical, irregular | 25 | Apoptosis | [41] | |
| 577 | Spherical | 200 | Loss of mitochondrial membrane potential, ROS generation, and apoptosis | [42] | |
| 577 | Spherical | 200 | Regulates histone deacetylases, downregulates oncogenes and upregulates tumor suppressor genes, intrinsic and extrinsic apoptosis, and downregulates inflammatory genes (TNF-α and COX-2) | [43] | |
| Ovarian cancer | |||||
| SKOV-3 | 20–50 | Spherical | 2.27 | Antioxidant, loss of membrane potential, and DNA fragmentation | [34] |
ROS, reactive oxygen species; NO, nitric oxide; TNF-α, tumor necrosis factor; IL-10, interleukin-10.
3.4. Wound Healing and Anti-Inflammatory Activity
Plant-mediated Cu/CuO NPs also demonstrate wound healing and anti-inflammatory properties. Studies indicate the potential of plant-mediated Cu/CuO NPs in reducing inflammation, relieving pain, and promoting wound healing [23][48].
4. Toxicity Evaluation
This aspect should be evaluated thoroughly before utilizing these NPs in medicine. Here, we review the toxicity of plant-based green-synthesized Cu and CuO NPs in vitro and in vivo studies. A previous study has demonstrated that plant-mediated Cu NPs are relatively safe on normal human cell lines (Table 2). These data revealed that the Cu and CuO NPs have minimal toxicity in normal cells, with a cytotoxicity effect on cancer cells at low concentrations, suggesting their safe application. Nevertheless, the toxicity and optimal dosage to be administrated should be evaluated. It is crucial to evaluate the toxicity of plant-mediated CuO NPs in various animal models.
Table 2. Effects of the plant-derived Cu and CuO NPs biosynthesized on cell lines and animal models.
| Types of Cells/ Cell Line/Animal |
Cu/CuO NPs | Toxicity (IC50) | Reference | |
|---|---|---|---|---|
| Size (nm) | Shapes | |||
| Human embryonic kidney cells | ||||
| HEK 293 | 32 ± 0.9 | Spherical | 410 μg/mL | [49] |
| Human umbilical vein endothelial cells | ||||
| HUVEC | 10–30 | Spherical | >1000 μg/mL | [48] |
| Human dermal fibroblast | ||||
| NHDF | 12 | Spherical | >100 μg/mL | [32] |
| HuFb | 20–50 | Spherical | 54.34 μg/mL | [34] |
| L929 | 20 | Spherical | >100 μg/mL | [45] |
| Animal | ||||
| Male Swiss albino mice (BALB/c strain) | 20–50 | Spherical | Lethal at 800 mg/kg | [34] |
| Zebrafish | 20–40 | Spherical | 500 ± 15 mg/L | [50] |
5. Comparison of the Efficacy of Plant-Based-Synthesized and Commercial Cu and CuO NPs
It was documented that the efficacy of these NPs in suppressing cancer proliferation was comparable to commercial CuO NPs or was even better in some of the cases (Table 3). These findings imply the potential of plant-based CuO NPs in cancer therapy without causing significant toxicity to normal cells.
Table 6. Comparison of the toxicity of plant-derived and commercialized CuO NPs.
| Cell Line/ Animal Model |
Plant-Mediated Cu/CuO NPs | Commercial Cu/CuO NPs | Reference | ||||
|---|---|---|---|---|---|---|---|
| Size (nm) | Shapes | Toxicity/ Area |
Size (nm) | Shapes | Toxicity/ Area |
||
| In vitro | |||||||
| MCF-7 | 12 | Spherical | 19.77 ± 0.98 μg/mL | 12 | Spherical | 27.44 ± 2.14 μg/mL | [32] |
| HeLa | 12 | Spherical | 20.32 ± 1.16 μg/mL | 12 | Spherical | 45.31 ± 2.44 μg/mL | [32] |
| A549 | 12 | Spherical | 18.11 ± 0.93 μg/mL | 12 | Spherical | 37.19 ± 2.82 μg/mL | [32] |
| In vivo | |||||||
| Zebrafish | 25–35 | Spherical | 175 ± 10 mg/L | 25–35 | Spherical | 45 ± 10 mg/L | [51] |
| Rat | 10–30 | Spherical | Wound area: 0.9 ± 0.2 cm2 | 10–30 | Spherical | Wound area: 2.1 ± 0.1 cm2 | [48] |
6. Future Perspective in Cancer Therapy of Cu and CuO NPs
Advancement in the green synthesis of NPs has created a new approach in biomedical applications. Plant-synthesized Cu and CuO NPs have many attractive properties, such as antibacterial, antifungal, anticancer, anti-inflammatory, and wound healing. It is important to note that the Cu/CuO NPs synthesized from different plants have different properties due to the diversity in metabolite composition. This diversity leads to variation in size, shape, and morphology of the NPs, which contributed to the alteration of their overall properties and activities. Additionally, further validation of the underlying mechanisms and pathways of Cu/CuO NPs at both the cellular and organism level is mandatory. Therefore, a comprehensive study on these NPs, including in vitro and in vivo assessments from different aspects, would drive their medicinal application. Although it seems that many trials and more research need to be conducted, the potential and prospects for developing Cu and CuO NPs as a future drug, especially in cancer therapy, are still very bright. The green technology of NPs, utilizing natural plant resources that will be developed into nanobiotechnology, is not very far off from a breakthrough.
