Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Connexins are proteins which comprise gap junctions in cells. These junctions can directly connect neighboring cells and the cell interior with the extracellular microenvironment and thus they act as tissue integrators. In addition, connexins perform a variety of non-channel functions. Alterations in connexin regulation can lead to unfavorable shifts in the tissue adhesive context thus eradicating the constraints of the normal tissue microenvironment, triggering (or enhancing) cancerogenesis and further tumor progression.
- tumor microenvironment
- connexins
- cell–cell contacts
- tumor stroma
- carcinogenesis
- tumor development
- metastasis
- tumor resistance
1. Introduction
Modern cancer research is focused on the identification of the mechanisms of cancer initiation, promotion and progression to further develop efficient treatment strategies. Accumulating experimental data testify to the tremendous role of the tissue microenvironment in tumor development and the formation of the unique tumor microenvironment (TME) [1][2][3]. TME is a complex and continuously evolving entity that includes immune cells, stromal cells, blood vessels, and non-cellular components such as extracellular matrix (ECM) and exosomes, and is characterized by specific physicochemical properties [4]. The crosstalk of TME constituents is executed by the simultaneous action of paracrine, autocrine, and endocrine signaling, and by the direct communication of adjacent and juxtaposed cells [5]. Direct intercellular communication and cell–matrix communication are maintained through intercellular junctions [6][7][8][9] and contacts with the extracellular matrix based on integrins and non-integrin receptors [10].
Connexins (Cxs) are proteins that form gap junctions (GJ) in cells of chordate organisms by assembling connexin monomers into hexameric hemichannels (connexins), that can function both autonomously, or undergo coupling to form a full-fledged channel that directly connects adjacent cells. To date 21 connexin isoforms have been discovered in humans [9]. The most widely used naming of connexin proteins is based on their predicted molecular weight in kilodaltons (e.g., Cx43, Cx32, etc.), whereas the classification of connexin genes was devised based on their sequence homology which falls into five subfamilies (α, β, γ, δ, ε) [11]. Connexins which have been found to play a significant role in cancer include Cx43 (the best studied), Cx25, Cx26, Cx32, Cx30, Cx31, Cx37, and Cx46.
Genes of connexins are shown to have one or multiple 5′UTR (untranslated region) exons which are separated from the exon containing the coding sequence by an intron of variable size. In some connexin genes, coding sequence may be interrupted by an additional intron. This allows a great variety of alternatively spliced connexin mRNA variants [12]. The transcription of connexin genes can be initiated by two promoters which are both regulated by universal and tissue-specific transcription factors. Among the universal regulators the most prominent are the Sp1, AP-1, cAMP and Wnt signaling pathways. Tissue specific regulation is provided by homeobox proteins, T-box and GATA transcription factors, hormones, etc. [13]. The production of connexins can be regulated epigenetically by hypermethylation of the promoter or acetylation/deacetylation of histones [14][15]. Concerning translation, connexins can be both silenced (via miRNA regulation) or facilitated (translation initiation via strong internal ribosome entry site (IRES)) [16][17].
The mutations in connexin gene family, both germline and somatic, have been established to be associated with multiple developmental abnormalities and syndromes. Nevertheless, the link between connexin mutations and cancer is still not clear. Several studies have revealed the association of certain type of tumors with mutated forms of connexins. Thus, the rate of mutated GJA1 gene (coding Cx43) is increased in colon adenocarcinomas [18], stomach adenocarcinomas and cutaneous melanoma [19]. Mutations of GJB2 (coding Cx26) might be associated with an increased propensity to develop skin cancer [20]; and germline mutations of this gene are assumed to increase the risk for early onset prostate cancer [21]. Mutated GJB6 (coding Cx30) and GJB7 (coding Cx25) might contribute to gastric and colorectal malignancies [22]. Cancer-specific rates of observed mutations support the important function of this group of proteins in the origin and development of tumors; however, their precise role and ability to drive carcinogenesis has to be further studied.
Connexins are tetraspan proteins, possessing four transmembrane domains, two extracellular loops and one intracellular loop; and intracellular N- and C-terminal tails (Figure 1). The transmembrane domains (TM1 to TM4) and amino terminus of connexins are relatively conserved, and form and maintain the connexin scaffold and transmembrane pore [23]. Early studies have shown that the vestibule of the connexin pore channels has a relatively large aperture ~40 Å, while it narrows in the membrane to ~15 Å due to a tilted domain orientation [24] and that the pore is formed by TM1 or TM3 [25][26][27]. Further studies have indicated that the N-terminus is also involved in the formation of the connexin funnel [28].
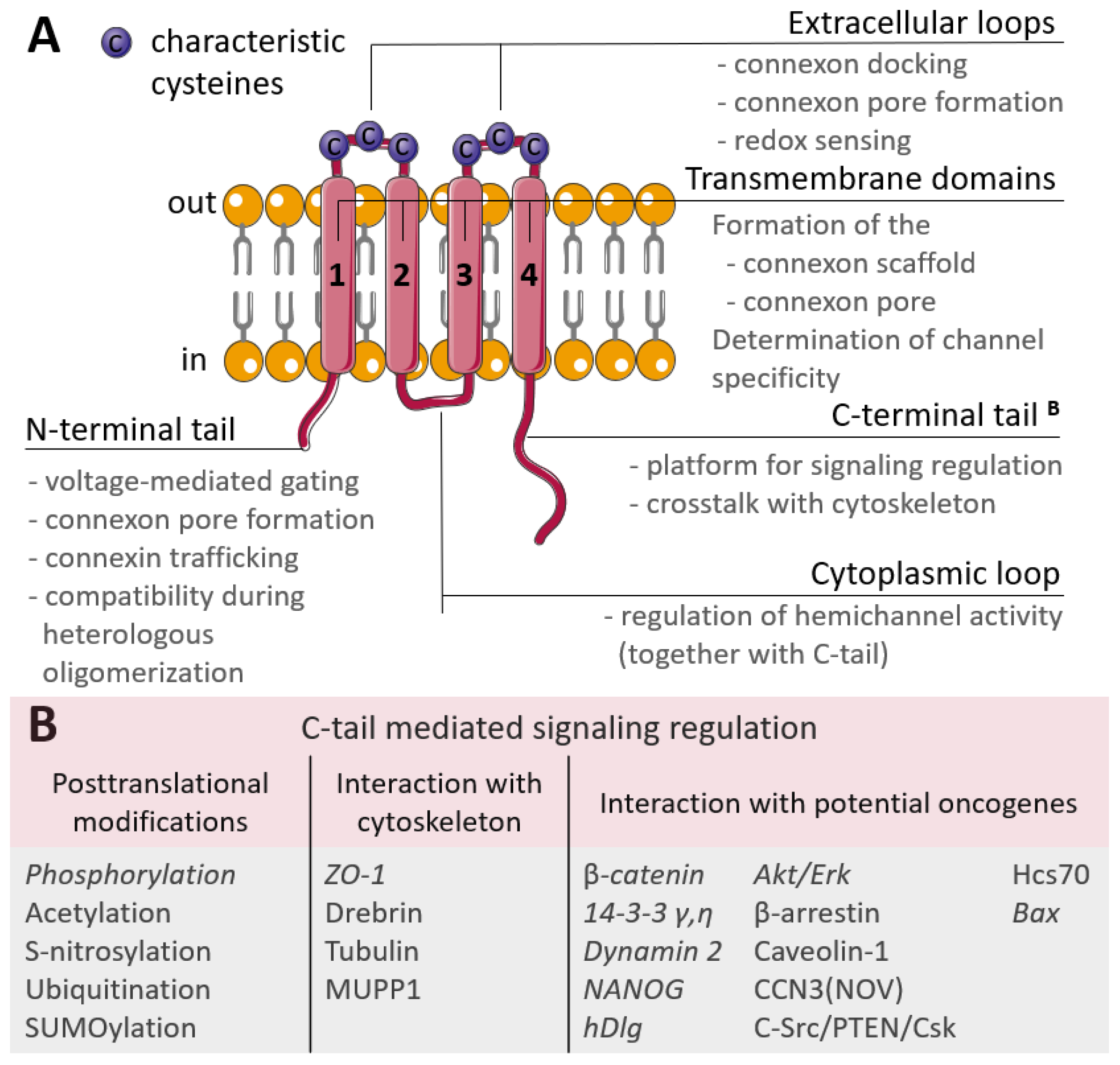
Figure 1. (A) Scheme of the connexin structure. Connexin is a tetraspan molecule which contains four transmembrane α-helices (1-4), two extracellular loops and one cytoplasmic loop; the amino- and carboxy-termini are located inside the cell. Transmembrane domains participate in the formation of the connexon scaffold and pore formation; extracellular loops are responsible for channel docking; the cytoplasmic loop and the N- and C-tails are the platform for the regulation of connexin functioning. (B) Participation of the connexin C-tail in regulation. The reported cancer-related signaling is represented in italic (explanations are in the text).
