1. Взаимодействие электромобилей с иммунными клетками
ВВ представляют собой частицы, которые естественным образом секретируются и поглощаются клетками. По этой причине ожидается, что эти носители будут биосовместимы. Действительно, было показано, что ЭВ вызывают лишь незначительные побочные эффекты на физиологию или выживаемость клеток [
67 ]. Однако известно, что ЭВ вносят значительный вклад в развитие иммунных ответов, особенно если они происходят из клеток иммунной системы. Появляется все больше доказательств того, что ЭВ, высвобождаемые неиммунными клетками, также могут участвовать в развитии иммунных ответов [
68 ]. Клетки крови часто выбирают для производства ЭВ, которые, как оказалось, могут стимулировать воспаление [
69 ,
70 ] .]. ЭВ, полученные из других клеточных источников, также могут вызывать умеренный провоспалительный ответ. Таким образом, EV, полученные из SKOV3 или HEK293, вызывали небольшую активацию продукции фактора некроза опухоли-α (TNF-α) или интерферона-α (INF-α) мононуклеарными клетками периферической крови (PBMC) [71
] . Представляется, что вне зависимости от происхождения ЭВ имеют тенденцию оказывать видимое влияние на клетки иммунной системы, и такое воздействие необходимо контролировать в случае длительного лечения.
Влияние неиммунных ВВ на иммунитет хозяина было хорошо проиллюстрировано ВВ, выделенными из коровьего молока и введенными самкам крыс Sprague-Dawley через желудочный зонд [
72 ]. Сегодня предпочтительна долгосрочная стратегия лечения с использованием хорошо переносимых доз терапевтических средств. Хотя это позволяет избежать острых реакций на терапию, в организме развиваются хронические реакции на воздействие. После длительного приема (1 раз в сутки в течение 15 дней) ЭВ молока (25 мг/кг массы тела) отмечено повышение уровня гранулоцитарно-макрофагального колониестимулирующего фактора (ГМ-КСФ) и снижение ~40% триглицериды были отмечены в сыворотке здоровых крыс, несмотря на отсутствие других серьезных изменений [
72 ].
Two models have been proposed to explain the action of milk EVs in the intestine. First, the uptake of EVs by enterocytes can cause the production of defective chylomicrons, which can be recognized and eliminated by intestinal macrophages. A more plausible explanation is that the macrophages engulf aggregates of chylomicrons and EVs. Recently, the associations of EVs and lipid particles, yielding aggregates, have been discussed [
73,
74,
75]. In any case, the reduced level of triglycerides in the rat’s blood is easily explained by the macrophage-mediated elimination of chylomicrons from the intestine that prevents chylomicron movement into the lacteal (lymph capillary) and subsequent entry into the venous circulation [
76].
Intrigue enough, these milk EVs (25 mg/kg b. wt.) had a long-term inhibitory effect (~4–5 weeks) on tumor growth in mice of human lung cancer (A549) xenografts exclusively after administration by oral gavage (three times a week), but not after intraperitoneal injection [
72]. It looks like the action of EVs in the intestine was the origin of their therapeutic effect. The positive effect of EVs administered through the digestive tract was also confirmed by other observations [
77].
2. EVs in the Bloodstream
Cancer is still one of the leading causes of death in the world, and EVs are considered promising carriers for therapeutics in oncology. Frequently, EVs are intravenously injected into the body based on the idea of their passive accumulation in a tumor site through the enhanced permeability and retention (EPR) effect [
78,
79]. However, the ability of EVs to overcome significant distances through the bloodstream seems questionable. Furthermore, the EPR effect seems to be overestimated in humans [
78,
79].
The first barrier on the way of EVs to the target sites (tumor) is blood phagocytes. A recent study showed that tumor EVs in the blood vessels of a zebrafish embryo were preferentially taken up by patrolling macrophages (functionally similar to human patrolling monocytes), endothelial cells, and putative hematopoietic stem cells [
80]. An interaction of EVs with non-cellular blood components, such as low-density lipoproteins (LDLs) [
73], also contributes to EV removal from the bloodstream. An electron microscopy assay of crude human plasma has shown that EVs frequently bind and fuse with lipoprotein-like structures [
75]. In vitro data indicate that EVs secreted by highly malignant breast cancer cells metastasizing to the brain are able to form aggregates with LDLs, which can subsequently be taken up by monocytes [
73].
Eventually, in addition to the removal of EVs directly from the blood, there is a risk of their accumulation in the reticuloendothelial-system-rich organs [
31]. This could result in EVs failing to meet their targets. The use of high doses of EVs can lead to their intensive accumulation in the lungs that can cause asphyxia, as has been found in experiments with mice [
81].