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931.
- Mandal, D.; Bolander, M.E.; Mukhopadhyay, D.; Sarkar, G.; Mukherjee, P. The use of microorganisms for the formation of metal nanoparticles and their application. Appl. Microbiol. Biotechnol. 2006, 69, 485–492.
- Kowshik, M.; Ashtaputre, S.; Kharrazi, S.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 2002, 14, 95.
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.; Alam, M.; Kumar, R. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis. Nano Lett. 2001, 1, 515–519.
- Chaudhary, R.; Nawaz, K.; Khan, A.K.; Hano, C.; Abbasi, B.H.; Anjum, S. An Overview of the Algae-Mediated Biosynthesis of Nanoparticles and Their Biomedical Applications. Biomolecules 2020, 10, 1498.
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650.
- Spadaro, D.; Gullino, M.L. Improving the efficacy of biocontrol agents against soilborne pathogens. Crop Protect. 2005, 24, 601–613.
- Saif Hasan, S.; Singh, S.; Parikh, R.Y.; Dharne, M.S.; Patole, M.S.; Prasad, B.; Shouche, Y.S. Bacterial synthesis of copper/copper oxide nanoparticles. J. Nanosci. Nanotechnol. 2008, 8, 3191–3196.
- Bukhari, S.I.; Hamed, M.M.; Al-Agamy, M.H.; Gazwi, H.S.; Radwan, H.H.; Youssif, A.M. Biosynthesis of Copper Oxide Nanoparticles Using Streptomyces MHM38 and Its Biological Applications. J. Nanomater. 2021, 2021.
- Sangeetha, S.; Kalaignan, G.P.; Anthuvan, J.T. Pulse electrodeposition of self-lubricating Ni–W/PTFE nanocomposite coatings on mild steel surface. Appl. Surf. Sci. 2015, 359, 412–419.
- Kumar, V.V.; Nithya, S.; Shyam, A.; Subramanian, N.S.; Anthuvan, J.T.; Anthony, S.P. Natural amino acid based phenolic derivatives for synthesizing silver nanoparticles with tunable morphology and antibacterial studies. Bull. Korean Chem. Soc. 2013, 34, 2702–2706.
- Ibrahim, S.; Jakaria, N.Z.; Rozali, S.; Ghazali, N.N.N.; Ab Karim, M.S.; Sabri, M.F.M. Biosynthesis of Copper Oxide Nanoparticles Using Camellia Sinensis Plant Powder. In Advances in Material Sciences and Engineering; Springer: Berlin, Germany, 2020; pp. 233–238.
- Singh, S.; Kumar, N.; Kumar, M.; Agarwal, A.; Mizaikoff, B. Electrochemical sensing and remediation of 4-nitrophenol using bio-synthesized copper oxide nanoparticles. Chem. Eng. J. 2017, 313, 283–292.
- Nasrollahzadeh, M.; Sajadi, S.M.; Rostami-Vartooni, A.; Hussin, S.M. Green synthesis of CuO nanoparticles using aqueous extract of Thymus vulgaris L. leaves and their catalytic performance for N-arylation of indoles and amines. J. Coll. Interface Sci. 2016, 466, 113–119.
- Minelli, C.; Shard, A.G. Chemical measurements of polyethylene glycol shells on gold nanoparticles in the presence of aggregation. Biointerphases 2016, 11, 04B306.
- Yugandhar, P.; Vasavi, T.; Devi, P.U.M.; Savithramma, N. Bioinspired green synthesis of copper oxide nanoparticles from Syzygium alternifolium (Wt.) Walp: Characterization and evaluation of its synergistic antimicrobial and anticancer activity. Appl. Nanosci. 2017, 7, 417–427.
- Wu, S.; Rajeshkumar, S.; Madasamy, M.; Mahendran, V. Green synthesis of copper nanoparticles using Cissus vitiginea and its antioxidant and antibacterial activity against urinary tract infection pathogens. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1153–1158.
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 1–24.
- Joshi, A.; Sharma, A.; Bachheti, R.K.; Husen, A.; Mishra, V.K. Plant-mediated synthesis of copper oxide nanoparticles and their biological applications. In Nanomaterials and Plant Potential; Springer: Berlin, Germany, 2019; pp. 221–237.