The extracellular loops are the most conserved regions of the connexin molecule, and are responsible for connexins docking. They possess consensus patterns with three characteristic cysteines in each loop [29]. These cysteines are not susceptible to post-translational modifications, namely to S-nitrosylation, and there is an assumption that these cysteines may also act as extracellular redox sensors [30]. Additionally, point mutations artificially induced in the extracellular loops led to the failed coupling of Cx43 hemichannels [31] and a permanently closed state of Cx46 hemichannels [32], but with no affection on their synthesis, membrane trafficking or non-channel-related properties. The variability in connexins is mostly due to the cytoplasmic loop and C-terminal tail which act as a highly versatile platform for connexin regulation and connexin-mediated signaling. Regulation is performed by various post-translational modifications of the C-tail and signaling is realized through a wide range of interactions with the cytoskeleton and potential oncogenes (Figure 1B) [33].
2. Connexin Localization and Its Role in Cancer
Connexin junctions directly connect cells in the TME, so connexin localization in the cell is an important issue as it determines cell behavior (Figure 2). The localization of connexins at the cell membrane can be considered in terms of their colocalization with other junctional proteins, contributing to cell polarity and cell surface protrusions. Connexins can also be localized intracellularly and be membrane-associated (in mitochondria) or represented in soluble form. A special case of localization of membrane-associated connexins is in exosomes.
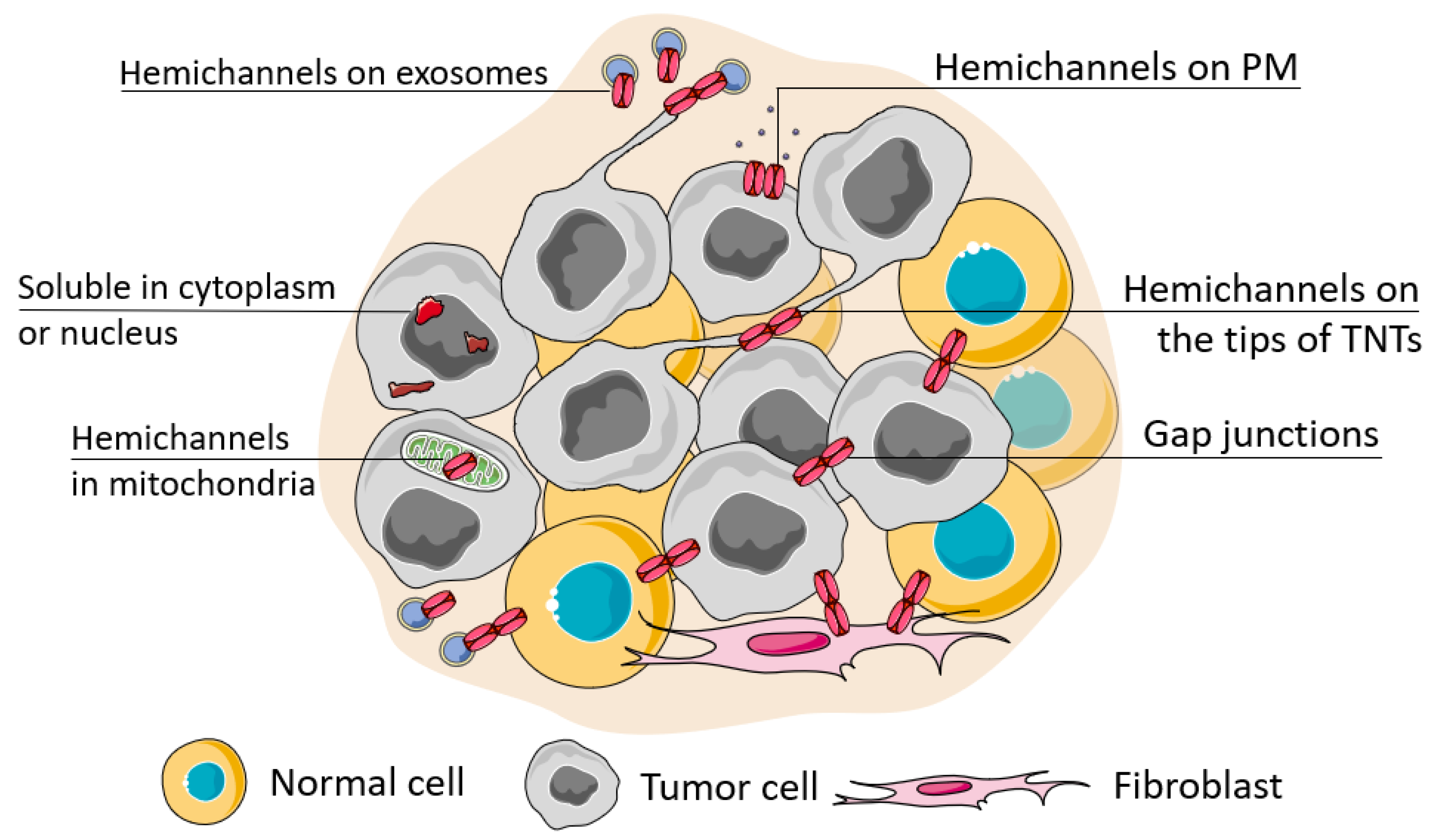
Figure 2. Possible localization of connexins in the cell. The localization of connexins in the cell can be attributed to its soluble forms (located in the nucleus or cytoplasm) or membrane-bound forms which can be observed during its trafficking, ultimately represented as a functional connexin residing in the cell membrane in the hemichannel or channel state (when docked with a connexin of a neighboring cell). The localization of connexin channels at the membrane can be considered relatively to their localization to the basal membrane (cell polarity), attributed to cell protrusions (e.g., tunneling nanotubes, TNTs) or extracellular vesicles (e.g., exosomes). Connexins can be also transferred to the inner membrane of mitochondria.
3. Connexin Participation in the Structural and Functional Integration of Malignant, Stromal, and Immune Cells within the Tumor
The coordinated actions of TME elements is one of the putative factors which determine tumor progression.
3.1. Connexins Integrate Tumor Cells
Connexin channels are direct bridges between neighboring cells, thus the presence of functional gap junctional plaques allows us to consider a network of such cells as a functional “syncytium”, somewhat similar to cardiac tissue [34]. The formation of such a structure provides metabolic cooperation and a platform for rapid signaling, both applicable to the cancer microenvironment to adapt to limitations. For example, such cooperation can rapidly regulate cell sizes in the actively proliferating tumor. Cells deep in the tumor experience great solid stress, thus their size is limited so water can be transported to cells of the outer layers accompanied by ion transport which proceeds through gap junctions. This causes swelling of the outer layer cells leading to increased cell proliferation [35]. Cx43-mediated glucose transfer reduces the size of the necrotic core in spheroids of colon cancer cells and elevates oxygenation and higher level of oxidative phosphorylation [36]. Cx43 located on the tips of TNTs possess integrative properties due to their capability to unite separate cells, as was indicated in vitro, such physical integration is relevant for the formation of metastatic foci and participates in angiogenesis due to the integration of endotheliocytes and pericytes.
3.2. Connexins Integrate into the Bone Metastaic Niche
Connexins coordinate the bone metastatic niche and lead to successful metastasis establishment due to the interaction of malignant cells with the osteoclast syncytium. In the osteoblast-conditioned microenvironment, membrane-localized Cx43 mediates tumor cell chemotaxis via its non-channel functions. At the leading edge of migrating cells, Cx43’s C-tail interacts with Rac1 and contractin, thus sustaining cell migration towards the osteogenic metastatic niche [37]. The direct interaction of cancer cells with osteoblasts through Cx43-based gap junctions provides a Ca2+ influx to the cancer cells enhancing their malignant potential [38]. By interacting with the osteoclast syncytium, cancer cells promote bone resorption during which a massive release of calcium and transforming growth factor-beta (TGFβ) takes place. TGFβ up-regulates Cx43, thus elevating the intracellular concentrations of calcium and enhancing GJ intercellular communications; in turn, Cx43 accelerates metastasis in the framework of this vicious cycle [39]. Taken together, the participation of connexin in bone metastasis may be considered a two-stage process with the connexin C-tail-mediated attraction of cancer cells and channel-related progression. At the same time it should be noted that in the bone microenvironment osteocytes perform purinergic signaling and can create an oxidative microenvironment by Cx43 hemichannel activity, thus combating tumor invasion [40][41]. Presumably, the overall role of connexins in bone as the potential soil for metastasis might depend on the ratio of the microenvironment participants.