3. EVs as a Part of the Cellular Secretory Apparatus
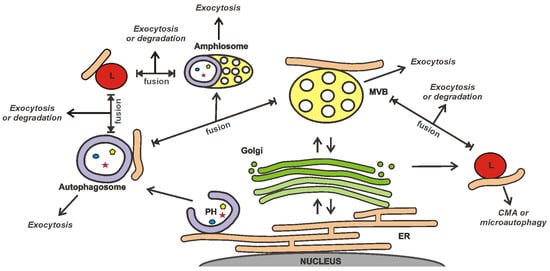
The release of EVs is closely integrated with other secretory and degradation processes in a cell (
Figure 1) [
82,
83,
84,
85,
86,
87]. This integration is suggested by the results of studies demonstrating that the secretion of EVs can be increased to compensate for lysosomal impairment [
84,
86] or autophagy inhibition [
83]. Sometimes, the loading of drugs into EVs can be performed using the cellular mechanisms of vesicle production. This technique is called “indirect”, “endogenous”, or “preloading”. A tNA or another cargo is added to cells, after which the secreted EVs are isolated. However, the intersections between EVs and other secretory/degradation processes inside cells can reduce the effectiveness of this drug loading strategy. Particular, the exocytosis of cargo can be expected to occur simultaneously via several secretory pathways; e.g., recently, the presence of overexpressed alpha-synuclein was simultaneously detected in endosomes, lysosomes, and autophagosomes [
85].
Figure 1. MVB interacts with other secretory and degradation processes in a cell. MVBs, autophagosomes, and amphisomes may undergo exocytosis or fusion with lysosomes, resulting in either degradation of their content or lysosomal exocytosis. It should be noted that some paths shown in the figure are hypothetical. The scheme was prepared on the basis of the reviews [
82,
84,
88,
89,
90]. MVB—multivesicular body; PH—phagophore; CMA—chaperone-mediated autophagy; ER—endoplasmic reticulum; L—lysosome.
The connection of EVs with other secretory/degradation processes may additionally provide new opportunities to control the EV release and the search for proteins suitable for selective loading of tNAs into EVs. An important question is the link between EV secretion and autophagy; e.g., the autophagy-associated protein Atg5 (autophagy protein 5) was found to control the release of EVs by the regulation of multivesicular body (MVB) acidification [
91]. Atg5 decreases the activity of V
1V
0-ATPase by detaching its Atp6v1e1 component [
91]. The deactivation of V
1V
0-ATPase reduces MVB acidity and promotes EV release. The sorting of Atp6v1e1 into intraluminal vesicles (ILVs) of MVBs depends on LC3 (microtubule-associated protein 1A/1B light chain 3). Most likely, the LC3 recruitment into ILVs is provided by Atg5. Eventually, both LC3 and Atp6v1e1 are released by cells via EVs [
91].
From a practical point of view, LC3 can be of interest for the selective loading of tNAs into EVs. Its lipidated form, LC3-II, can be involved in ribonucleoprotein (RNP) sorting into ILVs [
92]. LC3-II can recruit proteins containing the LC3-interaction region (LIR) [
87], although it is not clear whether it is absolutely required for the sorting of RNA-binding proteins (RBPs) into ILVs [
92]. Notably, LIR was not found in the previously mentioned protein, Atp6v1e1 [
91]. The use of LC3-mediated loading of RNPs into ILVs can be of interest to those aiming at the therapeutic application of small nucleolar RNAs (snoRNAs) [
92].
In addition to Atg5, there is growing evidence that proteins traditionally associated with autophagy can control MVB biogenesis. For instance, a recent report demonstrated the role of isoform A of lysosome-associated membrane protein 2 (LAMP2A) and heat shock 70-kDa protein 8 (HSC70) in the loading of proteins, containing the KFERQ-like motif, into EVs [
93]. Although it is well known that both LAMP2A and HSC70 are involved in chaperone-mediated autophagy (CMA) [
87,
90], it is quite possible that, in combination with other factors, they can change the fate of cargoes, directing them to EVs.
4. Influence of the Non-Cellular Environment on EVs
The interaction of exogenous EVs and cells is affected by various environmental factors. It has been found that the efficiency of EV internalization [
94] and the biological effects mediated by EVs [
95] can depend on the stiffness of the cell growth substrate; e.g., EVs secreted by highly malignant breast cancer cells can affect the activation, proliferation, motility, and contractility of fibroblasts only under conditions that mimic the stiffness of the tumor, but not healthy tissue [
95].
A general recommendation for EVs designed for the delivery of therapeutics to tumor cells is to obtain EVs under conditions close to the tumor microenvironment. Thus, it was noted that EVs secreted by metastatic melanoma cells were more efficiently internalized by their own cells, when EVs were produced under the acidic pH of the growth medium (pH = 6.0) [
96]. The rigidity of these “acidic” EVs was increased due to changes in the composition of the EV’s lipids [
96]. Similarly, hypoxic tumor EVs were better internalized by cancer cells than normoxic EVs [
97].