- Patra, J.K.; Baek, K.-H. Green nanobiotechnology: Factors affecting synthesis and characterization techniques. J. Nanomater. 2014, 2014.
- Baer, D. Surface Characterization of Nanoparticles: Critical needs and significant challenges. J. Surf. Anal. 2011, 17, 163–169.
- Saif, S.; Tahir, A.; Chen, Y. Green synthesis of iron nanoparticles and their environmental applications and implications. Nanomaterials 2016, 6, 209.
- Zhao, H.W.; Su, H.T.; Ahmeda, A.; Sun, Y.Q.; Li, Z.Y.; Zangeneh, M.M.; Nowrozi, M.; Zangeneh, A.; Moradi, R. Biosynthesis of copper nanoparticles using Allium eriophyllum Boiss leaf aqueous extract; characterization and analysis of their antimicrobial and cutaneous wound-healing potentials. Appl. Organomet. Chem. 2020.
- Prakash, S.; Elavarasan, N.; Venkatesan, A.; Subashini, K.; Sowndharya, M.; Sujatha, V. Green synthesis of copper oxide nanoparticles and its effective applications in Biginelli reaction, BTB photodegradation and antibacterial activity. Adv. Powder Technol. 2018, 29, 3315–3326.
- Fardood, S.T.; Ramazani, A.; Asiabi, P.; Joo, S. A novel green synthesis of copper oxide nanoparticles using a henna extract powder. J. Struct. Chem. 2018, 59, 1737–1743.
- Selvan, S.M.; Anand, K.V.; Govindaraju, K.; Tamilselvan, S.; Kumar, V.G.; Subramanian, K.S.; Kannan, M.; Raja, K. Green synthesis of copper oxide nanoparticles and mosquito larvicidal activity against dengue, zika and chikungunya causing vector Aedes aegypti. IET Nanobiotechnol. 2018, 12, 1042–1046.
- Park, Y.; Hong, Y.; Weyers, A.; Kim, Y.; Linhardt, R. Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IET Nanobiotechnol. 2011, 5, 69–78.
- Fazlzadeh, M.; Rahmani, K.; Zarei, A.; Abdoallahzadeh, H.; Nasiri, F.; Khosravi, R. A novel green synthesis of zero valent iron nanoparticles (NZVI) using three plant extracts and their efficient application for removal of Cr (VI) from aqueous solutions. Adv. Powder Technol. 2017, 28, 122–130.
- Akintelu, S.A.; Folorunso, A.S.; Folorunso, F.A.; Oyebamiji, A.K. Green synthesis of copper oxide nanoparticles for biomedical application and environmental remediation. Heliyon 2020, 6, e04508.
- Vijay, M.; Anu, Y. Anticancer activity of camellia Sinensis mediated copper nanoparticles against HT-29, MCF-7, and MOLT-4 human cancer cell lines. Asian J. Pharm. Clin. Res. 2017, 10, 82–88.
- Dey, A.; Manna, S.; Chattopadhyay, S.; Mondal, D.; Chattopadhyay, D.; Raj, A.; Das, S.; Bag, B.G.; Roy, S. Azadirachta indica leaves mediated green synthesized copper oxide nanoparticles induce apoptosis through activation of TNF-alpha and caspases signaling pathway against cancer cells. J. Saudi Chem. Soc. 2019, 23, 222–238.
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 89, 1067–1077.
- Ali, K.; Saquib, Q.; Ahmed, B.; Siddiqui, M.A.; Ahmad, J.; Al-Shaeri, M.; Al-Khedhairy, A.A.; Musarrat, J. Bio-functionalized CuO nanoparticles induced apoptotic activities in human breast carcinoma cells and toxicity against Aspergillus flavus: An In Vitro approach. Process. Biochem. 2020, 91, 387–397.
- Sulaiman, G.M.; Tawfeeq, A.T.; Jaaffer, M.D. Biogenic synthesis of copper oxide nanoparticles using olea europaea leaf extract and evaluation of their toxicity activities: An In Vivo and In Vitro study. Biotechnol. Prog. 2018, 34, 218–230.
- Nagaraj, E.; Karuppannan, K.; Shanmugam, P.; Venugopal, S. Exploration of Bio-synthesized Copper Oxide Nanoparticles Using Pterolobium hexapetalum Leaf Extract by Photocatalytic Activity and Biological Evaluations. J. Cluster Sci. 2019, 30, 1157–1168.
- Preeth, D.R.; Shairam, M.; Suganya, N.; Hootan, R.; Kartik, R.; Pierre, K.; Suvro, C.; Rajalakshmi, S. Green synthesis of copper oxide nanoparticles using sinapic acid: An underpinning step towards antiangiogenic therapy for breast cancer. J. Biol. Inorgan. Chem. 2019, 24, 633–645.