3.3. Connexins in the Brain Niche
In the brain microenvironment, connexins appear to be the instrument that cancer cells use to turn astrocytes to allies. The connexin C-tail-mediated inhibition of Src kinase down-regulates β-catenin, triggering the preferential differentiation of neural progenitor cells towards astrocytes (against neurons). While the establishment of connexin GJs between normal neural cells and tumor cells can attenuate cell proliferation due to miR-124-3p transfer [42], astrocytes are reported to possess pro-tumorigenic activities in the glioma microenvironment [43][44][45]. One of the possible reasons for this is the transfer of miRNA derived from glioma cells to astrocytes, which promotes pro-invasive behavior, as was shown for miR-5096 [46] and miR-19b [47]. In the case of miR-19b, invasiveness might be associated with the disruption of cell–matrix adhesion. The resulting shift in the microenvironment drives tissue malignization [48].
Connexins heavily contribute to successful brain metastasis. Cx43 GJ communication mediates the extravasation of cancer cells to the brain parenchyma via a transcellular way. This process is initiated by the interaction of cancer cells with endothelium. The extravasated tumor cells engage Cx43 contacts with astrocytes in favor of the formation of metastasis and then form Cx43 contacts with each other, thus establishing a metastatic node [49]. The preferential interaction of tumor cells with astrocytes has been shown to be promoted by c-MYC (cellular myelocytomatosis, a proto-oncogene) [50]. Thus, in the brain niche connexins may have anti-tumor properties in the case of early tumors, while in the case of metastasis from tumors of other organs, connexins of the brain microenvironment mostly provide the means for tumor cells to pass through the blood–brain barrier and accommodate in the brain parenchyma.
3.4. Connexins Mediate Interactions of Cancer Cells and Cells of the Immune System
An important aspect of the integrative functions of connexins in the TME is their contribution to the communication of tumor cells and cells of the immune system. Connexins participate in immunological synapses [51] and execute both pro- and anti-tumor activities [52]. The anti-tumor activity of connexins mostly consists of their involvement in antigen presentation. Cx43 has been shown to accumulate at the interfaces between dendritic cells (DC) and cytotoxic immune cells, such as natural killers and cytotoxic T-lymphocytes, and between cytotoxic immune cells and target cells. These interactions are key to the activation and execution of the cytotoxic functions towards tumor cells [53][54][55][56]. Of note, the connexin-mediated cytotoxic effect can also be directed towards normal cells. The transfer of peptides across the gap junctions between melanoma cells and endothelial cells, for example, may provoke the destruction of endotheliocytes by cytotoxic T cells, thus hindering tumor neovascularization and lowering the supply of oxygen and nutrients to growing tumors [57].
Immune cells can also participate in the microenvironmental adaptation of the tumor. It has been suggested that tumor-associated macrophages (TAMs) may begin to act as intermediaries of nutrients between the tumor and vessels, creating an extensive cellular network integrated by Cx43 channels. The resulting rise in tumor metabolism not only increases its adaptability, but also leads to higher aggressiveness [58].
The connexin-mediated cooperation of stromal cells with immune cells can contribute to tumor progression and manifests as a failed regeneration process. In the case of the giant-cell tumor of bone, Cx43 participates in the fusion of osteoclasts and monocytes. This abnormal cellular cooperation is most likely associated with an attempt by monocytes to regenerate the cancer-associated damage to bone tissues, which instead leads to an increase in the size of the tumor [59].
3.5. Connexins in the Interactions between Tumor Cells and Other Types of Stromal Cells
Connexin contacts are established between various stromal cells, characteristic of certain metastatic niches or immune cells. Fibroblasts are a vital tissue component which substantially participates in the TME. The participation of connexins in integrating fibroblasts into the TME is tumor suppressive in the case of the early tumor and pro-tumorigenic in the advanced stage. Normal fibroblasts of the skin inhibit keratinocyte colony formation by the establishment of Cx43 GJ intercellular communications. When normal fibroblasts are exposed to sublethal doses of hydrogen peroxide (e.g., in aging) they lose Cx43 expression and thus GJ intercellular communications, which leads to the activation of keratinocyte colony formation. When the transformed fibroblasts start to prevail in the tissue microenvironment this is the turning point towards tissue malignization [60]. Interestingly, during the development of melanoma, where Cx43 is lost in malignant cells, Cx43, Cx26 and Cx30 expression was reported to be enhanced in the surrounding normal epidermis and correlated with the grade of the tumor, while this was not observed in benign nevi, as shown by measuring the mRNA content [61] and the evaluation of the protein localized at the membrane [62]. Such an up-regulation of Cx26 and Cx30 occurs during wound healing and triggers keratinocyte proliferation [61]. It should also be noted that high levels of Cx43 is characteristic for basal keratinocytes, while during maturation the level of Cx43 decreases and Cxs 26, 30 and 31 start to increase, yet their levels are still relatively low [63].
Cells of squamous cell carcinoma down-regulate homologous GJ intercellular communications mediated by Cx43 between fibroblasts in vitro in a paracrine fashion with the involvement of calcium signaling [64]. In the same type of cancer in vivo, Cx43 expression was observed in cancer-associated fibroblasts (CAF) located in the perlecan-rich stroma which is characteristic of invasion. Interestingly, these fibroblasts were localized in areas deficient in vasculature, i.e., areas of limited oxygen and nutrient supply [65]. Tumor cells can also use fibroblasts as energy donors, triggering the process of cytoplasmic and organelle sharing between fibroblasts and melanoma cells by TNT formation [66].
Initial connexin contacts between tumor cells and non-fibroblast cells can induce profibrotic properties in them, the most prominent of which is the enhanced expression of type I collagen. This was indicated for stellate cells in hepatocellular [67] and pancreatic [68] carcinomas.
Apparently in the TME as long as connexins are predominantly used by non-tumor cells to successfully retrain tumor cells rather than by tumor cells to subjugate normal cells, they thus act as tumor suppressors. When normal stromal communication is lost, tumor cells multiply and start to prevail in the microenvironment and thus connexins become a means of tumor progression.
4. Involvement of Connexins in Cancer Initiation
Early studies in connexin-deficient mice revealed their increased susceptibility to carcinogens [69][70][71][72]. This phenomenological coincidence strongly indicated that connexins are involved in cancer initiation. Indeed, Cx43-knockout mice tended to be statistically more predisposed to developing lung cancer induced by urethane or DMBA (7,12-dimethylbenz[a]anthracene) than the wild-type mice [69][70]. Cx32-deficient mice had an increased incidence of liver tumors after exposure to chemical carcinogens (DEN, diethylnitrosamine) and radiation (X-rays), with a higher number and sizes of tumor nodes compared to wild-type mice [71][72].
Shifts in overall connexin abundance, but predominantly its loss, is associated with tissue malignization under the action of various transforming factors, such as metabolic disorders, inflammation, bacterial infection, etc. Thus, non-alcoholic hepatosteatosis can be accompanied by the down-regulated expression of Cx32 which eventually causes liver fibrosis and is followed by hepatocellular carcinoma [73]. In the inflammatory microenvironment, for example, in the presence of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) during aging [74] or prostaglandin E2 [75], the level of Cx43 decreases. Pre-tumorigenic cells which have lost Cx43 are susceptible to the inflammatory microenvironment and they acquire a motile phenotype [76]. Improper diet and related metabolic stresses are shown to induce malignant transformation of the intestinal epithelium.
The improper membrane localization of connexins can also become a tumor-initiating event. Infection with human papillomavirus 16 causes mutation in the human homolog of Drosophila discs large (hDlg)-binding motif of the Cx43 C-tail, which results in the disruption of proper Cx43 and hDlg trafficking to the membrane so Cx43 remains in the cytoplasm [77].
Another mechanism is the initiation of endometrial cancer in obese patients which can be due to the disruption of Cx43 GJ intercellular communications by hypermethylation of the Cx43 promoter in normal endometrial epithelium mediated by the microenvironment conditioned by adipose stromal cells which massively release plasminogen activator inhibitor 1 [78].
Considering not only the levels of individual connexins, but also taking into account their interplay with each other, relative changes in their levels are also important. For example, the turning point for the transformation of bile duct epithelium by Clonorchis sinensis metabolites and eventual cancer development is accompanied by the simultaneous decrease in the expression of Cx32 and up-regulation of Cx43 and Cx26. Cx43 in this case acts as a tumor promoter as its inhibition leads to a proliferation decrease [79]. Similarly, the altered expression and functionality of Cx43 and Cx32 in liver oval cells have been demonstrated to cause hepatocellular and cholangiocellular neoplasms due to the disruption of adequate cell differentiation [80]. This evidence may be supported by the fact that differentiation towards normal liver tissues in early development requires the sustained expression of Cx32 and down-regulation of Cx43 [81][82].