Sometimes, EVs show a greater affinity for their own cells [
98,
99]. In general, this is similar to how tumor cells prefer “acidic” or “hypoxic” EVs. In the case of tumor-derived EVs, this property of EVs has been proposed for use as a Trojan horse for drug delivery [
99]. This trend may persist even after endogenous loading of cargo into EVs [
100]. However, little is actually known about the effect of a cargo on the properties of EVs. Chemotherapeutic drugs can affect EVs after endogenous loading as they can influence the EV-secreting cells. It has been found that prolonged cell exposure to doxorubicin, sufficient for developing drug resistance, can change the protein profile of EVs secreted by these cells [
101]. Usually, drug loading into EVs requires short-term treatment, which is expected to have no effect on the properties of EVs.
In nature, the changes in the architecture of EVs can occur as a result of a viral infection. Viruses can penetrate inside EVs, thereby avoiding the host’s immune defense [
102] and providing viral ligands on the surface of EVs [
103]. Viruses need to control the EV composition to provide their entry into the type of cells that can ensure their propagation. Whether endogenously loaded cargo can affect the EV composition, such as viruses, is not entirely clear. The EV-mediated intercellular communication is believed to exist for targeted transfer of biological cargo from one cell to another [
104]. It is reasonable to assume that cells should control the EV specificity depending on the type of cargo and the type of target cells.
5. Manufacture of EVs
The low yield of EVs is the key problem limiting their clinical applications. Although some external stimuli (e.g., cell starvation, hypoxia, or an increase in Ca
2+ concentration in the culture medium) are used to increase the secretion of EVs [
105], the composition and properties of such EVs can differ significantly from those obtained under normal conditions. This has recently been confirmed for EVs secreted by starving cells, where starvation significantly affects the protein composition of EVs [
106]. The altering of the EV content in response to environmental stresses has also been discussed in a recently published review [
107]. In addition to activating the EV secretion in stress conditions, it is possible to overexpress some proteins in EV-secreting cells, which can enhance the EV release into the extracellular space [
108]. Although it may have a lesser influence on the content and features of EVs, the use of exogenous genetic constructs still raises concerns due to the risk of their inclusion into EVs, followed by insertional mutagenesis.
Isolation of EVs is considered a laborious and expensive process that is also poorly standardized. Conventional isolation approaches include ultracentrifugation, ultrafiltration, size exclusion chromatography, immunoaffinity, and polymer precipitation [
109]. Among these, ultracentrifugation is the most commonly used technique [
110]. Emerging technologies include membrane-based separation approaches and microfluidics [
109]. Finally, large-scale production of EVs is based on the use of bioreactors [
109,
111]. A convenient classification of methods for EV isolation is based on the degree of purity and yield [
112]. Подводя итог, чем выше степень очистки ЭВ, тем меньше его количество на выходе. Однако низкий выход ЭВ является проблемой не только для высокоочищенных препаратов ЭВ; даже стандартный протокол ультрацентрифугирования не всегда достаточен для удовлетворения количественных требований лабораторных исследований.
Из-за различных подходов, используемых для выделения и очистки EV, существуют значительные несоответствия в дозах EV, используемых в доклинических исследованиях [
61 ]. Количество ВВ обычно измеряют либо по концентрации общего белка, либо по количеству частиц в препаратах ВВ (методы характеристики ВВ обсуждались в обзорах [
40 ,
50 ,
113 ,
114 ,
115 ]). Однако разные методы выделения ЭВ дают разные уровни контаминации в препаратах ЭВ с невезикулярными структурами. Это может стать причиной искажения количественного определения ВВ с последующим разнообразием применяемых терапевтических доз [
61 ].
Для клинических исследований ВВ обычно получают от самих реципиентов, поскольку использование аутологичных ВВ снижает риск распознавания ВВ иммунной системой. Признание ЭВ потенциально опасными объектами может привести к их ускоренному обезвреживанию после введения. Недавно было замечено, что повторное внутривенное введение ЭВ, происходящих из Expi293F, нечеловеческим приматам (
Macaca nemestrina ) может вызывать специфические реакции антител к ЭВ, которые приводят к ускоренному клиренсу ЭВ [
116 ]. Поскольку время жизни первичных клеточных культур ограничено, может оказаться невозможным получить достаточное количество EV для терапевтической дозы. Таким образом, основным ограничением клинического применения ЭВ является отсутствие оптимального биологического источника для их получения.
This entry is adapted from the peer-reviewed paper 10.3390/ijms24087287