- Sukumar, S.; Rudrasenan, A.; Padmanabhan Nambiar, D. Green-Synthesized Rice-Shaped Copper Oxide Nanoparticles Using Caesalpinia bonducella Seed Extract and Their Applications. ACS Omega 2020, 5, 1040–1051.
- Nagajyothi, P.; Muthuraman, P.; Sreekanth, T.; Kim, D.H.; Shim, J. Green synthesis: In-Vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab. J. Chem. 2017, 10, 215–225.
- Gopinath, V.; Priyadarshini, S.; Al-Maleki, A.; Alagiri, M.; Yahya, R.; Saravanan, S.; Vadivelu, J. In Vitro toxicity, apoptosis and antimicrobial effects of phyto-mediated copper oxide nanoparticles. RSC Adv. 2016, 6, 110986–110995.
- Chung, I.M.; Abdul Rahuman, A.; Marimuthu, S.; Vishnu Kirthi, A.; Anbarasan, K.; Padmini, P.; Rajakumar, G. Green synthesis of copper nanoparticles using Eclipta prostrata leaves extract and their antioxidant and cytotoxic activities. Exp. Ther. Med. 2017, 14, 18–24.
- Chandrasekaran, R.; Yadav, S.A.; Sivaperumal, S. Phytosynthesis and Characterization of Copper Oxide Nanoparticles using the Aqueous Extract of Beta vulgaris L and Evaluation of their Antibacterial and Anticancer Activities. J. Cluster Sci. 2020, 31, 221–230.
- Sankar, R.; Maheswari, R.; Karthik, S.; Shivashangari, K.S.; Ravikumar, V. Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 234–239.
- Kalaiarasi, A.; Sankar, R.; Anusha, C.; Saravanan, K.; Aarthy, K.; Karthic, S.; Mathuram, T.L.; Ravikumar, V. Copper oxide nanoparticles induce anticancer activity in A549 lung cancer cells by inhibition of histone deacetylase. Biotechnol. Lett. 2018, 40, 249–256.
- Kiriyanthan, R.M.; Sharmili, S.A.; Balaji, R.; Jayashree, S.; Mahboob, S.; Al-Ghanim, K.A.; Al-Misned, F.; Ahmed, Z.; Govindarajan, M.; Vaseeharan, B. Photocatalytic, antiproliferative and antimicrobial properties of copper nanoparticles synthesized using Manilkara zapota leaf extract: A photodynamic approach. Photodiagn. Photodyn. Ther. 2020, 32, 102058.
- Rajamma, R.; Gopalakrishnan Nair, S.; Abdul Khadar, F.; Baskaran, B. Antibacterial and anticancer activity of biosynthesised CuO nanoparticles. IET Nanobiotechnol. 2020, 14, 833–838.
- Sukumar, K.; Arumugam, S.; Thangaswamy, S.; Balakrishnan, S.; Chinnappan, S.; Kandasamy, S. Eco-friendly cost-effective approach for synthesis of copper oxide nanoparticles for enhanced photocatalytic performance. Optik 2020, 202.
- Sundaram, C.S.; Kumar, J.S.; Kumar, S.S.; Ramesh, P.L.N.; Zin, T.; Rao, U.S.M. Antibacterial and anticancer potential of Brassica oleracea var acephala using biosynthesised copper nanoparticles. Med. J. Malaysia 2020, 75, 677–684.
- Hemmati, S.; Ahmeda, A.; Salehabadi, Y.; Zangeneh, A.; Zangeneh, M.M. Synthesis, characterization, and evaluation of cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing effects of copper nanoparticles using the aqueous extract of Strawberry fruit and L-Ascorbic acid. Polyhedron 2020, 180.
- Kumari, P.; Panda, P.K.; Jha, E.; Pramanik, N.; Nisha, K.; Kumari, K.; Soni, N.; Mallick, M.A.; Verma, S.K. Molecular insight to In Vitro biocompatibility of phytofabricated copper oxide nanoparticles with human embryonic kidney cells. Nanomedicine 2018, 13, 2415–2433.
- Santhosh Kumar, J.; Shanmugam, V. Green synthesis of copper oxide nanoparticles from magnolia champaca floral extract and its antioxidant & toxicity assay using Danio Rerio. Int. J. Recent Technol. Eng. 2020, 8, 5444–5449.
- Kumari, P.; Panda, P.K.; Jha, E.; Kumari, K.; Nisha, K.; Mallick, M.A.; Verma, S.K. Mechanistic insight to ROS and apoptosis regulated cytotoxicity inferred by green synthesized CuO nanoparticles from Calotropis gigantea to embryonic zebrafish. Sci. Rep. 2017, 7, 1–17.
 Encyclopedia
Encyclopedia