Another way to alter intercellular communication and hence the tissue context is the stable expression of connexins which are normally only transiently expressed in a tissue when necessary for precise functional needs. For example, in normal breast development Cx32 is expressed only during lactation precisely at the interfaces of luminal cells [83] but this is characteristic of metastatic breast cancer lesions in lymph nodes. Overexpression of Cx32 in the normal breast epithelium cell line MCF10A turned the cell morphology towards a mesenchymal phenotype and increased the migratory activity of these cells by triggering the expression of EMT markers [84].
5. Role of Connexins in Forming a Hypoxia-Resistant Cancer Cell Phenotype
Hypoxia is a biological condition characterized by insufficient oxygenation of tissues which, in the case of malignant tumors, is caused by rapid cell proliferation [85][86]. The oxygenation levels in tumors are lower compared to normal tissues. For example, it was demonstrated with the polarography method, that the oxygenation levels in well-oxygenated tissues, such as muscle, lie in the range of 20 to 70 mmHg, while in breast tumors it ranges between 3 and 30 mmHg, which indicates hypoxia [87].
Hypoxic conditions are, on the one hand, a circumstance that is destructive to tumor cells, which cells must cope with, and on the other hand, a powerful selection factor for their collective adaptation with the subsequent development of progression mechanisms. Connexins participate in both of these aspects.
Overcoming hypoxia on a large scale is realized in tumors by promoting angiogenesis mainly by establishing the proliferative and migrative phenotype of endothelial cells. This can be realized via different types of cell–cell interactions, i.e., between tumor cells, tumor cells and endotheliocytes and between stromal cells, including vascular cells, and is mostly provided by functional GJ intercellular communications. For example, transcriptional suppression of Cx43 and Cx26 in MDA-MB-231 breast cancer cells led to the down-regulation of GJ intercellular communications between cancer cells and cancer cells-and-endotheliocytes, accompanied by reduced migrative and invasive properties, as shown by real-time cell analysis [88]. These results suggest that GJ intercellular communications between tumor cells and endotheliocytes enhance their migration and proliferation. It has been shown that collectively migrating tumor cells that have formed Cx43 contacts with endothelial cells, i.e., pre-hypoxic micrometastases, trigger vascularization upon the onset of hypoxic conditions [89]. On the other hand, GJ intercellular communications between endothelial cells is vital for initial vessel integrity. Loss of Cx43 expression and hence its patchy membrane localization leads to increased permeability of the existing vessels and promotes angiogenesis, as shown for high-grade serous ovarian cancer [90]. Vascular permeability is reported to be the factor stimulating angiogenesis [91]. Another mechanism of angiogenesis stimulation due to Cx43 loss relies on the massive production of pro-angiogenic factors due to the elevated levels of hypoxia-induced factor alpha 1 (HIF1a), as Cx43 is responsible for HIF1a ubiquitination and degradation indicated in melanoma [92].
One of the characteristics of a severely hypoxic microenvironment that should be handled is acidosis, and functional GJ intercellular communications mediated by connexins allows its management. Thus, in spheroids of pancreatic cancer, it has been shown that connexin channels between hypoxic and normoxic cells allow the rapid distribution of bicarbonate ions to neutralize acidification in hypoxic areas [93]. More than this, tumors can protect themselves from acidification by another mechanism, specifically, transmitting lactate through Cx43-based channels [94]. It has been shown that acidosis management can be also carried out with the participation of the stroma. Hydrogen ions, produced by tumor cells, are captured from the extracellular space by the AE2 transporter on myofibroblasts and are then spread via Cx43 channels through the myofibroblast syncytium [95]. The transfer of ions occurs passively, which is energetically beneficial for hypoxic cells, as they retain ATP; moreover, a spread of lactate can additionally act as an alternative nutrient shared between cancer cells, as lactate is indirectly involved in the tri-carbon acid cycle [96]. Although as demonstrated in severe acidosis, when lactate concentrations are too high and high calcium concentrations are established, Cx43 channels close and uncouple, which leads to the interruption of the GJ intercellular communications in rat hepatocellular carcinoma and human glioblastoma A172 [97].
Thus, connexins participate in hypoxia resistance by providing a trans-cellular path for oxygen and nutrients in arranged cells which jut into the depths of the tissue from the perivascular space [58] or by providing the spread of alternative nutrients (lactate) simultaneously protecting cells from acidification. The loss of GJ intercellular communications in vascular cells triggers angiogenesis; and the re-establishment of GJ intercellular communications in impaired vessels facilitates the migration of tumor cells through the vessel wall, enabling the formation of metastatic units already possessing resistance to hypoxia.
6. The Multifaceted Role of Connexins in Both Tumor Progression and Suppression Due to the Intracellular Transfer of miRNA
miRNAs are one of the most potent factors of the TME and they are transmitted between both malignant and stromal cells through connexin-based gap junctions or via extracellular vesicles (exosomes). Thus, Cx43-based channels have been reported to transmit miRNA-145 from microvascular endothelial cells to colon cancer cells leading to the inhibition of angiogenesis [98]. A similar cancer-inhibiting effect was recently reported in glioma where the miR-152-3p transmitted from normal astrocytes to C6 glioma cells via Cx43-based channels attenuated their migration and invasion [99]; and in hepatocellular carcinoma where miR-142 and miR-233 were transferred from macrophages to tumor cells [100]. In glioma cells loaded with tumor-suppressive miR-124-3p, GJ intercellular communications enhanced the transfer and distribution of miRNA to neighboring cells which attenuated cell proliferation, as was shown in vitro and in vivo [42].
A considerable amount of data has been gathered on the pro-tumorigenic role of miRNA transfer by connexin channels. The miR-5096 derived from glioma cells possesses a pro-invasive effect when transferred to astrocytes [46], and a pro-angiogenic effect when transferred to microvascular endothelial cells along with the suppression of Cx43 expression [101]. Hypoxia-induced miR-192-5p transferred through Cx43-channels from melanoma cells to cytotoxic T-lymphocytes triggers the immune surveillance escape [102]. Bone marrow-derived miRNAs targeting CXCL12 were indicted to contribute to breast cancer quiescence [103].
In the case of the exosome-mediated transmission, connexin channels have been reported to recruit miRNAs to exosomes as they possess RNA-binding motifs in their structure [104]. The miRNA transmission mediated by exosomes containing connexins facilitates cancer progression in hypoxic conditions (Cx46-rich exosomes) [105]. It is interesting to note that Cx43-based channels have a higher permeability for various miRNAs compared to channels formed by other connexins, such as Cx26, Cx30, and Cx31 [106], or Cx32 and Cx37 [107].
Summarizing these data, the trend towards tumor progression or suppression may be determined by the type of miRNA which is being transferred and the direction of transfer (from a tumor cell to a normal cell or vice versa, or between tumor cells with different malignant potential). This can probably be determined by the presence, quantity, affinity, and conformational availability of RNA-binding motifs in various connexins.
7. Connexins and Cancer Stemness
Cancer stemness is an important factor of cancer progression, which determines cancer self-renewal, dormancy, and resistance to treatment [108]. Connexins are reported to participate in regulating cancer stemness in positive and negative ways, or utilized by cancer stem cells to perform their functions.
Connexins can reduce the stemness of cancer cells or provide assistance in cancer treatment aimed at resistant stem cells. Thus, ectopic expression of Cx43 in lung cancer cells reduces the abundance of cancer stem cells, as was shown by a reduction in tumor sphere formation and stemness markers in transfected cells [109]. Stemness attenuation was also reported for Cx30 in glioma due to its ability to interfere with the insulin-like growth factor 1 receptor, which is involved in maintaining self-renewal [110]. The assistance in coping with cancer stem cell drug resistance consists of the establishment of GJ communications, which was indicated in liver cancer, where simultaneous ectopic expression of Cx43 and SUMO1 resulted in a higher responsivity to treatment [111].
Connexins can also support the stemness features of cancer cells. The most prominent stemness feature supported by them is self-renewal which was established in the case of intracellular localization of connexins. For example, it has been shown that Cx26, Cx32 and Cx46 can form alternative signaling with the pluripotency transcription factor NANOG, up-regulate CD133 or markers of stemness Oct4 and Sox2, which leads to an increased cancer abundance of stem cells and the acquisition of an invasive tumor phenotype [112]. The functional GJ communications can also support stemness features, as was indicated for Cx46, which maintains self-renewal in glioblastoma [113]; and for Cx43 which is crucial for maintaining pluripotency and proliferation in embryonic stem cells [114] and maintaining dormancy in the bone marrow niche [115].
Connexins can also be used by cancer stem cells as a means of the realization of their aggressiveness. Thus, Cx43 was reported to mediate breast cancer immune escape by establishing communications between cancer stem cells and mesenchymal stem cells which results in a preferential Treg response against T-helper 17 cells [116]. Additionally, Cx43 facilitates lung cancer metastasis to the brain by establishing communications between cancer stem cells and astrocytes [117].
The recent conceptual papers by J.E. Trosko discussed the hypothesis that connexins may act as key molecules which underlie cancer stem cell origin and determine the cancer stem cell type due to their tissue integration properties with the most crucial point, cell differentiation. Cancer cell stemness, in this case, is assumed to be maintained on the one hand by the sustained expression of the Oct4A oncogene which prevents the expression of connexin genes, thus making it impossible for cells to differentiate at all, or in the case of when Oct4A oncogene is absent the stemness is maintained by other oncogenes which prevent proper connexin localization and the execution of their differentiation functions [118][119][120].
This entry is adapted from the peer-reviewed paper 10.3390/biology12020204
References
- Brücher, B.L.; Jamall, I.S. Somatic Mutation Theory—Why it’s Wrong for Most Cancers. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2016, 38, 1663–1680.
- Soto, A.M.; Sonnenschein, C. The tissue organization field theory of cancer: A testable replacement for the somatic mutation theory. BioEssays News Rev. Mol. Cell. Dev. Biol. 2011, 33, 332–340.
- Sonnenschein, C.; Soto, A.M. Over a century of cancer research: Inconvenient truths and promising leads. PLoS Biol. 2020, 18, e3000670.
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925.
- Zefferino, R.; Piccoli, C.; Di Gioia, S.; Capitanio, N.; Conese, M. How Cells Communicate with Each Other in the Tumor Microenvironment: Suggestions to Design Novel Therapeutic Strategies in Cancer Disease. Int. J. Mol. Sci. 2021, 22, 2550.
- Garrod, D.; Chidgey, M. Desmosome structure, composition and function. Biochim. Et Biophys. Acta 2008, 1778, 572–587.
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293.
- Harris, T.J.; Tepass, U. Adherens junctions: From molecules to morphogenesis. Nat. Rev. Mol. Cell Biol. 2010, 11, 502–514.
- Nielsen, M.S.; Axelsen, L.N.; Sorgen, P.L.; Verma, V.; Delmar, M.; Holstein-Rathlou, N.H. Gap junctions. Compr. Physiol. 2012, 2, 1981–2035.
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 533–548.
- Beyer, E.; Berthoud, V. The Family of Connexin Genes; Humana Press: Berlin/Heidelberg, Germany, 2009; pp. 3–26.
- Willecke, K.; Eiberger, J.; Degen, J.; Eckardt, D.; Romualdi, A.; Güldenagel, M.; Deutsch, U.; Söhl, G. Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 2002, 383, 725–737.
- Oyamada, M.; Takebe, K.; Oyamada, Y. Regulation of connexin expression by transcription factors and epigenetic mechanisms. Biochim. Et Biophys. Acta 2013, 1828, 118–133.
- Jayalakshmi, J.; Vanisree, A.J.; Ravisankar, S.; K, R. Site specific hypermethylation of CpGs in Connexin genes 30, 26 and 43 in different grades of glioma and attenuated levels of their mRNAs. Int. J. Neurosci. 2019, 129, 273–282.
- Sirnes, S.; Honne, H.; Ahmed, D.; Danielsen, S.A.; Rognum, T.O.; Meling, G.I.; Leithe, E.; Rivedal, E.; Lothe, R.A.; Lind, G.E. DNA methylation analyses of the connexin gene family reveal silencing of GJC1 (Connexin45) by promoter hypermethylation in colorectal cancer. Epigenetics 2011, 6, 602–609.
- Xiao, S.; Shimura, D.; Baum, R.; Hernandez, D.M.; Agvanian, S.; Nagaoka, Y.; Katsumata, M.; Lampe, P.D.; Kleber, A.G.; Hong, T.; et al. Auxiliary trafficking subunit GJA1-20k protects connexin-43 from degradation and limits ventricular arrhythmias. J. Clin. Investig. 2020, 130, 4858–4870.
- Basheer, W.A.; Xiao, S.; Epifantseva, I.; Fu, Y.; Kleber, A.G.; Hong, T.; Shaw, R.M. GJA1-20k Arranges Actin to Guide Cx43 Delivery to Cardiac Intercalated Discs. Circ. Res. 2017, 121, 1069–1080.
- Dubina, M.V.; Iatckii, N.A.; Popov, D.E.; Vasil’ev, S.V.; Krutovskikh, V.A. Connexin 43, but not connexin 32, is mutated at advanced stages of human sporadic colon cancer. Oncogene 2002, 21, 4992–4996.
- Gonzalez-Perez, A.; Perez-Llamas, C.; Deu-Pons, J.; Tamborero, D.; Schroeder, M.P.; Jene-Sanz, A.; Santos, A.; Lopez-Bigas, N. IntOGen-mutations identifies cancer drivers across tumor types. Nat. Methods 2013, 10, 1081–1082.
- Sakabe, J.; Yoshiki, R.; Sugita, K.; Haruyama, S.; Sawada, Y.; Kabashima, R.; Bito, T.; Nakamura, M.; Tokura, Y. Connexin 26 (GJB2) mutations in keratitis-ichthyosis-deafness syndrome presenting with squamous cell carcinoma. J. Derm. 2012, 39, 814–815.
- Tang, T.; Tan, X.; Wang, Z.; Wang, S.; Wang, Y.; Xu, J.; Wei, X.; Zhang, D.; Liu, Q.; Jiang, J. Germline Mutations in Patients With Early-Onset Prostate Cancer. Front. Oncol. 2022, 12.
- Son, H.J.; An, C.H.; Yoo, N.J.; Lee, S.H. Tight Junction-Related CLDN5 and CLDN6 Genes, and Gap Junction-Related GJB6 and GJB7 Genes Are Somatically Mutated in Gastric and Colorectal Cancers. Pathol. Oncol. Res. 2020, 26, 1983–1987.
- Kyle, J.W.; Minogue, P.J.; Thomas, B.C.; Domowicz, D.A.; Berthoud, V.M.; Hanck, D.A.; Beyer, E.C. An intact connexin N-terminus is required for function but not gap junction formation. J. Cell Sci. 2008, 121, 2744–2750.
- Weber, P.A.; Chang, H.C.; Spaeth, K.E.; Nitsche, J.M.; Nicholson, B.J. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys. J. 2004, 87, 958–973.
- Fleishman, S.J.; Unger, V.M.; Yeager, M.; Ben-Tal, N. A Calpha model for the transmembrane alpha helices of gap junction intercellular channels. Mol. Cell 2004, 15, 879–888.
- Skerrett, I.M.; Williams, J.B. A structural and functional comparison of gap junction channels composed of connexins and innexins. Dev. Neurobiol. 2017, 77, 522–547.
- Zhou, X.W.; Pfahnl, A.; Werner, R.; Hudder, A.; Llanes, A.; Luebke, A.; Dahl, G. Identification of a pore lining segment in gap junction hemichannels. Biophys. J. 1997, 72, 1946–1953.
- Kronengold, J.; Srinivas, M.; Verselis, V.K. The N-terminal half of the connexin protein contains the core elements of the pore and voltage gates. J. Membr. Biol. 2012, 245, 453–463.
- Bai, D.; Yue, B.; Aoyama, H. Crucial motifs and residues in the extracellular loops influence the formation and specificity of connexin docking. Biochim. Et Biophys. Acta. Biomembr. 2018, 1860, 9–21.
- Retamal, M.A.; García, I.E.; Pinto, B.I.; Pupo, A.; Báez, D.; Stehberg, J.; Del Rio, R.; González, C. Extracellular Cysteine in Connexins: Role as Redox Sensors. Front. Physiol. 2016, 7, 1.
- Olbina, G.; Eckhart, W. Mutations in the second extracellular region of connexin 43 prevent localization to the plasma membrane, but do not affect its ability to suppress cell growth. Mol. Cancer Res. MCR 2003, 1, 690–700.
- Fernández-Olivares, A.; Durán-Jara, E.; Verdugo, D.A.; Fiori, M.C.; Altenberg, G.A.; Stehberg, J.; Alfaro, I.; Calderón, J.F.; Retamal, M.A. Extracellular Cysteines Are Critical to Form Functional Cx46 Hemichannels. Int. J. Mol. Sci. 2022, 23, 7252.
- Leithe, E.; Mesnil, M.; Aasen, T. The connexin 43 C-terminus: A tail of many tales. Biochim. Et Biophys. Acta. Biomembr. 2018, 1860, 48–64.
- Carmeliet, E. Conduction in cardiac tissue. Historical reflections. Physiol. Rep. 2019, 7, e13860.
- McEvoy, E.; Han, Y.L.; Guo, M.; Shenoy, V.B. Gap junctions amplify spatial variations in cell volume in proliferating tumor spheroids. Nat. Commun. 2020, 11, 6148.
- Gong, K.; Hong, Q.; Wu, H.; Wang, F.; Zhong, L.; Shen, L.; Xu, P.; Zhang, W.; Cao, H.; Zhan, Y.Y.; et al. Gap junctions mediate glucose transfer to promote colon cancer growth in three-dimensional spheroid culture. Cancer Lett. 2022, 531, 27–38.
- Boucher, J.; Balandre, A.C.; Debant, M.; Vix, J.; Harnois, T.; Bourmeyster, N.; Péraudeau, E.; Chépied, A.; Clarhaut, J.; Debiais, F.; et al. Cx43 Present at the Leading Edge Membrane Governs Promigratory Effects of Osteoblast-Conditioned Medium on Human Prostate Cancer Cells in the Context of Bone Metastasis. Cancers 2020, 12, 3013.
- Wang, H.; Tian, L.; Liu, J.; Goldstein, A.; Bado, I.; Zhang, W.; Arenkiel, B.R.; Li, Z.; Yang, M.; Du, S.; et al. The Osteogenic Niche Is a Calcium Reservoir of Bone Micrometastases and Confers Unexpected Therapeutic Vulnerability. Cancer Cell 2018, 34, 823–839.e827.
- Waning, D.L.; Guise, T.A.; Mohammad, K.S. A “Connexin” Responsible for the Fatal Attraction of Cancer to Bone. Cell Metab. 2019, 29, 6–8.
- Tian, Y.; Riquelme, M.A.; Tu, C.; Quan, Y.; Liu, X.; Sun, L.-Z.; Jiang, J.X. Osteocytic Connexin Hemichannels Modulate Oxidative Bone Microenvironment and Breast Cancer Growth. Cancers 2021, 13, 6343.
- Zhou, J.Z.; Riquelme, M.A.; Gu, S.; Kar, R.; Gao, X.; Sun, L.; Jiang, J.X. Osteocytic connexin hemichannels suppress breast cancer growth and bone metastasis. Oncogene 2016, 35, 5597–5607.
- Suzhi, Z.; Liang, T.; Yuexia, P.; Lucy, L.; Xiaoting, H.; Yuan, Z.; Qin, W. Gap Junctions Enhance the Antiproliferative Effect of MicroRNA-124-3p in Glioblastoma Cells. J. Cell. Physiol. 2015, 230, 2476–2488.
- Brandao, M.; Simon, T.; Critchley, G.; Giamas, G. Astrocytes, the rising stars of the glioblastoma microenvironment. Glia 2019, 67, 779–790.
- Sin, W.C.; Aftab, Q.; Bechberger, J.F.; Leung, J.H.; Chen, H.; Naus, C.C. Astrocytes promote glioma invasion via the gap junction protein connexin43. Oncogene 2016, 35, 1504–1516.
- McCutcheon, S.; Spray, D.C. Abstract 2885: Connexin 43-dependent miRNA transfer drives perivascular glioma invasion through dysregulation of astrocytes. Cancer Res. 2021, 81, 2885.
- Hong, X.; Sin, W.C.; Harris, A.L.; Naus, C.C. Gap junctions modulate glioma invasion by direct transfer of microRNA. Oncotarget 2015, 6, 15566–15577.
- McCutcheon, S.; Spray, D.C. Glioblastoma–Astrocyte Connexin 43 Gap Junctions Promote Tumor Invasion. Mol. Cancer Res. 2021, 20, 319–331.
- Talaverón, R.; Matarredona, E.R.; Herrera, A.; Medina, J.M.; Tabernero, A. Connexin43 Region 266-283, via Src Inhibition, Reduces Neural Progenitor Cell Proliferation Promoted by EGF and FGF-2 and Increases Astrocytic Differentiation. Int. J. Mol. Sci. 2020, 21, 8852.
- Figueira, I.; Galego, S.; Custódio-Santos, T.; Vicente, R.; Molnár, K.; Haskó, J.; Malhó, R.; Videira, M.; Wilhelm, I.; Krizbai, I.; et al. Picturing Breast Cancer Brain Metastasis Development to Unravel Molecular Players and Cellular Crosstalk. Cancers 2021, 13, 910.
- Lee, H.Y.; Cha, J.; Kim, S.K.; Park, J.H.; Song, K.H.; Kim, P.; Kim, M.Y. c-MYC Drives Breast Cancer Metastasis to the Brain, but Promotes Synthetic Lethality with TRAIL. Mol. Cancer Res. MCR 2019, 17, 544–554.
- Tittarelli, A.; Navarrete, M.; Gleisner, M.A.; Gebicke-Haerter, P.; Salazar-Onfray, F. Connexin-Mediated Signaling at the Immunological Synapse. Int. J. Mol. Sci. 2020, 21, 3736.
- Tittarelli, A. Connexin channels modulation in pathophysiology and treatment of immune and inflammatory disorders. Biochim. Et Biophys. Acta. Mol. Basis Dis. 2021, 1867, 166258.
- Mendoza-Naranjo, A.; Bouma, G.; Pereda, C.; Ramírez, M.; Webb, K.F.; Tittarelli, A.; López, M.N.; Kalergis, A.M.; Thrasher, A.J.; Becker, D.L.; et al. Functional gap junctions accumulate at the immunological synapse and contribute to T cell activation. J. Immunol. 2011, 187, 3121–3132.
- Tittarelli, A.; Mendoza-Naranjo, A.; Farías, M.; Guerrero, I.; Ihara, F.; Wennerberg, E.; Riquelme, S.; Gleisner, A.; Kalergis, A.; Lundqvist, A.; et al. Gap junction intercellular communications regulate NK cell activation and modulate NK cytotoxic capacity. J. Immunol. 2014, 192, 1313–1319.
- Benlalam, H.; Carré, T.; Jalil, A.; Noman, Z.; Caillou, B.; Vielh, P.; Tittarelli, A.; Robert, C.; Chouaib, S. Regulation of gap junctions in melanoma and their impact on Melan-A/MART-1-specific CD8+ T lymphocyte emergence. J. Mol. Med. (Berl. Ger.) 2013, 91, 1207–1220.
- Hofmann, F.; Navarrete, M.; Álvarez, J.; Guerrero, I.; Gleisner, M.A.; Tittarelli, A.; Salazar-Onfray, F. Cx43-Gap Junctions Accumulate at the Cytotoxic Immunological Synapse Enabling Cytotoxic T Lymphocyte Melanoma Cell Killing. Int. J. Mol. Sci. 2019, 20, 4509.
- Benlalam, H.; Jalil, A.; Hasmim, M.; Pang, B.; Tamouza, R.; Mitterrand, M.; Godet, Y.; Lamerant, N.; Robert, C.; Avril, M.F.; et al. Gap junction communication between autologous endothelial and tumor cells induce cross-recognition and elimination by specific CTL. J. Immunol. 2009, 182, 2654–2664.
- Caillou, B.; Talbot, M.; Weyemi, U.; Pioche-Durieu, C.; Al Ghuzlan, A.; Bidart, J.M.; Chouaib, S.; Schlumberger, M.; Dupuy, C. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PloS One 2011, 6, e22567.
- Balla, P.; Maros, M.E.; Barna, G.; Antal, I.; Papp, G.; Sapi, Z.; Athanasou, N.A.; Benassi, M.S.; Picci, P.; Krenacs, T. Prognostic impact of reduced connexin43 expression and gap junction coupling of neoplastic stromal cells in giant cell tumor of bone. PLoS ONE 2015, 10, e0125316.
- Dilley, T.K.; Bowden, G.T.; Chen, Q.M. Novel mechanisms of sublethal oxidant toxicity: Induction of premature senescence in human fibroblasts confers tumor promoter activity. Exp. Cell Res. 2003, 290, 38–48.
- Kiszner, G.; Balla, P.; Wichmann, B.; Barna, G.; Baghy, K.; Nemeth, I.B.; Varga, E.; Furi, I.; Toth, B.; Krenacs, T. Exploring Differential Connexin Expression across Melanocytic Tumor Progression Involving the Tumor Microenvironment. Cancers 2019, 11, 165.
- Haass, N.K.; Ripperger, D.; Wladykowski, E.; Dawson, P.; Gimotty, P.A.; Blome, C.; Fischer, F.; Schmage, P.; Moll, I.; Brandner, J.M. Melanoma progression exhibits a significant impact on connexin expression patterns in the epidermal tumor microenvironment. Histochem. Cell Biol. 2010, 133, 113–124.
- Au, A.; Shao, Q.; White, K.K.; Lucaciu, S.A.; Esseltine, J.L.; Barr, K.; Laird, D.W. Comparative Analysis of Cx31 and Cx43 in Differentiation-Competent Rodent Keratinocytes. Biomolecules 2020, 10, 1443.
- Stuhlmann, D.; Ale-Agha, N.; Reinehr, R.; Steinbrenner, H.; Ramos, M.C.; Sies, H.; Brenneisen, P. Modulation of homologous gap junctional intercellular communication of human dermal fibroblasts via a paracrine factor(s) generated by squamous tumor cells. Carcinogenesis 2003, 24, 1737–1748.
- Essa, A.A.; Yamazaki, M.; Maruyama, S.; Abé, T.; Babkair, H.; Raghib, A.M.; Megahed, E.M.; Cheng, J.; Saku, T. Tumour-associated macrophages are recruited and differentiated in the neoplastic stroma of oral squamous cell carcinoma. Pathology 2016, 48, 219–227.
- Zoellner, H.; Paknejad, N.; Cornwell, J.A.; Chami, B.; Romin, Y.; Boyko, V.; Fujisawa, S.; Kelly, E.; Lynch, G.W.; Rogers, G.; et al. Potential Hydrodynamic Cytoplasmic Transfer between Mammalian Cells: Cell-Projection Pumping. Biophys. J. 2020, 118, 1248–1260.
- Khawar, I.A.; Park, J.K.; Jung, E.S.; Lee, M.A.; Chang, S.; Kuh, H.-J. Three Dimensional Mixed-Cell Spheroids Mimic Stroma-Mediated Chemoresistance and Invasive Migration in hepatocellular carcinoma. Neoplasia 2018, 20, 800–812.
- Masamune, A.; Suzuki, N.; Kikuta, K.; Ariga, H.; Hayashi, S.; Takikawa, T.; Kume, K.; Hamada, S.; Hirota, M.; Kanno, A.; et al. Connexins regulate cell functions in pancreatic stellate cells. Pancreas 2013, 42, 308–316.
- Avanzo, J.L.; Mesnil, M.; Hernandez-Blazquez, F.J.; Mackowiak, I.I.; Mori, C.M.; da Silva, T.C.; Oloris, S.C.; Gárate, A.P.; Massironi, S.M.; Yamasaki, H.; et al. Increased susceptibility to urethane-induced lung tumors in mice with decreased expression of connexin43. Carcinogenesis 2004, 25, 1973–1982.
- De Oliveira, K.D.; Tedardi, M.V.; Cogliati, B.; Dagli, M.L.Z. Higher incidence of lung adenocarcinomas induced by DMBA in connexin 43 heterozygous knockout mice. Biomed. Res. Int. 2013, 2013, 618475.
- Evert, M.; Ott, T.; Temme, A.; Willecke, K.; Dombrowski, F. Morphology and morphometric investigation of hepatocellular preneoplastic lesions and neoplasms in connexin32-deficient mice. Carcinogenesis 2002, 23, 697–703.
- King, T.J.; Lampe, P.D. Mice deficient for the gap junction protein Connexin32 exhibit increased radiation-induced tumorigenesis associated with elevated mitogen-activated protein kinase (p44/Erk1, p42/Erk2) activation. Carcinogenesis 2004, 25, 669–680.
- Naiki-Ito, A.; Kato, H.; Naiki, T.; Yeewa, R.; Aoyama, Y.; Nagayasu, Y.; Suzuki, S.; Inaguma, S.; Takahashi, S. A novel model of non-alcoholic steatohepatitis with fibrosis and carcinogenesis in connexin 32 dominant-negative transgenic rats. Arch. Toxicol. 2020, 94, 4085–4097.
- Sun, T.; Li, D.; Hu, S.; Huang, L.; Sun, H.; Yang, S.; Wu, B.; Ji, F.; Zhou, D. Aging-dependent decrease in the numbers of enteric neurons, interstitial cells of Cajal and expression of connexin43 in various regions of gastrointestinal tract. Aging 2018, 10, 3851–3865.
- Perrot, C.Y.; Herrera, J.L.; Fournier-Goss, A.E.; Komatsu, M. Prostaglandin E2 breaks down pericyte-endothelial cell interaction via EP1 and EP4-dependent downregulation of pericyte N-cadherin, connexin-43, and R-Ras. Sci. Rep. 2020, 10, 11186.
- Yassine, F.; Fostok, S.F.; Al Deen, N.N.; Talhouk, R.S. Endotoxin Triggers Tumor Initiation Events in Nontumorigenic Breast Epithelial Cells and Enhances Invasion-Related Phenotype in Pretumorigenic and Tumorigenic Breast Epithelial Cells. Int. J. Inflam 2021, 2021, 4666380.
- Sun, P.; Dong, L.; MacDonald, A.I.; Akbari, S.; Edward, M.; Hodgins, M.B.; Johnstone, S.R.; Graham, S.V. HPV16 E6 Controls the Gap Junction Protein Cx43 in Cervical Tumour Cells. Viruses 2015, 7, 5243–5256.
- Polusani, S.R.; Huang, Y.W.; Huang, G.; Chen, C.W.; Wang, C.M.; Lin, L.L.; Osmulski, P.; Lucio, N.D.; Liu, L.; Hsu, Y.T.; et al. Adipokines Deregulate Cellular Communication via Epigenetic Repression of Gap Junction Loci in Obese Endometrial Cancer. Cancer Res. 2019, 79, 196–208.
- Kim, E.M.; Bae, Y.M.; Choi, M.H.; Hong, S.T. Connexin 43 plays an important role in the transformation of cholangiocytes with Clonochis sinensis excretory-secretory protein and N-nitrosodimethylamine. PLoS Negl. Trop. Dis. 2019, 13, e0006843.
- Ruch, R.J.; Trosko, J.E. The role of oval cells and gap junctional intercellular communication in hepatocarcinogenesis. Anticancer Res. 1999, 19, 4831–4838.
- Qin, J.; Chang, M.; Wang, S.; Liu, Z.; Zhu, W.; Wang, Y.; Yan, F.; Li, J.; Zhang, B.; Dou, G.; et al. Connexin 32-mediated cell-cell communication is essential for hepatic differentiation from human embryonic stem cells. Sci. Rep. 2016, 6, 37388.
- Pei, H.; Zhai, C.; Li, H.; Yan, F.; Qin, J.; Yuan, H.; Zhang, R.; Wang, S.; Zhang, W.; Chang, M.; et al. Connexin 32 and connexin 43 are involved in lineage restriction of hepatic progenitor cells to hepatocytes. Stem Cell Res. Ther. 2017, 8, 252.
- Dianati, E.; Poiraud, J.; Weber-Ouellette, A.; Plante, I. Connexins, E-cadherin, Claudin-7 and β-catenin transiently form junctional nexuses during the post-natal mammary gland development. Dev. Biol. 2016, 416, 52–68.
- Adak, A.; Unal, Y.C.; Yucel, S.; Vural, Z.; Turan, F.B.; Yalcin-Ozuysal, O.; Ozcivici, E.; Mese, G. Connexin 32 induces pro-tumorigenic features in MCF10A normal breast cells and MDA-MB-231 metastatic breast cancer cells. Biochim. Et Biophys. Acta. Mol. Cell Res. 2020, 1867, 118851.
- Bache, M.; Kappler, M.; Said, H.M.; Staab, A.; Vordermark, D. Detection and specific targeting of hypoxic regions within solid tumors: Current preclinical and clinical strategies. Curr. Med. Chem. 2008, 15, 322–338.
- Walsh, J.C.; Lebedev, A.; Aten, E.; Madsen, K.; Marciano, L.; Kolb, H.C. The clinical importance of assessing tumor hypoxia: Relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid. Redox Signal. 2014, 21, 1516–1554.
- Orlova, A.G.; Kirillin, M.Y.; Volovetsky, A.B.; Shilyagina, N.Y.; Sergeeva, E.A.; Golubiatnikov, G.Y.; Turchin, I.V. Diffuse optical spectroscopy monitoring of oxygen state and hemoglobin concentration during SKBR-3 tumor model growth. Laser Phys. Lett. 2016, 14, 015601.
- Zibara, K.; Awada, Z.; Dib, L.; El-Saghir, J.; Al-Ghadban, S.; Ibrik, A.; El-Zein, N.; El-Sabban, M. Anti-angiogenesis therapy and gap junction inhibition reduce MDA-MB-231 breast cancer cell invasion and metastasis in vitro and in vivo. Sci. Rep. 2015, 5, 12598.
- Elzarrad, K.; Haroon, A.; Reed, D.; Al-Mehdi, A.B. Early incorporated endothelial cells as origin of metastatic tumor vasculogenesis. Clin. Exp. Metastasis 2009, 26, 589–598.
- Mikuła-Pietrasik, J.; Uruski, P.; Szubert, S.; Maksin, K.; Moszyński, R.; Szpurek, D.; Woźniak, A.; Sajdak, S.; Tykarski, A.; Książek, K. The Proangiogenic Capabilities of Malignant Ascites Generated by Aggressive Ovarian Tumors. Biomed. Res. Int. 2017, 2017, 2592496.
- Nagy, J.A.; Benjamin, L.; Zeng, H.; Dvorak, A.M.; Dvorak, H.F. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 2008, 11, 109–119.
- Wang, W.K.; Chen, M.C.; Leong, H.F.; Kuo, Y.L.; Kuo, C.Y.; Lee, C.H. Connexin 43 suppresses tumor angiogenesis by down-regulation of vascular endothelial growth factor via hypoxic-induced factor-1α. Int. J. Mol. Sci. 2014, 16, 439–451.
- Dovmark, T.H.; Hulikova, A.; Niederer, S.A.; Vaughan-Jones, R.D.; Swietach, P. Normoxic cells remotely regulate the acid-base balance of cells at the hypoxic core of connexin-coupled tumor growths. FASEB J. 2018, 32, 83–96.
- Dovmark, T.H.; Saccomano, M.; Hulikova, A.; Alves, F.; Swietach, P. Connexin-43 channels are a pathway for discharging lactate from glycolytic pancreatic ductal adenocarcinoma cells. Oncogene 2017, 36, 4538–4550.
- Hulikova, A.; Black, N.; Hsia, L.T.; Wilding, J.; Bodmer, W.F.; Swietach, P. Stromal uptake and transmission of acid is a pathway for venting cancer cell-generated acid. Proc. Natl. Acad. Sci. USA 2016, 113, E5344–E5353.
- De la Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front. Oncol. 2019, 9, 1143.
- Kucheryavykh, L.Y.; Benedikt, J.; Cubano, L.A.; Skatchkov, S.N.; Bukauskas, F.F.; Kucheryavykh, Y.V. Polyamines preserve connexin 43-mediated gap junctional communication during intracellular hypercalcemia and acidosis. Neuroreport 2017, 28, 208–213.
- Thuringer, D.; Jego, G.; Berthenet, K.; Hammann, A.; Solary, E.; Garrido, C. Gap junction-mediated transfer of miR-145-5p from microvascular endothelial cells to colon cancer cells inhibits angiogenesis. Oncotarget 2016, 7, 28160–28168.
- Fukuda, S.; Akiyama, M.; Niki, Y.; Kawatsura, R.; Harada, H.; Nakahama, K.I. Inhibitory effects of miRNAs in astrocytes on C6 glioma progression via connexin 43. Mol. Cell. Biochem. 2021, 476, 2623–2632.
- Aucher, A.; Rudnicka, D.; Davis, D.M. MicroRNAs transfer from human macrophages to hepato-carcinoma cells and inhibit proliferation. J. Immunol. 2013, 191, 6250–6260.
- Thuringer, D.; Boucher, J.; Jego, G.; Pernet, N.; Cronier, L.; Hammann, A.; Solary, E.; Garrido, C. Transfer of functional microRNAs between glioblastoma and microvascular endothelial cells through gap junctions. Oncotarget 2016, 7, 73925–73934.
- Tittarelli, A.; Navarrete, M.; Lizana, M.; Hofmann-Vega, F.; Salazar-Onfray, F. Hypoxic Melanoma Cells Deliver microRNAs to Dendritic Cells and Cytotoxic T Lymphocytes through Connexin-43 Channels. Int. J. Mol. Sci. 2020, 21, 7567.
- Lim, P.K.; Bliss, S.A.; Patel, S.A.; Taborga, M.; Dave, M.A.; Gregory, L.A.; Greco, S.J.; Bryan, M.; Patel, P.S.; Rameshwar, P. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011, 71, 1550–1560.
- Varela-Eirin, M.; Varela-Vazquez, A.; Rodríguez-Candela Mateos, M.; Vila-Sanjurjo, A.; Fonseca, E.; Mascareñas, J.L.; Eugenio Vázquez, M.; Mayan, M.D. Recruitment of RNA molecules by connexin RNA-binding motifs: Implication in RNA and DNA transport through microvesicles and exosomes. Biochim. Et Biophys. Acta. Mol. Cell Res. 2017, 1864, 728–736.
- Acuña, R.A.; Varas-Godoy, M.; Berthoud, V.M.; Alfaro, I.E.; Retamal, M.A. Connexin-46 Contained in Extracellular Vesicles Enhance Malignancy Features in Breast Cancer Cells. Biomolecules 2020, 10, 676.
- Zong, L.; Zhu, Y.; Liang, R.; Zhao, H.B. Gap junction mediated miRNA intercellular transfer and gene regulation: A novel mechanism for intercellular genetic communication. Sci. Rep. 2016, 6, 19884.
- Peng, Y.; Wang, X.; Guo, Y.; Peng, F.; Zheng, N.; He, B.; Ge, H.; Tao, L.; Wang, Q. Pattern of cell-to-cell transfer of microRNA by gap junction and its effect on the proliferation of glioma cells. Cancer Sci. 2019, 110, 1947–1958.
- Prasad, S.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Srivastava, S.K. Cancer cells stemness: A doorstep to targeted therapy. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165424.
- Ruch, R.J. Connexin43 Suppresses Lung Cancer Stem Cells. Cancers 2019, 11, 175.
- Arun, S.; Ravisankar, S.; Vanisree, A.J. Implication of connexin30 on the stemness of glioma: Connexin30 reverses the malignant phenotype of glioma by modulating IGF-1R, CD133 and cMyc. J. Neuro-Oncol. 2017, 135, 473–485.
- Shen, Y.; Li, Y.; Ma, X.; Wan, Q.; Jiang, Z.; Liu, Y.; Zhang, D.; Liu, X.; Wu, W. Connexin 43 SUMOylation improves gap junction functions between liver cancer stem cells and enhances their sensitivity to HSVtk/GCV. Int. J. Oncol. 2018, 52, 872–880.
- Thiagarajan, P.S.; Sinyuk, M.; Turaga, S.M.; Mulkearns-Hubert, E.E.; Hale, J.S.; Rao, V.; Demelash, A.; Saygin, C.; China, A.; Alban, T.J.; et al. Cx26 drives self-renewal in triple-negative breast cancer via interaction with NANOG and focal adhesion kinase. Nat. Commun. 2018, 9, 578.
- Mulkearns-Hubert, E.E.; Torre-Healy, L.A.; Silver, D.J.; Eurich, J.T.; Bayik, D.; Serbinowski, E.; Hitomi, M.; Zhou, J.; Przychodzen, B.; Zhang, R.; et al. Development of a Cx46 Targeting Strategy for Cancer Stem Cells. Cell Rep. 2019, 27, 1062–1072.e1065.
- Todorova, M.G.; Soria, B.; Quesada, I. Gap junctional intercellular communication is required to maintain embryonic stem cells in a non-differentiated and proliferative state. J. Cell. Physiol. 2008, 214, 354–362.
- Sinha, G.; Ferrer, A.I.; Ayer, S.; El-Far, M.H.; Pamarthi, S.H.; Naaldijk, Y.; Barak, P.; Sandiford, O.A.; Bibber, B.M.; Yehia, G.; et al. Specific N-cadherin-dependent pathways drive human breast cancer dormancy in bone marrow. Life Sci. Alliance 2021, 4.
- Patel, S.A.; Dave, M.A.; Bliss, S.A.; Giec-Ujda, A.B.; Bryan, M.; Pliner, L.F.; Rameshwar, P. T(reg)/Th17 polarization by distinct subsets of breast cancer cells is dictated by the interaction with mesenchymal stem cells. J. Cancer Stem Cell Res. 2014, 2014, e1003.
- Kuramoto, K.; Yamamoto, M.; Suzuki, S.; Sanomachi, T.; Togashi, K.; Seino, S.; Kitanaka, C.; Okada, M. AS602801, an Anti-Cancer Stem Cell Drug Candidate, Suppresses Gap-junction Communication Between Lung Cancer Stem Cells and Astrocytes. Anticancer Res. 2018, 38, 5093–5099.
- Trosko, J.E. Cancer Prevention and Therapy of Two Types of Gap Junctional Intercellular Communication-Deficient “Cancer Stem Cell”. Cancers 2019, 11, 87.
- Trosko, J.E. On the potential origin and characteristics of cancer stem cells. Carcinogenesis 2021, 42, 905–912.
- Trosko, J.E. In Search of a Unifying Concept in Human Diseases. Diseases 2021, 9, 68.
This entry is offline, you can click here to edit this entry!
